Evaluating the Efficacy of Electrical Vestibular Stimulation (VeNS) on Insomnia Adults: Study Protocol of a Double-Blinded, Randomized, Sham-Controlled Trial
Abstract
:1. Introduction
1.1. Prevalence of Insomnia in Different Countries
1.2. Prevalence of Sleep Problems during COVID-19 Pandemic
1.3. Existing Treatment Modality for Insomnia and Past Research Findings
1.4. Vestibular Stimulation
1.5. What Is Cranial Electrotherapy Stimulation (CES)? and Past Research Using CES on Insomnia
1.6. Objectives
1.7. Hypotheses
1.7.1. Primary Hypothesis
1.7.2. Secondary Hypotheses
2. Materials and Methods
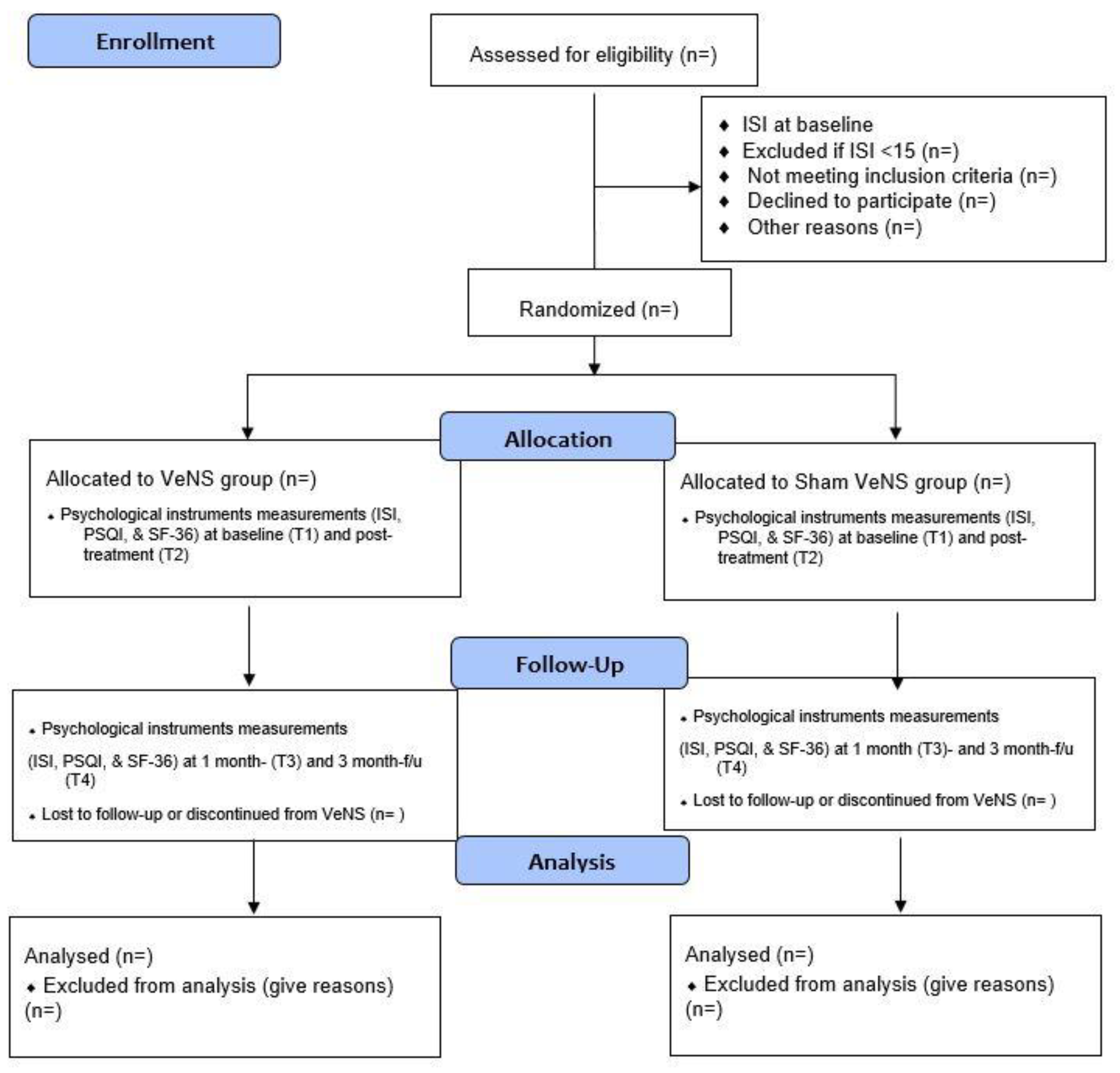
2.1. Trial Design
2.2. Subjects
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Sample Size
2.6. Screening and Self-Administered Questionnaire
2.7. Randomization, Allocation and Masking
2.8. Interventions
2.8.1. VeNS group
2.8.2. Sham VeNS
2.9. Fidelity
2.10. Safety, Adverse Effects and Risk Indicators
2.11. Ethical and Data Security Considerations
2.12. Outcome Evaluation (Primary and Secondary Outcomes)
2.12.1. Primary Outcome
Insomnia Severity
2.12.2. Secondary Outcomes
Sleep Quality
Quality of Life
3. Statistical Analyses
4. Significance/Novelty of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, T.; Mayleben, D.; Feldman, N.; Lankford, A.; Grant, T.; Nofzinger, E. A novel forehead temperature-regulating device for insomnia: A randomized clinical trial. Sleep 2018, 41, zsy045. [Google Scholar] [CrossRef] [Green Version]
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and Coding Manual, 3rd ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2014. [Google Scholar]
- Sutton, E.L. Insomnia. Ann. Intern. Med. 2021, 174, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Lucena, L.; Polesel, D.N.; Poyares, D.; Bittencourt, L.; Andersen, M.L.; Tufik, S.; Hachul, H. The association of insomnia and quality of life: Sao Paulo epidemiologic sleep study (EPISONO). Sleep Health 2020, 6, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Guo, N.; Lewis, T.T.; Sofer, T.; Williams, D.; Sims, M.; Wilson, J.G.; Redline, S. 0336 Associations of Psychosocial Factors, Short Sleep and Insomnia, and Hypertension Control among African-Americans: The Jackson Heart Sleep Study (JHSS). Sleep 2019, 42 (Suppl. 1S), A137–A138. [Google Scholar] [CrossRef]
- Nieters, A.; Blagitko-Dorfs, N.; Peter, H.-H.; Weber, S. Psychophysiological insomnia and respiratory tract infections: Results of an infection-diary-based cohort study. Sleep 2019, 42, zsz098. [Google Scholar] [CrossRef]
- Larsson, S.C.; Markus, H.S. Genetic liability to insomnia and cardiovascular disease risk. Circulation 2019, 140, 796–798. [Google Scholar] [CrossRef]
- Harsh, J.R.; Hayduk, R.; Rosenberg, R.; Wesnes, K.A.; Walsh, J.K.; Arora, S.; Niebler, G.E.; Roth, T. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr. Med. Res. Opin. 2006, 22, 761–774. [Google Scholar] [CrossRef]
- Sailesh, K.S.; Mukkadan, J. Controlled vestibular stimulation: A novel treatment for insomnia. System 2013, 12, 13. [Google Scholar]
- Sailesh, K.S.; Mukkadan, J. Can controlled vestibular stimulation reduce stress. Health Sci. 2013, 2, JS001. [Google Scholar]
- Davis, S.F.; Derbenev, A.V.; Williams, K.W.; Glatzer, N.R.; Smith, B.N. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004, 1017, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, S.S.; Blanchard, J.; Morin, L.P. Medial vestibular connections with the hypocretin (orexin) system. J. Comp. Neurol. 2005, 487, 127–146. [Google Scholar] [CrossRef]
- Conway, S.; Richardson, L.; Speciale, S.; Moherek, R.; Mauceri, H.; Krulich, L. Interaction between norepinephrine and serotonin in the neuroendocrine control of growth hormone release in the rat. Endocrinology 1990, 126, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, M.; Van Cauter, E.; Van Onderbergen, A.; Caufriez, A.; Thorner, M.O.; Copinschi, G. Sleep-promoting effects of growth hormone-releasing hormone in normal men. Am. J. Physiol. Endocrinol. Metab. 1993, 264, E594–E598. [Google Scholar] [CrossRef] [PubMed]
- Snowball, R.K.; Dampney, R.; Lumb, B.M. Responses of neurones in the medullary raphe nuclei to inputs from visceral nociceptors and the ventrolateral periaqueductal grey in the rat. Exp. Physiol. Transl. Integr. 1997, 82, 485–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, A.; Pompeiano, O. Vestibular control of autonomic functions during the rapid eye movements of desynchronized sleep. Arch. Ital. De Biol. 1970, 108, 154–180. [Google Scholar]
- Yates, B.J. Autonomic reaction to vestibular damage. Otolaryngol. Head Neck Surg. 1998, 119, 106–112. [Google Scholar] [CrossRef]
- Iber, C.; Davies, S.F.; Mahowald, M.W. Nocturnal rocking bed therapy: Improvement in sleep fragmentation in patients with respiratory muscle weakness. Sleep 1989, 12, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Woodward, S.; Tauber, E.S.; Spielman, A.J.; Thorpy, M.J. Effects of otolithic vestibular stimulation on sleep. Sleep 1990, 13, 533–537. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Ivers, H.; Beaulieu-Bonneau, S.; Morin, C.M. Complementary and alternative treatments for insomnia/insomnia-depression-anxiety symptom cluster: Meta-analysis of English and Chinese literature. Sleep Med. Rev. 2021, 58, 101445. [Google Scholar] [CrossRef]
- Geng, F.; Liang, Y.; Li, Y.; Fang, Y.; Pham, T.S.; Liu, X.; Fan, F. Bidirectional associations between insomnia, posttraumatic stress disorder, and depressive symptoms among adolescent earthquake survivors: A longitudinal multiwave cohort study. Sleep 2019, 42, zsz162. [Google Scholar] [CrossRef]
- Li, R.; Wing, Y.; Ho, S.; Fong, S. Gender differences in insomnia—A study in the Hong Kong Chinese population. J. Psychosom. Res. 2002, 53, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.-T.; Ma, X.; Cai, Z.-J.; Li, S.-R.; Xiang, Y.-Q.; Guo, H.-L.; Hou, Y.-Z.; Li, Z.-B.; Li, Z.-J.; Tao, Y.-F. The prevalence of insomnia, its sociodemographic and clinical correlates, and treatment in rural and urban regions of Beijing, China: A general population-based survey. Sleep 2008, 31, 1655–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, C.-C.; Huang, C.-J.; Wang, M.-Y.; Tsai, P.-S. Insomnia: Prevalence and its impact on excessive daytime sleepiness and psychological well-being in the adult Taiwanese population. Qual. Life Res. 2008, 17, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Yamaoka, K.; Nakao, M.; Yano, E. Impact of insomnia on individual health dissatisfaction in Japan, South Korea, and Taiwan. Sleep 2005, 28, 1328–1332. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.-F.; Yeung, W.-F.; Ho, F.Y.-Y.; Yung, K.-P.; Yu, Y.-M.; Kwok, C.-W. Cross-cultural and comparative epidemiology of insomnia: The Diagnostic and statistical manual (DSM), International classification of diseases (ICD) and International classification of sleep disorders (ICSD). Sleep Med. 2015, 16, 477–482. [Google Scholar] [CrossRef]
- Yu, B.Y.-M.; Yeung, W.-F.; Lam, J.C.-S.; Yuen, S.C.-S.; Lam, S.C.; Chung, V.C.-H.; Chung, K.-F.; Lee, P.H.; Ho, F.Y.-Y.; Ho, J.Y.-S. Prevalence of sleep disturbances during COVID-19 outbreak in an urban Chinese population: A cross-sectional study. Sleep Med. 2020, 74, 18–24. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Lam, S.P.; Yu, M.W.M.; Li, S.X.; Zhou, J.; Chan, J.W.Y.; Chan, N.Y.; Li, A.M.; Wing, Y.-K. Help-seeking behaviors for insomnia in Hong Kong Chinese: A community-based study. Sleep Med. 2016, 21, 106–113. [Google Scholar] [CrossRef]
- Ho, F.Y.-Y.; Choi, W.-T.; Yeung, W.-F.; Lam, H.-K.; Lau, W.-Y.; Chung, K.-F. The efficacy of integrated cognitive behavioral therapy (CBT) and acupressure versus CBT for insomnia: A three-arm pilot randomized controlled trial. Sleep Med. 2021, 87, 158–167. [Google Scholar] [CrossRef]
- Albathi, M.; Agrawal, Y. Vestibular vertigo is associated with abnormal sleep duration. J. Vestib. Res. 2017, 27, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Feusner, J.D.; Madsen, S.; Moody, T.D.; Bohon, C.; Hembacher, E.; Bookheimer, S.Y.; Bystritsky, A. Effects of cranial electrotherapy stimulation on resting state brain activity. Brain Behav. 2012, 2, 211–220. [Google Scholar] [CrossRef]
- Yennu, S.; Kang, D.H.; Hwu, W.-J.; Padhye, N.S.; Masino, C.; Liu, D.D.; Dibaj, S.; Williams, J.L.; Lu, Z.; Bruera, E. Cranial Electrotherapy Stimulation for the Management of Depression, Anxiety, Sleep Disturbance, and Pain in Patients with Advanced Cancer: A Preliminary Study; American Society of Clinical Oncology: Alexandria, VA, USA, 2017. [Google Scholar]
- Imran, M.; Kwakkenbos, L.; McCall, S.J.; McCord, K.A.; Fröbert, O.; Hemkens, L.G.; Zwarenstein, M.; Relton, C.; Rice, D.B.; Langan, S.M. Methods and results used in the development of a consensus-driven extension to the Consolidated Standards of Reporting Trials (CONSORT) statement for trials conducted using cohorts and routinely collected data (CONSORT-ROUTINE). BMJ Open 2021, 11, e049093. [Google Scholar] [CrossRef] [PubMed]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Goothy, S.S.K.; McKeown, J. Modulation of sleep using electrical vestibular nerve stimulation prior to sleep onset: A pilot study. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.-F.; Yu, B.Y.-M.; Yuen, J.W.-M.; Ho, J.Y.S.; Chung, K.-F.; Zhang, Z.-J.; Mak, D.S.Y.; Suen, L.K.-P.; Ho, L.-M. Semi-individualized acupuncture for insomnia disorder and oxidative stress: A randomized, double-Blind, sham-controlled trial. Nat. Sci. Sleep 2021, 13, 1195. [Google Scholar] [CrossRef]
- Grewal, T.; James, C.; Macefield, V.G. Frequency-dependent modulation of muscle sympathetic nerve activity by sinusoidal galvanic vestibular stimulation in human subjects. Exp. Brain Res. 2009, 197, 379–386. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, E.; Goothy, S.S.K.; McKeown, J. Electrical vestibular nerve stimulation (VeNS): A follow-up safety assessment of long-term usage. J. Basic Clin. Physiol. Pharmacol. 2022, 33, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.S. Insomnia Severity Index: Psychometric properties with Chinese community-dwelling older people. J. Adv. Nurs. 2010, 66, 2350–2359. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Ho, K.Y.; Lam, K.K.; Xia, W.; Chung, J.; Cheung, A.T.; Ho, L.L.; Chiu, S.Y.; Chan, G.C.; Li, W.H. Psychometric properties of the Chinese version of the Pittsburgh Sleep Quality Index (PSQI) among Hong Kong Chinese childhood cancer survivors. Health Qual. Life Outcomes 2021, 19, 176. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Shen, Y. Development and psychometric tests of a Chinese version of the SF-36 Health Survey Scales. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.] 2002, 36, 109–113. [Google Scholar]
- Liu, J.; Qu, B.; Hu, B.; Jiang, N.; Wang, D. The quality of life of men who have sex with men in China: Reliability and validity testing of the SF-36 questionnaire. PLoS ONE 2013, 8, e83362. [Google Scholar] [CrossRef] [PubMed]
- Morriss, R.; Xydopoulos, G.; Craven, M.; Price, L.; Fordham, R. Clinical effectiveness and cost minimisation model of Alpha-Stim cranial electrotherapy stimulation in treatment seeking patients with moderate to severe generalised anxiety disorder. J. Affect. Disord. 2019, 253, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombach, I.; Gray, A.M.; Jenkinson, C.; Murray, D.W.; Rivero-Arias, O. Multiple imputation for patient reported outcome measures in randomised controlled trials: Advantages and disadvantages of imputing at the item, subscale or composite score level. BMC Med. Res. Methodol. 2018, 18, 87. [Google Scholar]
| Level | Stimulation Level |
|---|---|
| 0 | No stimulation applied (0 mA) |
| 1–3 | Stimulation applied (0.5 mA) |
| 4–7 | Stimulation applied (0.7 mA) |
| 8–10 | Stimulation applied (1 mA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheung, T.; Lam, J.Y.T.; Fong, K.H.; Cheng, C.P.-W.; Ho, A.; Sittlington, J.; Xiang, Y.-T.; Li, T.M.H. Evaluating the Efficacy of Electrical Vestibular Stimulation (VeNS) on Insomnia Adults: Study Protocol of a Double-Blinded, Randomized, Sham-Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 3577. https://doi.org/10.3390/ijerph20043577
Cheung T, Lam JYT, Fong KH, Cheng CP-W, Ho A, Sittlington J, Xiang Y-T, Li TMH. Evaluating the Efficacy of Electrical Vestibular Stimulation (VeNS) on Insomnia Adults: Study Protocol of a Double-Blinded, Randomized, Sham-Controlled Trial. International Journal of Environmental Research and Public Health. 2023; 20(4):3577. https://doi.org/10.3390/ijerph20043577
Chicago/Turabian StyleCheung, Teris, Joyce Yuen Ting Lam, Kwan Hin Fong, Calvin Pak-Wing Cheng, Alex Ho, Julie Sittlington, Yu-Tao Xiang, and Tim Man Ho Li. 2023. "Evaluating the Efficacy of Electrical Vestibular Stimulation (VeNS) on Insomnia Adults: Study Protocol of a Double-Blinded, Randomized, Sham-Controlled Trial" International Journal of Environmental Research and Public Health 20, no. 4: 3577. https://doi.org/10.3390/ijerph20043577






