Motor Imagery Training Is Beneficial for Motor Memory of Upper and Lower Limb Tasks in Very Old Adults
Abstract
:1. Introduction
2. Methods
2.1. Participants
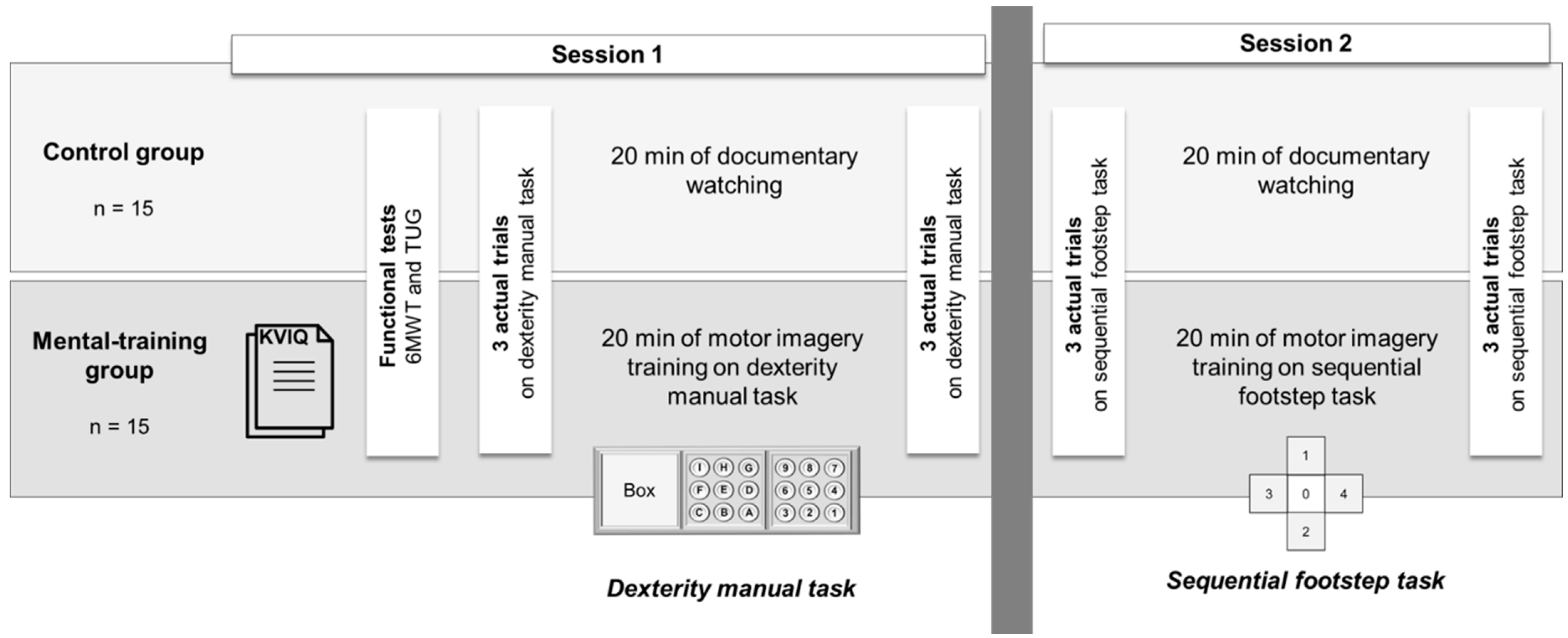
2.2. Experimental Procedures
2.3. Imagery Ability
2.4. Functional Tests
2.5. Experiment on Old Adults
2.6. Statistical Analysis
3. Results
3.1. Cognitive and Functional Capacities
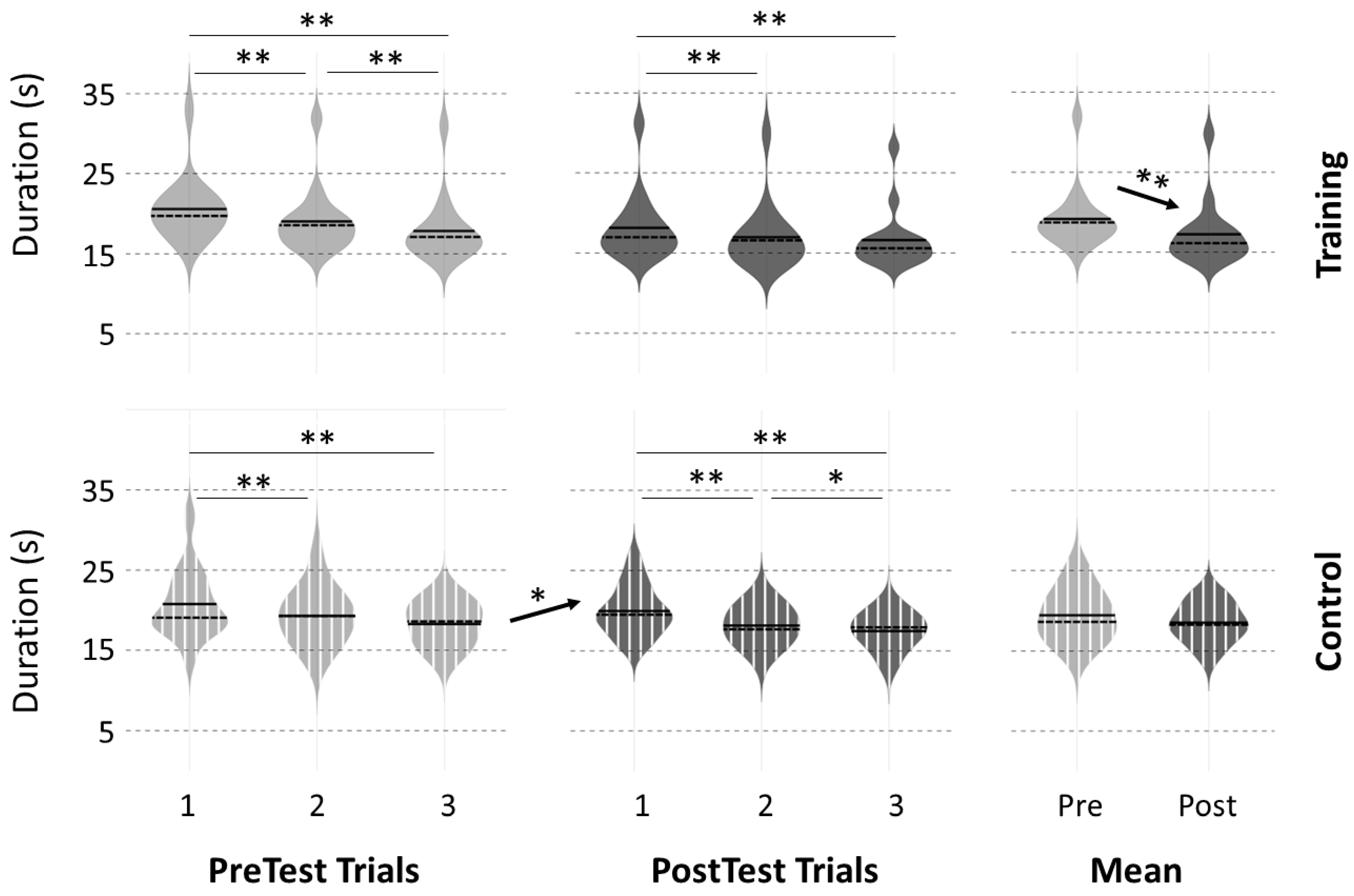
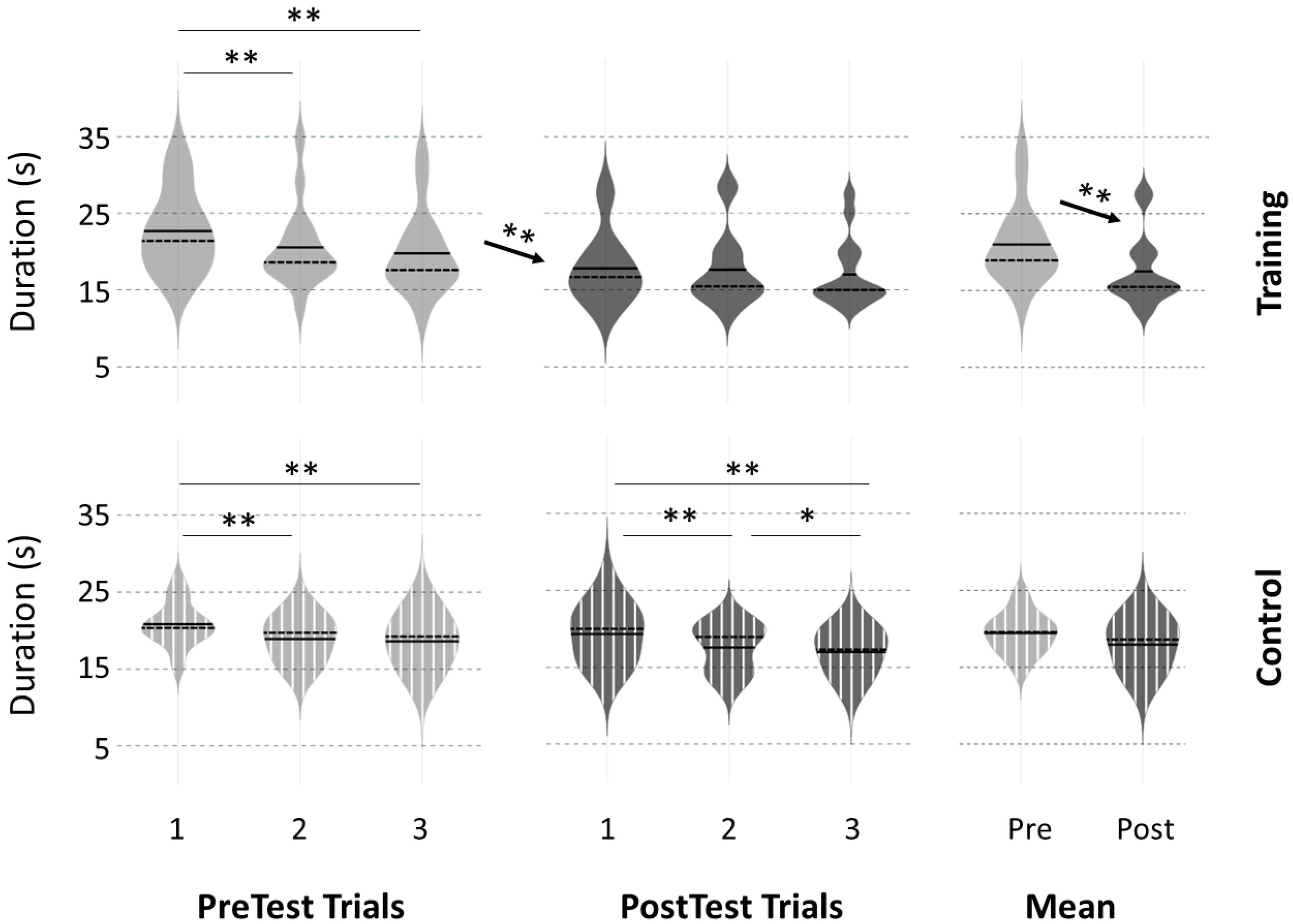
3.2. Motor Performance at PreTests
3.3. Motor Performance Following the Intervention
3.4. Comparison with Old Adults
4. Discussion
4.1. Performance Improvement after Physical Practice
4.2. Effects of the 20 Min Break Period on Performance
4.3. Effects of Mental Training on Performance
4.4. Efficiency of Mental Training across Ages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrews-Hanna, J.R.; Snyder, A.Z.; Vincent, J.L.; Lustig, C.; Head, D.; Raichle, M.E.E.; Buckner, R.L. Disruption of Large-Scale Brain Systems in Advanced Aging. Neuron 2007, 56, 924–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaVoie, D.J.; Cobia, D.J. Recollecting, Recognizing, and Other Acts of Remembering: An Overview of Human Memory. J. Neurol. Phys. Ther. 2007, 31, 135–144. [Google Scholar] [CrossRef]
- Robertson, E.M.; Pascual-Leone, A.; Miall, R.C. Current Concepts in Procedural Consolidation. Nat. Rev. Neurosci. 2004, 5, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Classen, J.; Liepert, J.; Wise, S.P.; Hallett, M.; Cohen, L.G. Rapid Plasticity of Human Cortical Movement Representation Induced by Practice. J. Neurophysiol. 1998, 79, 1117–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anguera, J.A.; Reuter-Lorenz, P.A.; Willingham, D.T.; Seidler, R.D. Failure to Engage Spatial Working Memory Contributes to Age-Related Declines in Visuomotor Learning. J. Cogn. Neurosci. 2011, 23, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Ahmed, A.A. Older Adults Learn Less, but Still Reduce Metabolic Cost, during Motor Adaptation. J. Neurophysiol. 2014, 111, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Ruffino, C.; Bourrelier, J.; Papaxanthis, C.; Mourey, F.; Lebon, F. The Use of Motor Imagery Training to Retain the Performance Improvement Following Physical Practice in the Elderly. Exp. Brain Res. 2019, 237, 1375–1382. [Google Scholar] [CrossRef]
- Malone, L.A.; Bastian, A.J. Age-Related Forgetting in Locomotor Adaptation. Neurobiol. Learn. Mem. 2016, 128, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Flöel, A.; Breitenstein, C.; Hummel, F.; Celnik, P.; Gingert, C.; Sawaki, L.; Knecht, S.; Cohen, L.G. Dopaminergic Influences on Formation of a Motor Memory. Ann. Neurol. 2005, 58, 121–130. [Google Scholar] [CrossRef]
- Jeannerod, M. The Representing Brain: Neural Correlates of Motor Intention and Imagery. Behav. Brain Sci. 1994, 17, 187. [Google Scholar] [CrossRef] [Green Version]
- Decety, J.; Perani, D.; Jeannerod, M.; Bettinardi, V.; Tadary, B.; Woods, R.; Mazziotta, J.C.; Fazio, F. Mapping Motor Representations with Positron Emission Tomography. Nature 1994, 371, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Gerardin, E.; Sirigu, A.; Lehéricy, S.; Poline, J.B.; Gaymard, B.; Marsault, C.; Agid, Y.; Le Bihan, D. Partially Overlapping Neural Networks for Real and Imagined Hand Movements. Cereb. Cortex 2000, 10, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.; Collet, C.; Nguyen, V.A.; Malouin, F.; Richards, C.; Doyon, J. Brain Activity during Visual versus Kinesthetic Imagery: An FMRI Study. Hum. Brain Mapp. 2009, 30, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.; Collet, C. Construction of the Motor Imagery Integrative Model in Sport: A Review and Theoretical Investigation of Motor Imagery Use. Int. Rev. Sport Exerc. Psychol. 2008, 1, 31–44. [Google Scholar] [CrossRef]
- Yue, G.; Cole, K.J. Strength Increases from the Motor Program: Comparison of Training with Maximal Voluntary and Imagined Muscle Contractions. J. Neurophysiol. 1992, 67, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Zijdewind, I.; Toering, S.T.; Bessem, B.; Van der Laan, O.; Diercks, R.L. Effects of Imagery Motor Training on Torque Production of Ankle Plantar Flexor Muscles. Muscle Nerve 2003, 28, 168–173. [Google Scholar] [CrossRef]
- Feltz, D.L.; Landers, D.M. The Effects of Mental Practice on Motor Skill Learning and Performance: A Meta-Analysis. J. Sport Psychol. 1983, 5, 25–57. [Google Scholar] [CrossRef]
- Gentili, R.; Papaxanthis, C.; Pozzo, T. Improvement and Generalization of Arm Motor Performance through Motor Imagery Practice. Neuroscience 2006, 137, 761–772. [Google Scholar] [CrossRef]
- Saimpont, A.; Malouin, F.; Tousignant, B.; Jackson, P.L. Motor Imagery and Aging. J. Mot. Behav. 2013, 45, 21–28. [Google Scholar] [CrossRef]
- Hamel, M.F.; Lajoie, Y. Mental Imagery. Effects on Static Balance and Attentional Demands of the Elderly. Aging Clin. Exp. Res. 2005, 17, 223–228. [Google Scholar] [CrossRef]
- Varesco, G.; Luneau, E.; Féasson, L.; Lapole, T.; Rozand, V. Very Old Adults Show Impaired Fatigue Resistance Compared to Old Adults Independently of Sex during a Knee-Extensors Isometric Test. Exp. Gerontol. 2022, 161, 111732. [Google Scholar] [CrossRef] [PubMed]
- Schott, N.; Munzert, J. Temporal Accuracy of Motor Imagery in Older Women. Int. J. Sport Psychol. 2007, 38, 304–320. [Google Scholar]
- Skoura, X.; Papaxanthis, C.; Vinter, A.; Pozzo, T. Mentally Represented Motor Actions in Normal Aging: I. Age Effects on the Temporal Features of Overt and Covert Execution of Actions. Behav. Brain Res. 2005, 165, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA—J. Am. Med. Assoc. 2011, 305, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Rozand, V.; Lebon, F.; Stapley, P.J.; Papaxanthis, C.; Lepers, R. A Prolonged Motor Imagery Sessions Alter Imagined and Actual Movement Durations: Potential Implications for Neurorehabilitation. Behav. Brain Res. 2016, 297, 67–75. [Google Scholar] [CrossRef]
- Malouin, F.; Richards, C.L.; Jackson, P.L.; Lafleur, M.F.; Durand, A.; Doyon, J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for Assessing Motor Imagery in Persons with Physical Disabilities: A Reliability and Construct Validity Study. J. Neurol. Phys. Ther. 2007, 31, 20–29. [Google Scholar] [CrossRef]
- Brooks, D.; Solway, S.; Gibbons, W.J. ATS Statement on Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2003, 167, 1287. [Google Scholar] [CrossRef]
- Mathias, S.; Nayak, U.S.L.; Isaacs, B. Balance in Elderly Patients: The “get-up and Go” Test. Arch. Phys. Med. Rehabil. 1986, 67, 387–389. [Google Scholar]
- Coats, R.O.; Wilson, A.D.; Snapp-Childs, W.; Fath, A.J.; Bingham, G.P. The 50s Cliff: Perceptuo-Motor Learning Rates across the Lifespan. PLoS ONE 2014, 9, e85758. [Google Scholar] [CrossRef]
- Cirillo, J.; Todd, G.; Semmler, J.G. Corticomotor Excitability and Plasticity Following Complex Visuomotor Training in Young and Old Adults. Eur. J. Neurosci. 2011, 34, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Bickford, P. Motor Learning Deficits in Aged Rats Are Correlated with Loss of Cerebellar Noradrenergic Function. Brain Res. 1993, 620, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age- and Gender-Related Test Performance in Community-Dwelling Elderly People: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and Gait Speeds. Phys. Ther. 2002, 82, 128–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muellbacher, W.; Zlemann, U.; Wissel, J.; Dang, N.; Kofler, M.; Facchini, S.; Boroojerdi, B.; Poewe, W.; Hallett, M. Early Consolidation in Human Primary Motor Cortex. Nature 2002, 415, 640–644. [Google Scholar] [CrossRef]
- Galea, J.M.; Vazquez, A.; Pasricha, N.; Orban De Xivry, J.J.; Celnik, P. Dissociating the Roles of the Cerebellum and Motor Cortex during Adaptive Learning: The Motor Cortex Retains What the Cerebellum Learns. Cereb. Cortex 2011, 21, 1761–1770. [Google Scholar] [CrossRef] [Green Version]
- Jouvenceau, A.; Dutar, P.; Billard, J.M. Alteration of NMDA Receptor-Mediated Synaptic Responses in CA1 Area of the Aged Rat Hippocampus: Contribution of GABAergic and Cholinergic Deficits. Hippocampus 1998, 8, 627–637. [Google Scholar] [CrossRef]
- Sawaki, L.; Yaseen, Z.; Kopylev, L.; Cohen, L.G. Age-Dependent Changes in the Ability to Encode a Novel Elementary Motor Memory. Ann. Neurol. 2003, 53, 521–524. [Google Scholar] [CrossRef]
- Nicholson, V.; Watts, N.; Chani, Y.; Keogh, J.W. Motor Imagery Training Improves Balance and Mobility Outcomes in Older Adults: A Systematic Review. J. Physiother. 2019, 65, 200–207. [Google Scholar] [CrossRef]
- Ruffino, C.; Papaxanthis, C.; Lebon, F. The Influence of Imagery Capacity in Motor Performance Improvement. Exp. Brain Res. 2017, 235, 3049–3057. [Google Scholar] [CrossRef]
- Taube, W.; Lorch, M.; Zeiter, S.; Keller, M. Non-Physical Practice Improves Task Performance in an Unstable, Perturbed Environment: Motor Imagery and Observational Balance Training. Front. Hum. Neurosci. 2014, 8, 972. [Google Scholar] [CrossRef] [Green Version]
- Gentili, R.; Han, C.E.; Schweighofer, N.; Papaxanthis, C. Motor Learning without Doing: Trial-by-Trial Improvement in Motor Performance during Mental Training. J. Neurophysiol. 2010, 104, 774–783. [Google Scholar] [CrossRef]
- Dahm, S.; Rieger, M. Is Imagery Better than Reality? Performance in Imagined Dart Throwing. Hum. Mov. Sci. 2019, 66, 38–52. [Google Scholar] [CrossRef]
- Kilteni, K.; Andersson, B.J.; Houborg, C.; Ehrsson, H.H. Motor Imagery Involves Predicting the Sensory Consequences of the Imagined Movement. Nat. Commun. 2018, 9, 1617. [Google Scholar] [CrossRef] [Green Version]
- Ruffino, C.; Rannaud Monany, D.; Papaxanthis, C.; Hilt, P.M.; Gaveau, J.; Lebon, F. Smoothness Discriminates Physical from Motor Imagery Practice of Arm Reaching Movements. Neuroscience 2022, 483, 24–31. [Google Scholar] [CrossRef]
- Ruffino, C.; Papaxanthis, C.; Lebon, F. Neural Plasticity during Motor Learning with Motor Imagery Practice: Review and Perspectives. Neuroscience 2017, 341, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Grosprêtre, S.; Ruffino, C.; Lebon, F. Motor Imagery and Cortico-Spinal Excitability: A Review. Eur. J. Sport Sci. 2016, 16, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Avanzino, L.; Gueugneau, N.; Bisio, A.; Ruggeri, P.; Papaxanthis, C.; Bove, M. Motor Cortical Plasticity Induced by Motor Learning through Mental Practice. Front. Behav. Neurosci. 2015, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturelli, M.; Reggiani, C.; Richardson, R.S.; Schena, F. Skeletal Muscle Function in the Oldest-Old: The Role of Intrinsic and Extrinsic Factors. Exerc. Sport Sci. Rev. 2018, 46, 188–194. [Google Scholar] [CrossRef] [PubMed]

| Mental-Training Group | Control Group | |
|---|---|---|
| MMSE | 29 ± 1 | 29 ± 2 |
| KVIQ | 86 ± 15 | |
| 6MWT (m) | 440 ± 68 | 426 ± 69 |
| TUG (s) | 9.0 ± 1.5 | 9.4 ± 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilt, P.M.; Bertrand, M.F.; Féasson, L.; Lebon, F.; Mourey, F.; Ruffino, C.; Rozand, V. Motor Imagery Training Is Beneficial for Motor Memory of Upper and Lower Limb Tasks in Very Old Adults. Int. J. Environ. Res. Public Health 2023, 20, 3541. https://doi.org/10.3390/ijerph20043541
Hilt PM, Bertrand MF, Féasson L, Lebon F, Mourey F, Ruffino C, Rozand V. Motor Imagery Training Is Beneficial for Motor Memory of Upper and Lower Limb Tasks in Very Old Adults. International Journal of Environmental Research and Public Health. 2023; 20(4):3541. https://doi.org/10.3390/ijerph20043541
Chicago/Turabian StyleHilt, Pauline M., Mathilde F. Bertrand, Léonard Féasson, Florent Lebon, France Mourey, Célia Ruffino, and Vianney Rozand. 2023. "Motor Imagery Training Is Beneficial for Motor Memory of Upper and Lower Limb Tasks in Very Old Adults" International Journal of Environmental Research and Public Health 20, no. 4: 3541. https://doi.org/10.3390/ijerph20043541






