Endocrine Disruption of Propylparaben in the Male Mosquitofish (Gambusia affinis): Tissue Injuries and Abnormal Gene Expressions of Hypothalamic-Pituitary-Gonadal-Liver Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Care
2.2. PrP Exposure
2.3. Sample Collection
2.4. Tissue Sections and HE Staining
2.5. RNA Extraction and qPCR
2.6. Statistical Analysis and Cluster Heat Map Analysis
3. Results
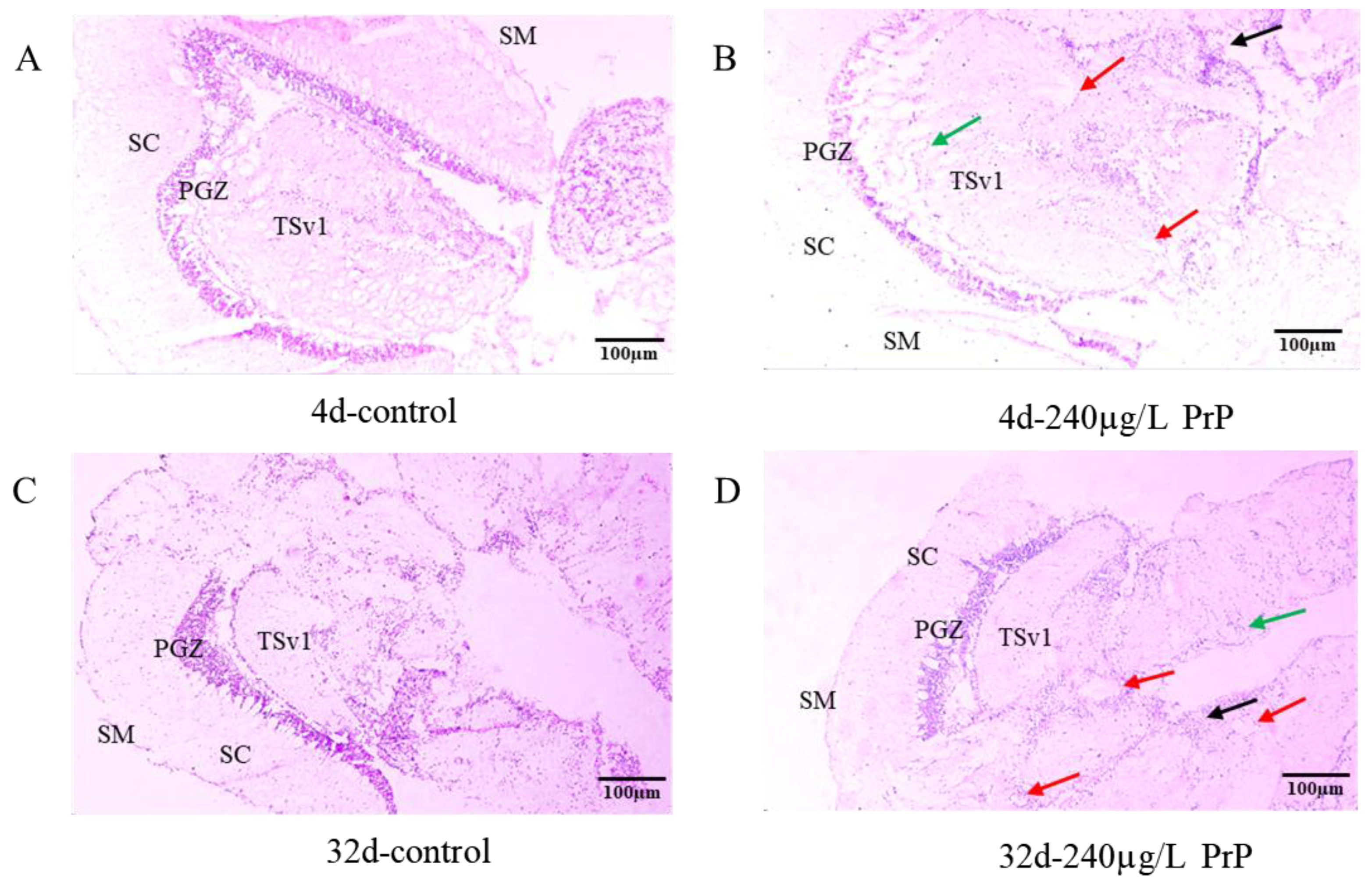
3.1. Brain Histopathology and Gene Expression Changes of HPGL Axis
3.1.1. Brain Histopathology
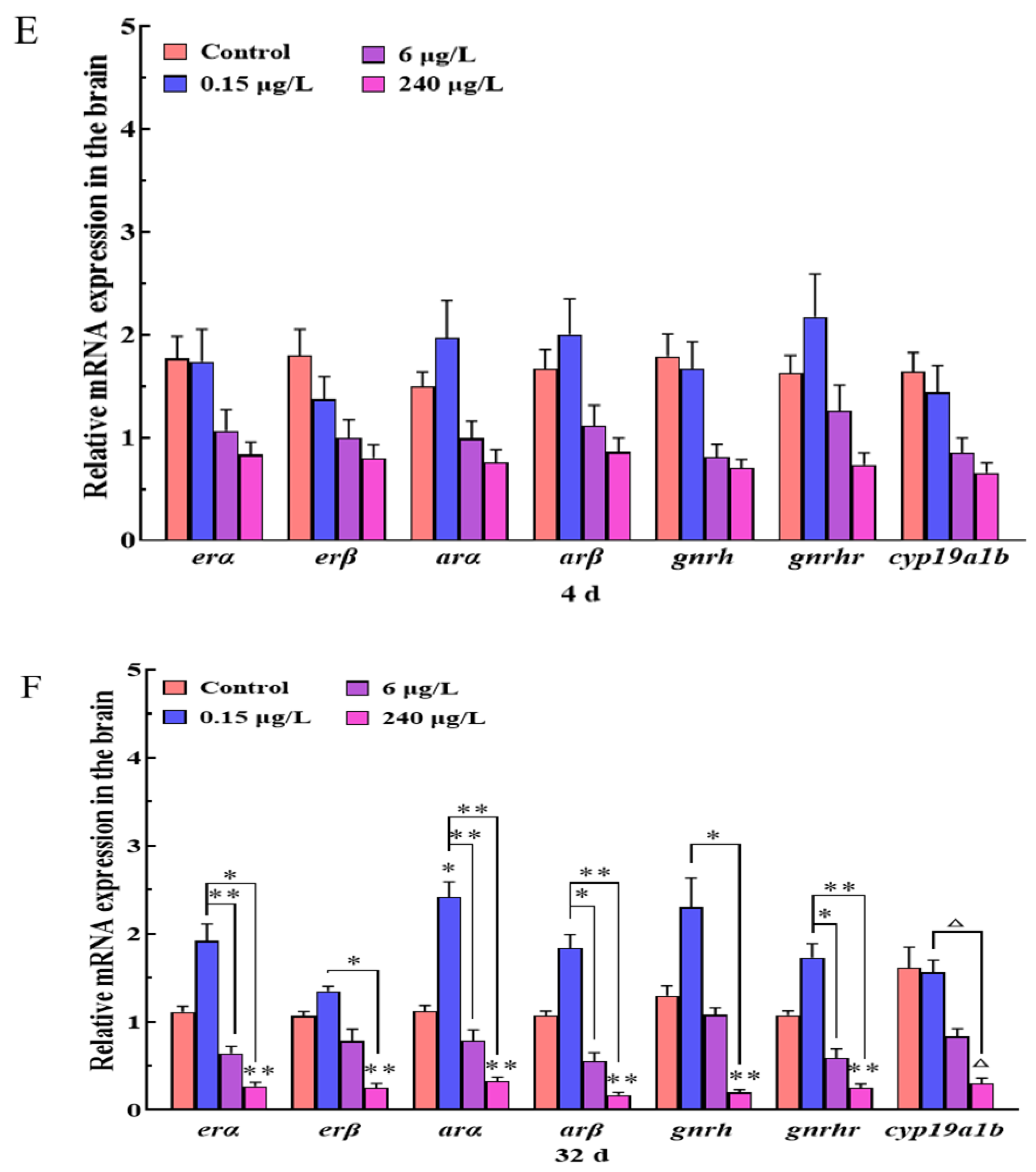
3.1.2. Gene Expression Changes of HPGL Axis in the Brain
3.2. Liver Histopathology and Gene Expression Changes of HPGL Axis
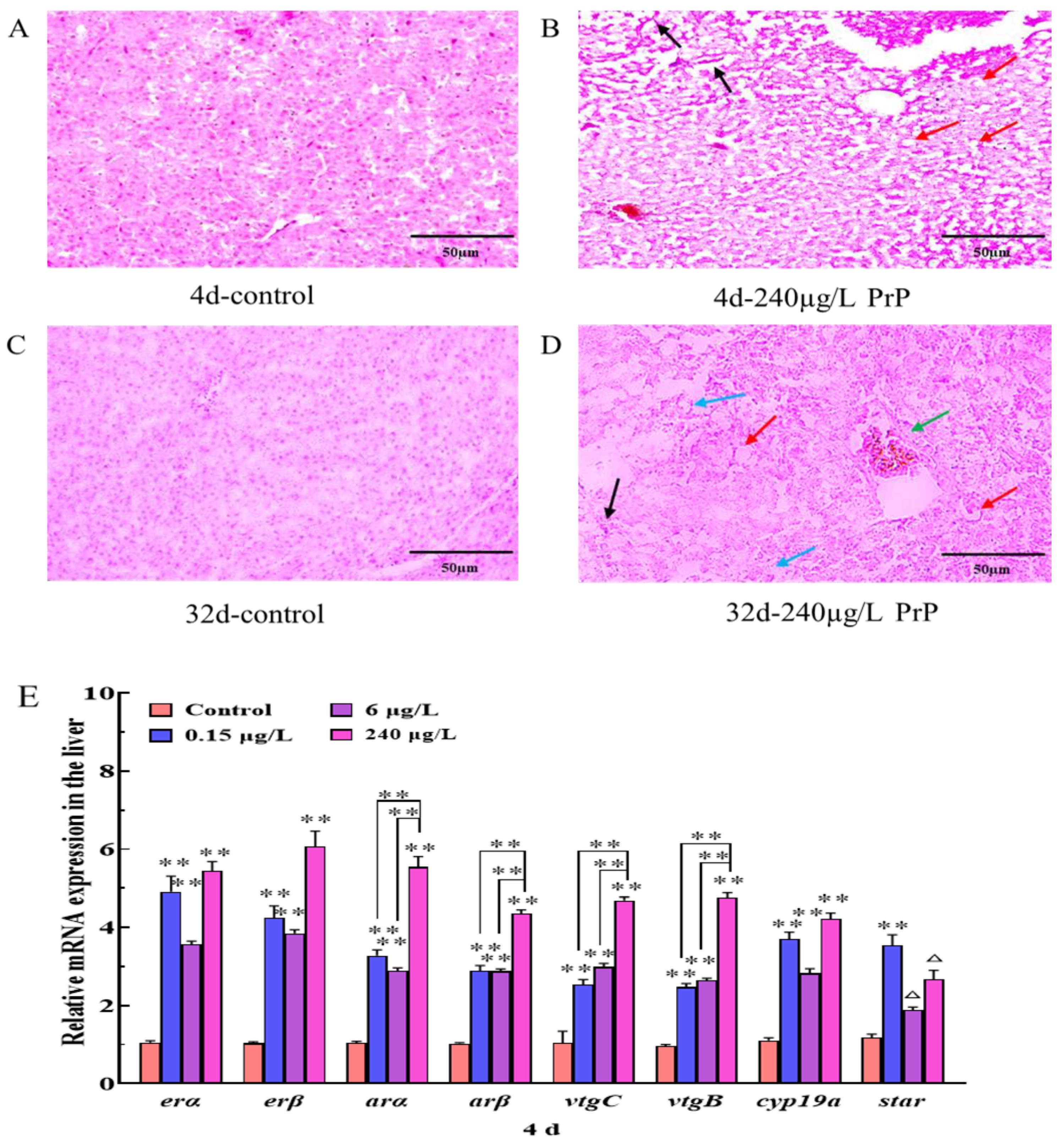
3.2.1. Liver Histopathology
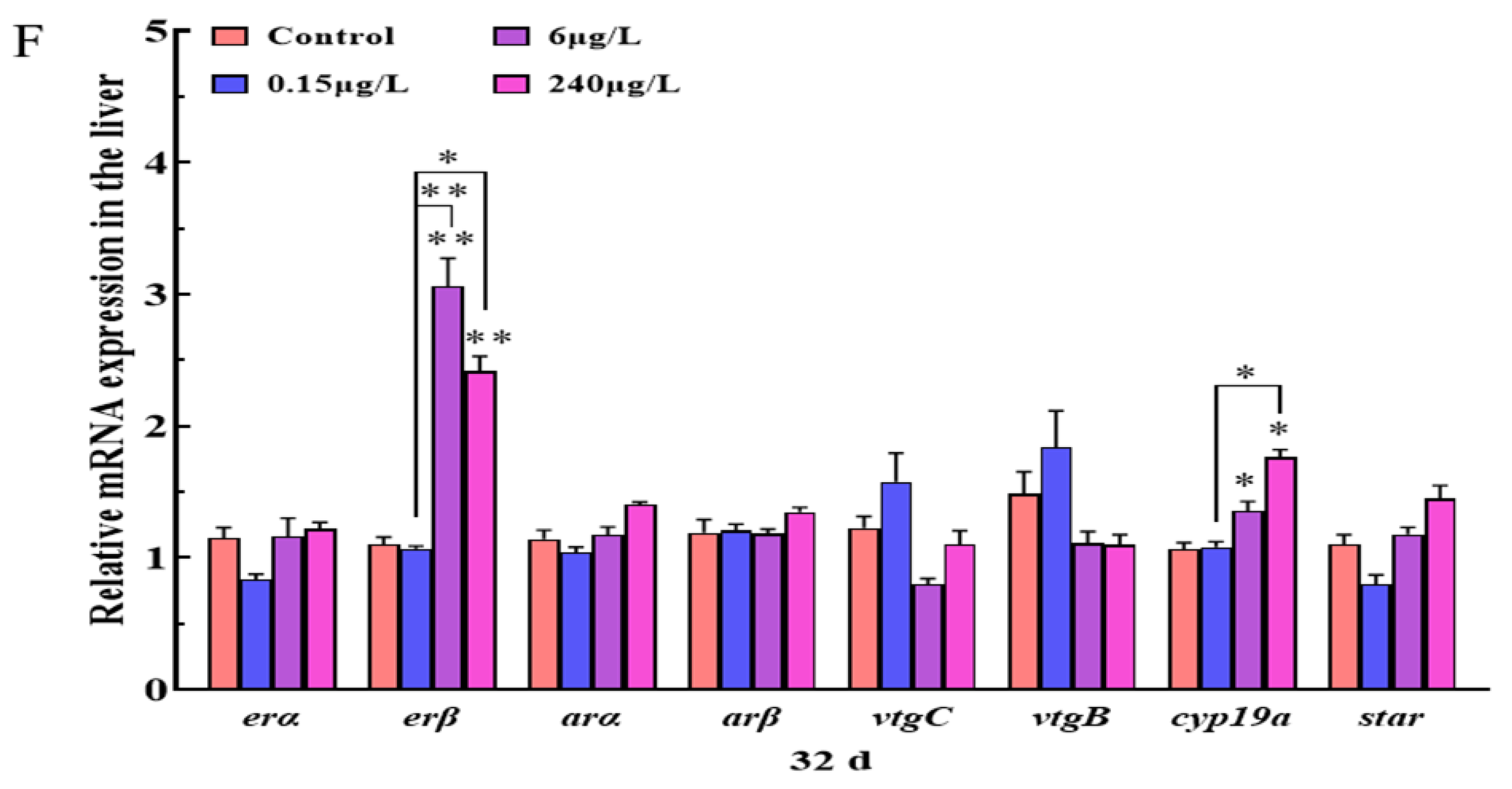
3.2.2. Gene Expression Changes of HPGL Axis in the Liver
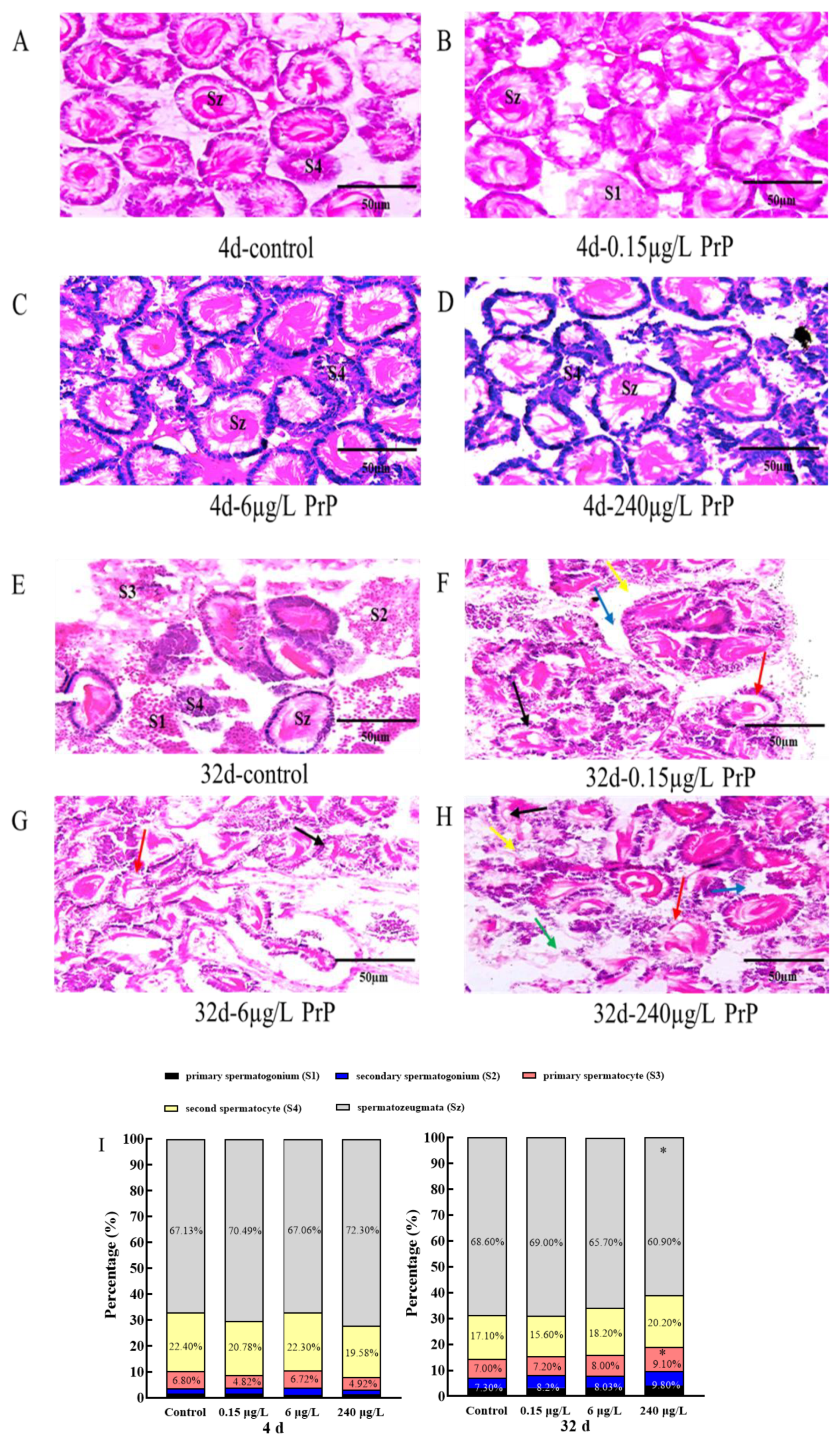
3.3. Testes Histopathology and Gene Expression Changes of HPGL Axis
3.3.1. Testes Histopathology and Morphometry
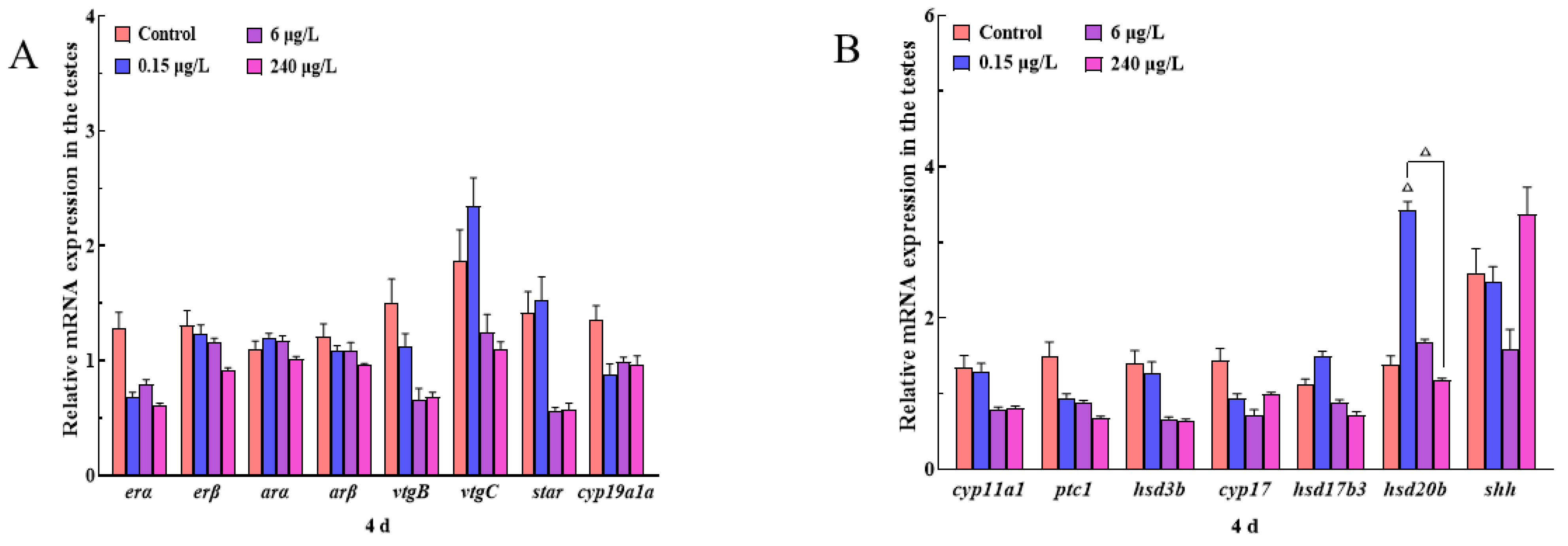
3.3.2. Gene Expression Changes of HPGL Axis in the Testes
3.4. Cluster Heat Map Analysis of HPGL Axis Related Genes in the Male Mosquitofish
3.5. The Time and Dose Dependent Manner of HPGL Axis Related Genes in the Male Mosquitofish
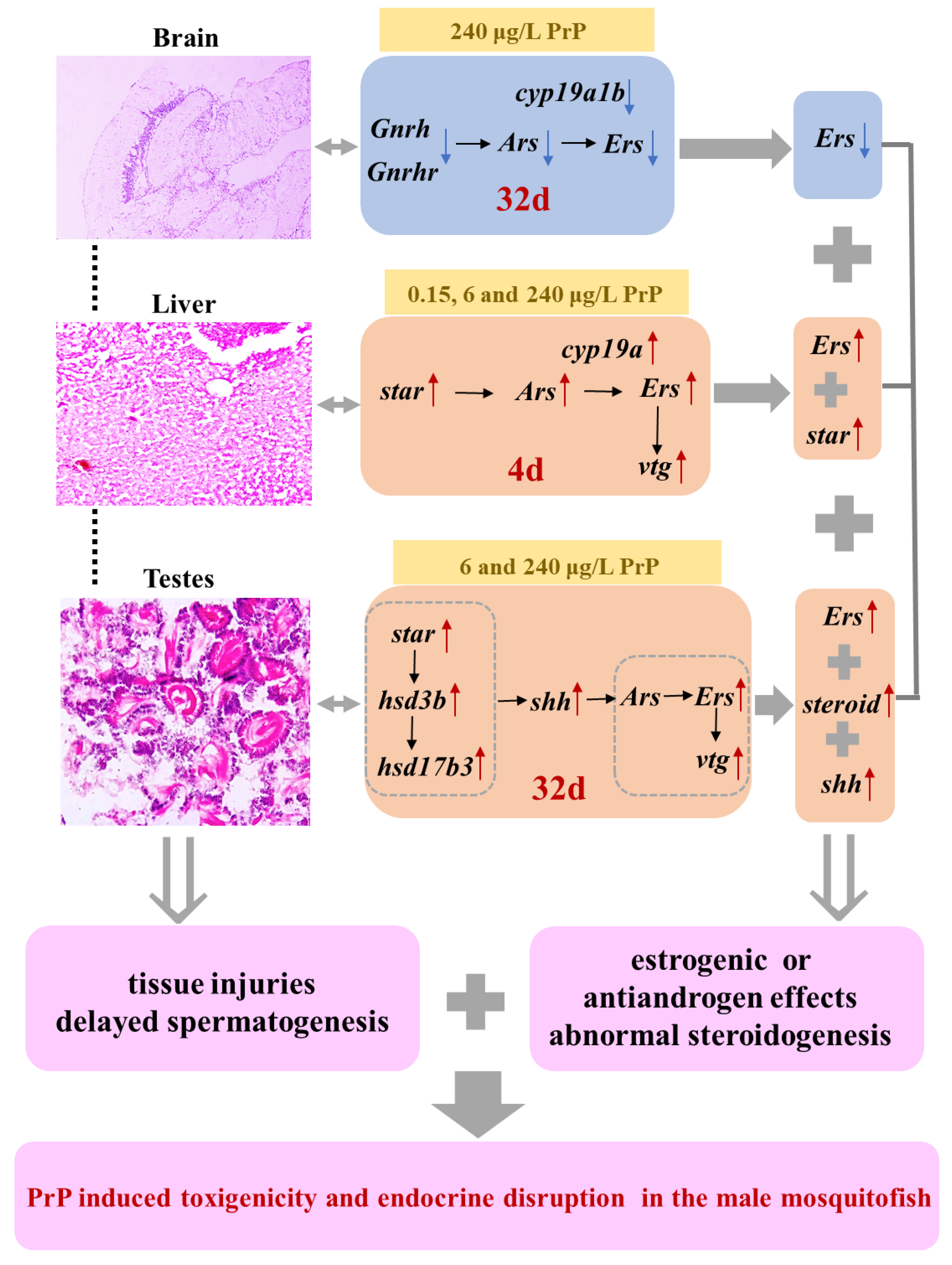
4. Discussion
4.1. PrP Induced Injuries in the Brain, Liver and Testes of Male Mosquitofish
4.2. Deleterious Impacts of PrP Stress on Endocrine Markers in HPGL Axis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haman, C.; Dauchy, X.; Rosin, C.; Munoz, J.-F. Occurrence, fate and behavior of parabens in aquatic environments: A review. Water Res. 2015, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Błędzka, D.; Gromadzińska, J.; Wąsowicz, W. Parabens. From environmental studies to human health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef]
- Lu, S.; Wang, N.; Ma, S.; Hu, X.; Kang, L.; Yu, Y. Parabens and triclosan in shellfish from Shenzhen coastal waters: Bioindication of pollution and human health risks. Environ. Pollut. 2019, 246, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Le, T.M.; Pham, P.T.; Nguyen, T.Q.; Bui, M.Q.; Nguyen, H.Q.; Vu, N.D.; Kannan, K.; Tran, T.M. A survey of parabens in aquatic environments in Hanoi, Vietnam and its implications for human exposure and ecological risk. Environ. Sci. Pollut. Res. 2022, 29, 46767–46777. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, L.; Shi, Y.; Wang, Y.; Liu, J.; Cai, Y. Spatial distribution, temporal variation and risks of parabens and their chlorinated derivatives in urban surface water in Beijing, China. Sci. Total. Environ. 2016, 539, 262–270. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total. Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, J.-L.; Liu, Y.-S.; Zhang, Q.-Q.; Jiang, Y.-X.; Liu, S.; Liu, W.-R.; Yang, Y.-Y.; Ying, G.-G. Personal care products in wild fish in two main Chinese rivers: Bioaccumulation potential and human health risks. Sci. Total. Environ. 2018, 621, 1093–1102. [Google Scholar] [CrossRef]
- Bolujoko, N.B.; Ogunlaja, O.O.; Alfred, M.O.; Okewole, D.M.; Ogunlaja, A.; Olukanni, O.D.; Msagati, T.A.; Unuabonah, E.I. Occurrence and human exposure assessment of parabens in water sources in Osun State, Nigeria. Sci. Total. Environ. 2022, 814, 152448. [Google Scholar] [CrossRef]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef]
- Bodin, L.; Rogiers, V.; Bernauer, U.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Ezendam, J.; Gaffet, E.; Galli, C.L.; Granum, B.; et al. Opinion of the Scientific Committee on Consumer safety (SCCS)—Final opinion on propylparaben (CAS No 94-13-3, EC No 202-307-7). Regul. Toxicol. Pharmacol. 2021, 125, 105005. [Google Scholar] [CrossRef]
- Hajizadeh, Y.; Feizabadi, G.K.; Feizi, A. Dietary habits and personal care product use as predictors of urinary concentrations of parabens in Iranian adolescents. Environ. Toxicol. Chem. 2020, 39, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Quan, Q.; Li, X.; Sun, W.; Zhu, K.; Wang, X.; Sun, X.; Zhan, M.; Xu, W.; Lu, L.; et al. Occurrence of parabens and their metabolites in the paired urine and blood samples from Chinese university students: Implications on human exposure. Environ. Res. 2020, 183, 109288. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.P.; Mendola, P.; von Ehrenstein, O.S.; Ye, X.; Calafat, A.M.; Fenton, S.E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2015, 54, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bräuner, E.V.; Uldbjerg, C.S.; Lim, Y.-H.; Gregersen, L.S.; Krause, M.; Frederiksen, H.; Andersson, A.-M. Presence of parabens, phenols and phthalates in paired maternal serum, urine and amniotic fluid. Environ. Int. 2022, 158, 106987. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Y.; Li, J.; Sun, X.; Liu, H.; Jiang, Y.; Peng, Y.; Zhao, H.; Xia, W.; Li, Y.; et al. Parabens exposure in early pregnancy and gestational diabetes mellitus. Environ. Int. 2019, 126, 468–475. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.; Park, J.; Kim, H.-J.; Lee, J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.-B.; et al. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci. Total. Environ. 2013, 461–462, 214–221. [Google Scholar] [CrossRef]
- Shekhar, S.; Sood, S.; Showkat, S.; Lite, C.; Chandrasekhar, A.; Vairamani, M.; Barathi, S.; Santosh, W. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen. Comp. Endocrinol. 2017, 241, 100–107. [Google Scholar] [CrossRef]
- Galinaro, C.A.; Pereira, F.M.; Vieira, E.M. Determination of parabens in surface water from Mogi Guaçu River (São Paulo, Brazil) using dispersive liquid-liquid microextraction based on low density solvent and LC-DAD. J. Braz. Chem. Soc. 2015, 26, 2205–2213. [Google Scholar] [CrossRef]
- Galinaro, C.A.; Spadoto, M.; de Aquino, F.W.B.; de Souza Pelinson, N.; Vieira, E.M. Environmental risk assessment of parabens in surface water from a Brazilian river: The case of Mogi Guaçu Basin, São Paulo State, under precipitation anomalies. Environ. Sci. Pollut. Res. 2022, 29, 8816–8830. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, J.; Xi, N.; Guo, W.; Sun, J. Parabens and their metabolite in surface water and sediment from the Yellow River and the Huai River in Henan Province: Spatial distribution, seasonal variation and risk assessment. Ecotoxicol. Environ. Saf. 2019, 172, 480–487. [Google Scholar] [CrossRef]
- Radwan, E.K.; Ibrahim, M.B.; Adel, A.; Farouk, M. The occurrence and risk assessment of phenolic endocrine-disrupting chemicals in Egypt’s drinking and source water. Environ. Sci. Pollut. Res. 2020, 27, 1776–1788. [Google Scholar] [CrossRef]
- Yao, L.; Lv, Y.-Z.; Zhang, L.-J.; Liu, W.-R.; Zhao, J.-L.; Yang, Y.-Y.; Jia, Y.-W.; Liu, Y.-S.; He, L.-Y.; Ying, G.-G. Bioaccumulation and risks of 24 personal care products in plasma of wild fish from the Yangtze River, China. Sci. Total. Environ. 2019, 665, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.L.; Pedersen, S.N.; Christiansen, L.B.; Korsgaard, B.; Bjerregaard, P. The preservatives ethyl-, propyl- and butylparaben are oestrogenic in an in vivo fish assay. Pharmacol. Toxicol. 2000, 86, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, L.L.; Usenko, S.; Brain, R.A.; Brooks, B.W. Probabilistic ecological hazard assessment of parabens using Daphnia magna and Pimephales promelas. Environ. Toxicol. Chem. 2009, 28, 2744–2753. [Google Scholar] [CrossRef] [PubMed]
- González-Doncel, M.; García-Mauriño, J.E.; Segundo, L.S.; Beltrán, E.M.; Sastre, S.; Torija, C.F. Embryonic exposure of medaka (Oryzias latipes) to propylparaben: Effects on early development and post-hatching growth. Environ. Pollut. 2014, 184, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Merola, C.; Amorena, M.; D’Angelo, M.; Cimini, A.; Benedetti, E. Sublethal exposure to propylparaben leads to lipid metabolism impairment in zebrafish early-life stages. J. Appl. Toxicol. 2020, 40, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Lite, C.; Guru, A.; Juliet, M.; Arockiaraj, J. Embryonic exposure to butylparaben and propylparaben induced developmental toxicity and triggered anxiety-like neurobehavioral response associated with oxidative stress and apoptosis in the head of zebrafish larvae. Environ. Toxicol. 2022, 37, 1988–2004. [Google Scholar] [CrossRef] [PubMed]
- Bereketoglu, C.; Pradhan, A. Comparative transcriptional analysis of methylparaben and propylparaben in zebrafish. Sci. Total. Environ. 2019, 671, 129–139. [Google Scholar] [CrossRef]
- Mikula, P.; Kruzikova, K.; Dobsikova, R.; Haruštiaková, D.; Svobodova, Z. Influence of propylparaben on vitellogenesis and sex ratio in juvenile zebrafish (Danio rerio). Acta Vet. Brno 2009, 78, 319–326. [Google Scholar] [CrossRef]
- Kang, H.-M.; Kim, M.-S.; Hwang, U.-K.; Jeong, C.-B.; Lee, J.-S. Effects of methylparaben, ethylparaben, and propylparaben on life parameters and sex ratio in the marine copepod Tigriopus japonicus. Chemosphere 2019, 226, 388–394. [Google Scholar] [CrossRef]
- Brand, W.; Boon, P.E.; Hessel, E.V.S.; Meesters, J.A.J.; Weda, M.; Schuur, A.G. Exposure to and toxicity of methyl-, ethyl- and propylparaben: A literature review with a focus on endocrine-disrupting properties. RIVM Rep. 2018, 2017-0028, 1–109. [Google Scholar] [CrossRef]
- Nowak, K.; Ratajczak–Wrona, W.; Górska, M.; Jabłońska, E. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.M.; Gregoraszczuk, E.Ł. Actions of methyl-, propyl- and butylparaben on estrogen receptor-α and -β and the progesterone receptor in MCF-7 cancer cells and non-cancerous MCF-10A cells. Toxicol. Lett. 2014, 230, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, L.; Hu, Y.; Jiao, J.; Hu, J. Inverse antagonist activities of parabens on human oestrogen-related receptor γ (ERRγ): In vitro and in silico studies. Toxicol. Appl. Pharmacol. 2013, 270, 16–22. [Google Scholar] [CrossRef]
- Ma, D.; Chen, L.; Zhu, X.; Li, F.; Liu, C.; Liu, R. Assessment of combined antiandrogenic effects of binary parabens mixtures in a yeast-based reporter assay. Environ. Sci. Pollut. Res. 2014, 21, 6482–6494. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, E.; Barlas, N.; Çetinkaya, M.A. Assessing the antiandrogenic properties of propyl paraben using the Hershberger bioassay. Toxicol. Res. 2018, 7, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, A.M.; Said, M.M.; Badawi, M.M.; Rabo, M.M.A. Subchronic toxicity of propyl paraben in adult male rats. Egypt. J. Biochem. Mol. Biol. 2013, 31, 1–20. [Google Scholar]
- Bao, M.; Zheng, S.; Liu, C.; Huang, W.; Xiao, J.; Wu, K. Perfluorooctane sulfonate exposure alters sexual behaviors and transcriptions of genes in hypothalamic–pituitary–gonadal–liver axis of male zebrafish (Danio rerio). Environ. Pollut. 2020, 267, 115585. [Google Scholar] [CrossRef]
- Dang, Y.; Wang, J.; Giesy, J.P.; Liu, C. Responses of the zebrafish hypothalamic–pituitary–gonadal–liver axis PCR array to prochloraz are dependent on timing of sampling. Aquat. Toxicol. 2016, 175, 154–159. [Google Scholar] [CrossRef]
- OECD. Guidelines for the Testing of Chemicals, Test No. 229: Fish Short Term Reproduction Assay; OECD Publishing: Paris, France, 2012. [Google Scholar]
- Song, X.; Wang, X.; Li, X.; Yan, X.; Liang, Y.; Huang, Y.; Huang, L.; Zeng, H. Histopathology and transcriptome reveals the tissue-specific hepatotoxicity and gills injury in mosquitofish (Gambusia affinis) induced by sublethal concentration of triclosan. Ecotoxicol. Environ. Saf. 2021, 220, 112325. [Google Scholar] [CrossRef]
- Ullmann, J.F.; Cowin, G.; Kurniawan, N.D.; Collin, S.P. A three-dimensional digital atlas of the zebrafish brain. Neuroimage 2010, 51, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.M.; Teles, M.C.; Oliveira, R.F.; Van der Linden, A.; Verhoye, M. A three-dimensional stereotaxic MRI brain atlas of the cichlid fish Oreochromis mossambicus. PLoS ONE 2012, 7, e44086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macêdo, A.K.S.; dos Santos, K.P.E.; Brighenti, L.S.; Windmöller, C.C.; Barbosa, F.A.R.; Ribeiro, R.I.M.D.A.; dos Santos, H.B.; Thomé, R.G. Histological and molecular changes in gill and liver of fish (Astyanax lacustris Lütken, 1875) exposed to water from the Doce basin after the rupture of a mining tailings dam in Mariana, MG, Brazil. Sci. Total. Environ. 2020, 735, 139505. [Google Scholar] [CrossRef] [PubMed]
- Agamy, E. Histopathological changes in the livers of rabbit fish (Siganus canaliculatus) following exposure to crude oil and dispersed oil. Toxicol. Pathol. 2012, 40, 1128–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leusch, F.D.L.; Chapman, H.F.; Kay, G.W.; Gooneratne, S.R.; Tremblay, L.A. Anal fin morphology and gonadal histopathology in mosquitofish (Gambusia holbrooki) exposed to treated municipal sewage effluent. Arch. Environ. Contam. Toxicol. 2006, 50, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Unlu, E. Histopathological changes in the gill and liver tissues of freshwater fish, Cirrhinus mrigala exposed to dichlorvos. Braz. Arch. Biol. Technol. 2009, 52, 1291–1296. [Google Scholar] [CrossRef]
- Cengiz, E.I.; Unlu, E. Sublethal effects of commercial deltamethrin on the structure of the gill, liver and gut tissues of mosquitofish, Gambusia affinis: A microscopic study. Environ. Toxicol. Pharmacol. 2006, 21, 246–253. [Google Scholar] [CrossRef]
- Shirdel, I.; Kalbassi, M.R.; Esmaeilbeigi, M.; Tinoush, B. Disruptive effects of nonylphenol on reproductive hormones, antioxidant enzymes, and histology of liver, kidney and gonads in Caspian trout smolts. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 232, 108756. [Google Scholar] [CrossRef]
- Zhong, L.; Liang, Y.Q.; Lu, M.; Pan, C.G.; Dong, Z.; Zhao, H.; Li, C.; Lin, Z.; Yao, L. Effects of dexamethasone on the morphology, gene expression and hepatic histology in adult female mosquitofish (Gambusia affinis). Chemosphere 2021, 274, 129797. [Google Scholar] [CrossRef]
- Fang, G.-Z.; Huang, G.-Y.; Ying, G.-G.; Qiu, S.-Q.; Shi, W.-J.; Xie, L.; Yang, Y.-Y.; Ma, D.-D. Endocrine disrupting effects of binary mixtures of 17β-estradiol and testosterone in adult female western mosquitofish (Gambusia affinis). Ecotoxicol. Environ. Saf. 2021, 208, 111566. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Chen, S.; Chen, H.; Ying, G.; Chen, D.; Liu, J.; Liang, Y.; Wu, R.; Fang, X.; Zhang, C.; et al. Rapid masculinization and effects on the liver of female western mosquitofish (Gambusia affinis) by norethindrone. Chemosphere 2019, 216, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ou, R.; Wu, X.; Peijia, K.; Lan, W.; Tian, S.; Liang, X.; Nie, X. Cloning of cat, gapdh and gst genes of Gambusia affinis and its application in ecotoxicology. Asian J. Ecotoxicol. 2015, 10, 83–92. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Merola, C.; Vremere, A.; Fanti, F.; Iannetta, A.; Caioni, G.; Sergi, M.; Compagnone, D.; Lorenzetti, S.; Perugini, M.; Amorena, M. Oxysterols profile in zebrafish embryos exposed to triclocarban and propylparaben—A preliminary study. Int. J. Environ. Res. Public Health 2022, 19, 1264. [Google Scholar] [CrossRef]
- Crinnion, W.J. Toxic effects of the easily avoidable phthalates and parabens. Altern. Med. Rev. 2010, 15, 190–196. [Google Scholar]
- Hou, J.; Li, L.; Wu, N.; Su, Y.; Lin, W.; Li, G.; Gu, Z. Reproduction impairment and endocrine disruption in female zebrafish after long-term exposure to MC-LR: A life cycle assessment. Environ. Pollut. 2016, 208, 477–485. [Google Scholar] [CrossRef]
- Yildirim, M.Z.; Benlı, A.K.; Selvı, M.; Özkul, A.; Erkoç, F.; Koçak, O. Acute toxicity, behavioral changes, and histopathological effects of deltamethrin on tissues (gills, liver, brain, spleen, kidney, muscle, skin) of Nile tilapia (Oreochromis niloticus L.) fingerlings. Environ. Toxicol. 2006, 21, 614–620. [Google Scholar] [CrossRef]
- Szeląg, S.; Zabłocka, A.; Trzeciak, K.; Drozd, A.; Baranowska-Bosiacka, I.; Kolasa, A.; Goschorska, M.; Chlubek, D.; Gutowska, I. Propylparaben-induced disruption of energy metabolism in human HepG2 cell line leads to increased synthesis of superoxide anions and apoptosis. Toxicol. Vitr. 2016, 31, 30–34. [Google Scholar] [CrossRef]
- Silva, D.C.; Serrano, L.; Oliveira, T.M.; Mansano, A.S.; Almeida, E.A.; Vieira, E.M. Effects of parabens on antioxidant system and oxidative damages in Nile tilapia (Oreochromis niloticus). Ecotoxicol. Environ. Saf. 2018, 162, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Lara-Valderrábano, L.; Rocha, L.; Galván, E.J. Propylparaben reduces the excitability of hippocampal neurons by blocking sodium channels. Neurotoxicology 2016, 57, 183–193. [Google Scholar] [CrossRef]
- Pisera-Fuster, A.; Otero, S.; Talevi, A.; Bruno-Blanch, L.; Bernabeu, R. Anticonvulsant effect of sodium cyclamate and propylparaben on pentylenetetrazol-induced seizures in zebrafish. Synapse 2017, 71, e21961. [Google Scholar] [CrossRef] [PubMed]
- Atli, E. The effects of ethylparaben and propylparaben on the development and fecundity of Drosophila melanogaster. Environ. Toxicol. Pharmacol. 2022, 92, 103856. [Google Scholar] [CrossRef] [PubMed]
- Calma, M.L.; Medina, P.M.B. Acute and chronic exposure of the holometabolous life cycle of Aedes aegypti L. to emerging contaminants naproxen and propylparaben. Environ. Pollut. 2020, 266, 115275. [Google Scholar] [CrossRef] [PubMed]
- García-Espiñeira, M.C.; Tejeda-Benítez, L.P.; Olivero-Verbel, J. Toxic effects of bisphenol A, propyl paraben, and triclosan on Caenorhabditis elegans. Intern. J. Environ. Res. Public Health 2018, 15, 684. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Li, M.; Guo, Q.; Li, X.; Zhou, S.; Dai, J.; Zhang, J.; Wu, M.; Tang, W.; Wen, J.; et al. Chronic exposure to propylparaben at the humanly relevant dose triggers ovarian aging in adult mice. Ecotoxicol. Environ. Saf. 2022, 235, 113432. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova, L.; Vitku, J.; Hampl, R.; Adamcova, K.; Skodova, T.; Simkova, M.; Parizek, A.; Starka, L.; Duskova, M. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ. Res. 2018, 163, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.; Martins, F.C.; Oliveira, P.J.; Ramalho-Santos, J.; Peixoto, F.P. Parabens in male infertility-Is there a mitochondrial connection? Reprod. Toxicol. 2009, 27, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Oishi, S. Effects of propyl paraben on the male reproductive system. Food Chem. Toxicol. 2002, 40, 1807–1813. [Google Scholar] [CrossRef]
- Gazin, V.; Marsden, E.; Marguerite, F. Oral propylparaben administration to juvenile male Wistar rats did not induce toxicity in reproductive organs. Toxicol. Sci. 2013, 136, 392–401. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, N. Histopathological evaluation of the zebrafish (Danio rerio) testis following exposure to methyl paraben. Int. J. Aquat. Biol. 2017, 5, 71–78. [Google Scholar] [CrossRef]
- Meijide, F.J.; Vázquez, G.R.; Piazza, Y.G.; Babay, P.A.; Itria, R.F.; Nostro, F.L.L. Effects of waterborne exposure to 17β-estradiol and 4-tert-octylphenol on early life stages of the South American cichlid fish Cichlasoma dimerus. Ecotoxicol. Environ. Saf. 2016, 124, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, F.; Chen, W.; Xu, R.; Wang, W. Effects of triclosan (TCS) on hormonal balance and genes of hypothalamus-pituitary- gonad axis of juvenile male Yellow River carp (Cyprinus carpio). Chemosphere 2018, 193, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Campos, M.S.; Lima, R.F.; Gomes, L.S.; Marques, M.R.; Taboga, S.R.; Biancardi, M.F.; Brito, P.V.A.; Santos, F.C.A. Endocrine-disrupting effects of methylparaben on the adult gerbil prostate. Environ. Toxicol. 2017, 32, 1801–1812. [Google Scholar] [CrossRef]
- Wei, F.; Cheng, H.; Sang, N. Comprehensive assessment of estrogenic activities of parabens by in silico approach and in vitro assays. Sci. Total. Environ. 2022, 845, 157194. [Google Scholar] [CrossRef]
- Dennis, M.K.; Bowles, H.J.; MacKenzie, D.A.; Burchiel, S.W.; Edwards, B.S.; Sklar, L.A.; Prossnitz, E.R.; Thompson, T.A. A multifunctional androgen receptor screening assay using the high-throughput Hypercyt® flow cytometry system. Cytom. Part A 2008, 73A, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Peng, T.; Liu, F.; Ren, L.; Peng, Z.; Ji, G.; Zhou, Y.; Fu, Z. Transcriptional responses in male Japanese medaka exposed to antiandrogens and antiandrogen/androgen mixtures. Environ. Toxicol. 2015, 31, 1591–1599. [Google Scholar] [CrossRef]
- Pan, X.; Liu, Y.; Zhou, K.; Mu, X.; Zheng, S.; Liu, C.; Hu, Y. Tissue expression and bioinformatics analysis of the vitellogenin gene of Asian arowana (Scleropages formosus). J. Appl. Ichthyol. 2019, 35, 970–977. [Google Scholar] [CrossRef]
- Mills, L.J.; Gutjahr-Gobell, R.E.; Horowitz, D.B.; Denslow, N.D.; Chow, M.C.; Zaroogian, G.E. Relationship between reproductive success and male plasma vitellogenin concentrations in cunner, Tautogolabrus adspersus. Environ. Health Perspect. 2003, 111, 93–99. [Google Scholar] [CrossRef]
- Inui, M.; Adachi, T.; Takenaka, S.; Inui, H.; Nakazawa, M.; Ueda, M.; Watanabe, H.; Mori, C.; Iguchi, T.; Miyatake, K. Effect of UV screens and preservatives on vitellogenin and choriogenin production in male medaka (Oryzias latipes). Toxicology 2003, 194, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, P.; Andersen, D.N.; Pedersen, K.L.; Pedersen, S.N.; Korsgaard, B. Estrogenic effect of propylparaben (propylhydroxybenzoate) in rainbow trout Oncorhynchus mykiss after exposure via food and water. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, H.; He, J.; Wu, X.; Wang, L.; Liu, X.; Lin, H. Interaction of nuclear ERs and GPER in vitellogenesis in zebrafish. J. Steroid Biochem. Mol. Biol. 2019, 189, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Koutková, Z.; Blahová, J.; Svobodová, Z. Vitellogenin—Biomarker of endocrine disruption in fish. Chem. Listy 2020, 114, 746–752. [Google Scholar]
- Zhang, W.; Sheng, N.; Wang, M.; Zhang, H.; Dai, J. Zebrafish reproductive toxicity induced by chronic perfluorononanoate exposure. Aquat. Toxicol. 2016, 175, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-J.; Lin, J.-C.; Chung, B.-C. Zebrafish cyp11a1 and hsd3b genes: Structure, expression and steroidogenic development during embryogenesis. Mol. Cell. Endocrinol. 2009, 312, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Mindnich, R.; Deluca, D.; Adamski, J. Identification and characterization of 17β-hydroxysteroid dehydrogenases in the zebrafish, Danio rerio. Molcular Cell. Endocrinol. 2004, 215, 19–30. [Google Scholar] [CrossRef]
- Liang, Y.-Q.; Huang, G.-Y.; Lin, Z.; Li, J.; Yang, J.-W.; Zhong, L.-Y.; Ying, G.-G. Reproductive effects of synthetic progestin norgestrel in zebrafish (Danio rerio). Chemosphere 2018, 190, 17–24. [Google Scholar] [CrossRef]
- Gal, A.; Gedye, K.; Craig, Z.R.; Ziv-Gal, A. Propylparaben inhibits mouse cultured antral follicle growth, alters steroidogenesis, and upregulates levels of cell-cycle and apoptosis regulators. Reprod. Toxicol. 2019, 89, 100–106. [Google Scholar] [CrossRef]
- Zou, C.; Wang, L.; Zou, Y.; Wu, Z.; Wang, W.; Liang, S.; Wang, L.; You, F. Characteristics and sex dimorphism of 17β-hydroxysteroid dehydrogenase family genes in the olive flounder Paralichthys olivaceus. J. Steroid Biochem. Mol. Biol. 2020, 199, 105597. [Google Scholar] [CrossRef]
- Ogino, Y.; Miyagawa, S.; Katoh, H.; Prins, G.S.; Iguchi, T.; Yamada, G. Essential functions of androgen signaling emerged through the developmental analysis of vertebrate sex characteristics. Evol. Dev. 2011, 13, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Podlasek, C.A.; Barnett, D.H.; Clemens, J.Q.; Bak, P.M.; Bushman, W. Prostate development requires sonic hedgehog expressed by the urogenital sinus epithelium. Dev. Biol. 1999, 209, 28–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagawa, S.; Matsumaru, D.; Murashima, A.; Omori, A.; Satoh, Y.; Haraguchi, R.; Motoyama, J.; Iguchi, T.; Nakagata, N.; Hui, C.-C.; et al. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology 2011, 152, 2894–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migone, F.F.; Hung, P.-H.; Cowan, R.G.; Selvaraj, V.; Suarez, S.S.; Quirk, S.M. Overactivation of hedgehog signaling in the developing Müllerian duct interferes with duct regression in males and causes subfertility. Reproduction 2017, 153, 481–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Wang, Y.; Chen, T.; Song, P.; Xin, D.; Ping, P.; Huang, Y.; Li, Z.; Hu, H. Ectopic expression of sonic hedgehog in a cryptorchid man with azoospermia: A case report. J. Int. Med. Res. 2014, 42, 589–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallah, H.P.; Rodrigues, M.S.; Corchuelo, S.; Nóbrega, R.H.; Habibi, H.R. Role of GnRH isoforms in paracrine/autocrine control of zebrafish (Danio rerio) spermatogenesis. Endocrinology 2020, 161, bqaa004. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yu, J.; Qu, P.; Ma, P.; Yu, Z. The negative effects of chronic exposure to isoflurane on spermatogenesis via breaking the hypothalamus-pituitary-gonadal equilibrium. Inhal. Toxicol. 2015, 27, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L.; Wilson, G.; Huhtaniemi, I. Hormonal treatment after cytotoxic therapy stimulates recovery of spermatogenesis. Cancer Res. 1999, 59, 3557–3560. [Google Scholar]


| Tissues | Injuries | 4d | 32d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.15 μg/L | 6 μg/L | 240 μg/L | Control | 0.15 μg/L | 6 μg/L | 240 μg/L | ||
| Brain | Cell cavitation | - | - | - | + | - | + | ++ | ++ |
| Cytomorphosis | - | - | - | + | - | + | ++ | ++ | |
| Blurred cell boundaries | - | - | - | + | - | + | ++ | ++ | |
| Liver | Hepatic sinus dilatation | - | + | ++ | ++ | - | ++ | ++ | ++ |
| Cytoplasmic vacuolation | - | + | + | + | - | + | ++ | ++ | |
| Cytolysis | - | - | - | - | - | + | ++ | +++ | |
| Nuclear aggregation | - | - | - | - | - | + | + | +++ | |
| Testes | Spermatorgenic cell lesion | - | - | - | - | - | + | ++ | +++ |
| Decreased mature seminal vesicle | - | - | - | - | - | ++ | ++ | +++ | |
| Sperm cells aggregation | - | - | - | - | - | ++ | ++ | +++ | |
| Seminiferous tubules disorder | - | - | - | - | - | ++ | ++ | +++ | |
| Dilated intercellular space | - | - | - | - | - | ++ | ++ | +++ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Li, Y.; Song, X.; Yang, T.; Wang, H.; Liang, Y.; Huang, L.; Zeng, H. Endocrine Disruption of Propylparaben in the Male Mosquitofish (Gambusia affinis): Tissue Injuries and Abnormal Gene Expressions of Hypothalamic-Pituitary-Gonadal-Liver Axis. Int. J. Environ. Res. Public Health 2023, 20, 3557. https://doi.org/10.3390/ijerph20043557
Ma Y, Li Y, Song X, Yang T, Wang H, Liang Y, Huang L, Zeng H. Endocrine Disruption of Propylparaben in the Male Mosquitofish (Gambusia affinis): Tissue Injuries and Abnormal Gene Expressions of Hypothalamic-Pituitary-Gonadal-Liver Axis. International Journal of Environmental Research and Public Health. 2023; 20(4):3557. https://doi.org/10.3390/ijerph20043557
Chicago/Turabian StyleMa, Yun, Yujing Li, Xiaohong Song, Tao Yang, Haiqin Wang, Yanpeng Liang, Liangliang Huang, and Honghu Zeng. 2023. "Endocrine Disruption of Propylparaben in the Male Mosquitofish (Gambusia affinis): Tissue Injuries and Abnormal Gene Expressions of Hypothalamic-Pituitary-Gonadal-Liver Axis" International Journal of Environmental Research and Public Health 20, no. 4: 3557. https://doi.org/10.3390/ijerph20043557





