1. Introduction
Anaemia is a significant public health problem worldwide, with adverse effects on health and socio-economic development [
1]. Global anaemia prevalence in 2019 was 23.2%, affecting 1.8 billion people, resulting in an estimated 50.3 million years lived with disability [
2]. Dietary iron deficiency is the most prevalent cause globally, followed by inherited hemoglobinopathies and haemolytic anaemias [
2]. Iron deficiency (ID) is recognised as a significant contributor to anaemia and ill-health in patients with common chronic inflammatory conditions including chronic kidney disease (CKD) [
3], heart failure [
4], and cancer [
5]. There is also evidence of inadequate investigation of anaemia in primary care [
6,
7,
8].
Anaemia is common in South Africa, with community rates between 22% and 31% in women and 12% and 17% in men older than 15 years [
9,
10]. In addition, South Africa has 8.2 million persons living with human immunodeficiency virus infection (PLWHIV) [
11] and an associated anaemia prevalence up to 72% [
12]. South Africa also has a high tuberculosis (TB) prevalence estimated at 737 per 100,000 in 2018 [
13]. Anaemia is a risk factor for TB [
12,
14], and TB is associated with a high prevalence of anaemia (61.5%) [
15].
ID is probably the most common cause in South Africa [
9]. Anaemia of inflammation (AI), which is synonymous with anaemia of chronic disease, is the second most common cause globally in clinical practice [
16] and, most probably, in South Africa [
17,
18]. In persons living with HIV (PLWHIV), causes may be multifactorial, but anaemia of chronic disease is the predominant cause [
19]. In Southern Africa, TB is probably the most common cause of moderate-to-severe anaemia in PLWHIV [
12,
20,
21,
22].
The relative contributions of all causes of anaemia are not well characterised in patients attending primary care and district hospitals in South Africa. In a community-based study in the Free State Province, AI was the most prevalent cause of anaemia in women with or without HIV [
17]; and in a district hospital (GF Jooste) in Cape Town, >95% of patients with HIV-associated TB had AI and <3% had IDA [
23]. However, no testing was done for other causes of anaemia in either study.
The aims of this study were to determine the causes of anaemia in adults in primary care and a district hospital setting in Mthatha, South Africa. To determine the overall causes in a resource-efficient manner, the study focused on moderate and severe anaemia.
3. Results
A total of 21 declined participation in the study. The most common reason (5) was unreadiness to know their HIV status.
Table 1 summarises the demographic characteristics of patients who participated, and it shows that 1327 participants were enrolled into the study, of which 44.6% (592) were seen at MRH, and 63.5% (842) were female. The combined median age was 48 years (IQR = 31 years). There was statistical homogeneity between males and females (
p-value = 0.100), the CD4 count (
p-value = 0.539), and viral load (
p-value = 0.235). The median ages of patients were statistically different depending on their recruiting health facility (
p-value = 0.0003). Even though the combined HIV prevalence was 20.9% (277/1327), it ranged from 5.5% (22/399) in CHC1 to 35.8% (212/592) at MRH, and this was statistically significant (
p-value < 0.0001). The HemoCue also showed anaemia prevalences of 48.4% (147/304) and 46.1% (246/534) for males and females 40 years old and older, respectively.
Of the 1327 patients, 471 (35.5%) qualified to have a laboratory evaluation of their anaemia due to HemoCue blood levels of moderate or severe anaemia status (
Table 2). However, only 463/471 (98.3%) participants had laboratory haemoglobin results, of whom 49.0% (
n = 227) had moderate and 24.0% (
n = 111) severe anaemia, comprising 25.5% (
n = 338) of those screened. Chronic diseases that can cause anaemia (HIV, TB, CKD, cancer, and heart failure) and non-communicable diseases (NCDs) of high prevalence were assessed on the 471 participants who had progressed for a laboratory evaluation. The median haemoglobin levels were statistically different between the three health facilities (
p-value = 0.002), and the difference was due to the lower median of MRH (9.4) when compared to CHC1 (10.9), which was statistically significant (
p-value < 0.001). Disease prevalences were as follows: tuberculosis (16.6%,
n = 78), hypertension (20.0%,
n = 94), chronic kidney disease (5.9%, 28), and cancers (2.6%,
n = 12). There was a statistically significant difference in the hypertension status depending on the health facility of recruitment (
p-value < 0.001). Of those with confirmed TB, 54/78 (69.2%) had pulmonary TB, 13/78 (16.7%) extra-pulmonary TB, 7/78 (9.0%) had both, and in 4/78 (5.1%), the primary site could not be confirmed. In addition to TB, there were only two patients with CDC-Stage-3-defining illnesses [
48], namely cervical cancer and cryptococcal antigenaemia. More than two-thirds of the participants with HemoCue severe and moderate anaemia who had HIV were in CDC Stage 3. The CD4 nadir, median, mean, and zenith were 1, 103, 191, and 2440, respectively. Viral replication was not suppressed in the majority of PLWHIV.
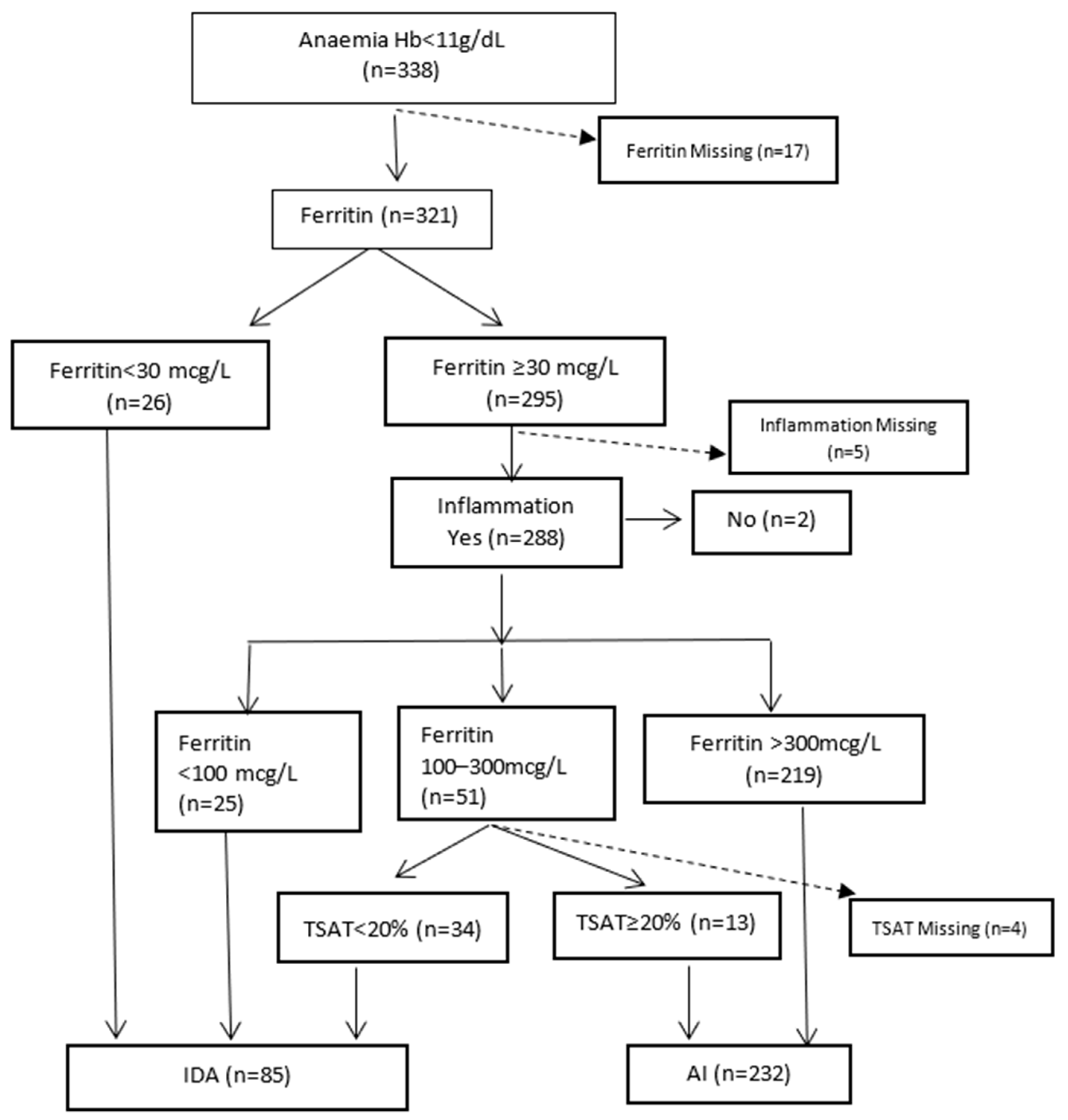
Figure 1 illustrates the biochemical diagnosis of IDA and AI. The category AI includes those with specific causes of anaemia (e.g., vitamin deficiencies and haematological malignancies) who also had inflammation. Excluding 17 with missing ferritin results, the percentages of AI and IDA were 72.3% (232/321) and 26.5% (85/321), respectively. In patients with CKD, 8/25 (32%) had IDA. Of nine patients with cancer, 1/6 (16.7%) had IDA. Four patients with cardiac failure had AI.
Only 399 individuals had a full record of the time from venesection to FBC analysis. The time a specimen was received at the laboratory was used as a proxy for the time to analysis, as reports did not specify analysis times. With a median of 2.6 h (IQR = 2.2 h), all FBC analyses were undertaken within 24 h.
Figure 2 shows no statistically significant difference in the median MCV in those analysed <8 h and ≥8 h (
p = 0.766) and in the median %HPO for those analysed <6 h and ≥6 h (
p = 0.176).
Using RHC to diagnose IDA, as shown in
Table 3, sensitivity was 34.7%, specificity 89.4%, and positive predictive value 64.7%.
Table 4 shows the use of HRC to diagnose IDA. Sensitivity was 29.7%, specificity 87.9%, and positive predictive value 92.2%.
Table 5 shows that patients with ID had predominantly normocytic anaemia, as also did patients with folate deficiency. Patients with vitamin B12 deficiency had equal proportions of microcytic, normocytic, and macrocytic anaemia. In patients 65 years old and older with IDA, 35.3% (6/17) had microcytosis.
In eight patients with vitamin B12 deficiency, two had neurological disorders consistent with deficiency. One had acute confusion (vitamin B12, 50 pmol/L) and one lumbar radiculopathy (vitamin B12, 75 pmol/L). They had normal MCV and no blood film features of megaloblastic anaemia. Another patient was on metformin, which may cause deficiency. Five had no neurological or haematological features to diagnose deficiency. Of three patients with possible vitamin B12 deficiency (range 147.6–258.3 pmol/L), one had acute psychosis with HIV and hepatitis B virus infections, one had paraparesis of unknown cause, and one PLWHIV had TB meningitis. Overall, 2.5% (8/324 results) had vitamin B12 deficiency.
In patients with moderate and severe anaemias and a vitamin B12 level <147.6 pmol/L, none had hyper-segmented neutrophils or Howell–Jolly bodies. One patient had macrocytosis and ovalocytes with a vitamin B12 level of 109 pmol/L and a folate level of 2.1 pmol/L. The prevalence of folate deficiency was 6.1% (18/294 results). None of the patients with folate deficiency were taking a medication causing deficiency.
3.1. Macrocytosis
Of 25 patients with macrocytosis, 10 had incomplete drug data and 15 were PLWHIV. The putative causes of macrocytosis in 15 were lamivudine, 8; folate deficiency, 3; vitamin B12 deficiency, 2; cotrimoxazole, 2; valproate, 2; zidovudine, 2; liver disease, 1; and multiple myeloma, 1 (four patients had two causes each, and one had three).
3.2. Microcytosis
Of 78 patients with microcytosis, one had a Mentzer index value < 13. That patient had IDA (TSAT 4%, ferritin 46 mcg/L).
3.3. Multiple Causes of Anaemia
For each of the eight major causes, the numbers (percentage) of patients with moderate/severe anaemia due solely to that cause were iron deficiency 32/85 (37.6), vitamin B12 deficiency 4/8 (50), folate 1/18 (5.6), HIV 136/219 (62.1), cancer 2/12 (16.7), CKD 8/28 (28.6), TB 6/75 (8.0), and heart failure 2/4 (50). Overall, 191/449 (42.5%) had a single cause of anaemia, ranging from 5.6% for patients with folate deficiency to 62.1% with HIV. The remainder (57.5%) had two or more causes.
In the bivariable model, those who had severe anaemia were 70% (OR = 1.7, 95%CI = 1.0–2.7;
p-value = 0.039) and 90% (OR = 1.9, 95%CI = 1.1–3.2;
p-value = 0.017) more likely to have HIV and TB, respectively (
Table 6). However, the multivariable best-fitting model that was adjusted for other chronic conditions and the anaemia markers did not show an association between the severity of anaemia and an HIV-positive result (
p-value = 0.880). Instead, the measure of effect increased for TB, wherein patients with severe anaemia were found to have been three times more likely to have had TB, and this was also statistically significant (OR = 3.1, 95% CI = 1.5–6.5;
p-value = 0.002). Similarly, patients with severe anaemia were 5.5 times more likely to present in a hospital setting than a primary care setting, and this was statistically significant as well (OR = 5.5, 95%CI = 1.5–19.7;
p-value = 0.009).
The multivariable analysis further shows that an increase of 1 in the reticulocyte production index increased the odds of severe anaemia by 5.5 (co-efficient = 1.7, 95%CI = 0.9–2.5; p-value < 0.0001); and a 1 g/dL increase in the mean corpuscular haemoglobin concentration (MCHC) increases the odds of having severe anaemia by 65% (co-efficient = 0.5, 95%CI = 0.4–0.7; p-value < 0.0001), and both these were statistically significant.
Even though PLWHIV with moderate anaemia had a statistically lower median Hb than HIV-negative patients (
p-value < 0.0004), there was no statistical difference between the median Hb of patients with a negative HIV status and PLWHIV in patients with severe anaemia (
p-value = 0.623) (
Table 7). TB showed an opposite effect where patients with severe anaemia had a higher median Hb, and this was statistically significant (
p-value = 0.005). The median MCHC was lower than normal in patients with moderate and severe anaemia and lower still in patients with severe anaemia and HIV or TB. Other markers of statistical significance included the following: a higher median CRP for PLWHIV with moderate anaemia (
p-value = 0.003); higher median CRP values for both TB-positive patients with moderate (median = 146;
p-value = 0.005) and severe anaemia (median = 163;
p-value = 0.0004); higher median transferrin saturation severe anaemia in both HIV-positive (median = 19.6%;
p-value = 0.0004) and TB-positive patients (median = 19.2%;
p-value = 0.015); lower median RPIs for PLWHIV with moderate (median = 0.5;
p-value = 0.005) and severe anaemia (median = 0.3;
p-value = 0.036); and higher median ferritin levels for all categories of HIV-positive and TB-positive patients (
p-value < 0.05). Vitamin B12 values were higher in PLWHIV with moderate or severe anaemia and in TB with moderate anaemia. Folate levels were unchanged with HIV and TB.
4. Discussion
There was an overall high (45.9%) prevalence of anaemia in the screened population, with the highest prevalence at MRH. Similar to attendees in primary care in South Africa [
49], two-thirds of patients screened for anaemia in this study were female. In this study, the age group 40–49 years had the largest number screened. This may be due to the exclusion of pregnant women and the inclusion of sicker patients attending MRH. In older adults (≥40 years old), 48.4% of men and 46.1% of women had anaemia. High rates of anaemia were also found in community-living older adults in Mpumalanga Province, South Africa, i.e., 40.1% of men and 43% of women [
50]. Prevalence of HIV infection was similar, at 20.9% in this study and 20.7% in Mpumalanga Province.
Overall, the largest category of anaemia on screening was moderate, followed by severe. In contrast, in the national community surveys, the largest category was mild followed by moderate [
9,
10]. The skewed distribution in this study is most likely due to selection of participants from health care facilities, which will likely have more sicker individuals than those in the general population. The overall prevalence of moderate and severe anaemia was 35.5% on HemoCue testing but 25.5% on laboratory testing. HemoCue 201 overestimates mean Hb concentrations by 0.1–1.2 g/dL [
51]. Furthermore, capillary finger-prick usually produces higher Hb values by 0.2–0.9 g/dL compared to venous blood [
51].
In patients with laboratory-confirmed moderate and severe anaemia, AI was the predominant type at 72.6%, reflecting the high prevalences of HIV (59.5%) and TB (16.6%). In Cape Town, >95% of patients with anaemia and HIV-associated TB had AI [
23]. In patient populations where there are very low levels of HIV and TB diagnoses, AI was the commonest type of anaemia, but prevalences were much lower at 25.7% in elderly inpatients in South Africa [
18] and 41.9% of medical inpatients in Italy [
52]. In the U.S., one-third of elderly community-dwellers with anaemia had AI, one-third nutrient deficiencies, and one-third unexplained anaemia [
53].
The 26.5% prevalence of IDA is similar to prevalences of 24.3% in older inpatients in South Africa [
18] and 20% in older community-dwellers in the U.S. [
53] but higher than 14.7% in internal medicine inpatients in Italy [
52]. IDA in this study comprised 15.9%, with absolute ID (ferritin < 100 mcg/L [
54]) and 10.5% functional ID (ferritin ≥ 100–300 mcg/L and TSAT < 20%) [
39,
43]. However, absolute and functional ID overlap such that patients who have ferritin > 100 mcg/L may have absolute iron deficiency. In a systematic review utilising 38 studies, the mean ferritin level in absolute iron deficiency was 82.4 mcg/L (range of means 34–158 mcg/L) in patients with inflammatory diseases, using bone marrow iron as the gold standard [
55]. While there are various definitions of ID [
23], the “pragmatic” definition of Camaschella and Girelli [
39] was used in this study, combining ferritin and TSAT. TSAT is considered an accurate test for ID in patients with inflammation [
39,
56]. Newer tests for ID include hepcidin and soluble transferrin receptor (sTfR) levels [
40] but are not routinely available in South Africa.
Forty-one per cent (41%) of patients with IDA had microcytosis. In patients 65 years old and older, 35.3% had microcytosis. Studies in older patients with absolute IDA in developed countries show <30% have microcytosis [
57]. These data strongly support biochemical testing for ID in all patients with anaemia irrespective of MCV values. Only one with microcytosis had a Mentzer index value < 13 [
58] but had concomitant ID. This suggests that the thalassaemia trait is uncommon in the study population. While the α-thalassaemia trait is present in some communities in South Africa, with a prevalence of 3.8% [
59] and 16% in non-random samples [
60], testing is mainly done if there is unexplained microcytosis [
60,
61].
All MCV measurements in the laboratory were done within 24 h of venesection, with no difference between those measured before and after eight hours. While MCV stored at 4 °C remains unchanged for 24 h, it increases significantly after eight hours at room temperature (standard mean difference −0.30, CI −0.50, −0.10)) [
62].
RHC and %HYPO had low sensitivity for IDA, implying that the tests are not suitable for ruling out IDA in the study setting. RHC can perform as well as standard tests for the diagnosis of ID [
35]. %HPO has mainly been used to diagnose ID in the setting of CKD [
35,
39]. While %HPO should be analysed within six hours of venesection [
34],
Figure 2 shows that there was no significant difference between blood samples drawn before or after six hours. Further research is needed into the sub-optimal performance of these tests in similar settings to this study.
The major disease categories associated with moderate and severe anaemia were, in order, HIV, TB, CKD, and cancer, as shown in
Table 2. Anaemia is common in PLWHIV from multifactorial causes [
63]. While worldwide prevalences in PLWHIV are 21.6%, 22.6%, and 6.2% for mild, moderate, and severe anaemia, respectively [
64], in South Africa, prevalences are mostly higher at 26.7%, 41.1%, and 4.3% [
12]. In this study, 59.5% of those with moderate/severe anaemia were PLWHIV. The majority had low mean CD4 counts, unsuppressed VLs, and CDC Stage 3, which are indicative of advanced HIV disease and a high risk of anaemia. The low prevalence of reported OIs other than TB in this study may be due to limited investigations. Compared to low- and middle-income countries in Latin America and Asia, adults in Sub-Saharan Africa have markedly lower incidences of
Pneumocystis jirovecii pneumonia and cerebral toxoplasmosis in ART-naïve patients [
65], most likely due to a lack of resources for diagnosis rather than a true difference in infection rates [
66].
Patients with severe anaemia were three times more likely to have a diagnosis of TB compared to the other major diagnostic categories (HIV, cancer, and CKD) as demonstrated in the multivariate analysis (
Table 6, which only considers patients with laboratory moderate or severe anaemia). While
Table 7 shows that in patients with TB and severe anaemia, Hb was higher in those with TB compared to those without, the multivariate analysis is the most accurate summation due to accounting for multiple variables.
Table 7 is an analysis of indices to assist in characterising findings in HIV and TB, which are the most prevalent causes, compared to all other causes in patients with moderate/severe anaemia. In PLWHIV with moderate (but not severe) anaemia, Hb was significantly lower compared to those without HIV. HIV frequently causes impaired haematopoiesis, and anaemia is the most common manifestation, increasing in frequency and severity with disease progression [
63]. PLWHIV with moderate or severe anaemia had overall low CD4 counts (median values < 200 cells/µL) indicative of more advanced disease [
67]. PLWHIV with TB and severe anaemia had lower CD4 counts and higher VL compared to those without TB. This is consistent with HIV as the most potent immunosuppressive risk factor for active TB [
68]. HIV infection was associated with a slightly lower RPI that is not clinically significant. Patients with or without HIV or TB and moderate and severe anaemia had normal MCV and low MCHC (indicating hypochromia). However, MCHC is considered of little clinical relevance in interpreting anaemias [
69]. Median TSAT was also very low (<16%) in both moderate and severe anaemia without HIV or TB, which is suggestive of absolute ID [
56]. Serum iron was lower in severe anaemia without HIV or TB. Serum iron is depressed in both ID and AI and cannot differentiate between the two conditions [
70]. Serum transferrin was significantly lower in patients with moderate and severe anaemia and HIV or TB. It is an acute-phase reactant that deceases in inflammation but increases in ID [
41]. Ferritin and CRP, both inflammatory markers, were significantly higher in patients with either HIV or TB and moderate or severe anaemia. CRP can be used for diagnosis of pulmonary TB [
71] and as an indicator of disease severity [
72] and higher mortality [
73]. Median CRP levels in patients with TB in this study, i.e., 146 mg/L (IQR 214 mg/L) in moderate and 163 mg/L (IQR 122 mg/L) in severe anaemia, were considerably higher than those of 40 mg/L (IQR 83 mg/L) in U.K. [
72] and 17.3 (±37.2 mg/L, SD) in Taiwanese [
73] studies and indicative of more severe disease, possibly due to delayed diagnoses.
CKD stages 3–5 were present in 6.5% of patients with moderate and severe anaemia. Prevalences of CKD stages 3–5 in Africa are 4.6% in the general population and 13% in high-risk populations, e.g., HIV, diabetes, and hypertension [
74], compared with 10.6% in the general population worldwide [
75]. The low prevalence of CKD may be due to the strict application of the criteria to define CKD, i.e., abnormalities of kidney structure or function, present for >3 months [
46], and non-performance of regular renal evaluations, in patients at high risk, e.g., diabetes mellitus as elsewhere in South Africa [
76], and the non-availability of prior patient records with creatinine results. The actual prevalence is most likely double this, as all creatinine-based GFR-estimating equations underestimate GFR in African populations [
77]. While cystatin C-based equations may be superior, cystatin C is unaffordable in clinical practice in developing countries [
78]. In this study, 32% with CKD had IDA. This compares to a 35.3% IDA prevalence in non-dialysis CKD outpatients in Johannesburg [
79] and 15–72.8% in developed countries [
80]. It is important that IDA is recognised and treated to improve quality of life [
81]. Most patients with stable CKD can be managed in primary care [
82].
In patients with cancer, one of six had IDA. The prevalences of anaemia and ID in different cancers was reported as 29–46% and 7–42%, respectively, in developed countries (ID prevalence at 60% was highest in colorectal cancer) [
5]. No patient had colorectal cancer in this study.
There was a low overall prevalence (2.5%) of vitamin B12 deficiency (<147.6 pmol/L) compared to 8.8% in non-pregnant women of childbearing age elsewhere in South Africa [
83]. While NHLS normal values for both sexes were 133–675 pmol/L, the lower limit of normal cannot be used as a cut-off to define deficiency due to frequent false-positive and false-negative results [
36] Methylmalonic acid and homocysteine levels are required to confirm vitamin B12 deficiency [
36], but testing was not mandated in the public-sector guidelines [
84]. As seen in this study, neuropsychiatric disorders due to deficiency often occur without haematological changes [
36]. While vitamin B12 deficiency can be found in up to 30% of PLWHIV [
85], there is little evidence of overt clinical disease [
86,
87]. A minority of patients with vitamin B12 deficiency exhibited macrocytic changes. This is well documented, particularly in patients with iron deficiency or inflammation [
88]. Vitamin B12 values were significantly higher in patients at MRH compared to the CHCs. Levels were higher also in moderate and severe anaemia in PLWHIV and patients with TB. There are reports of higher mortality with elevated vitamin B12 levels, namely >250 pmol/L [
89] and >700 pmol/L [
90] by mechanisms yet to be elucidated.
Low community folate-deficiency prevalences of 0.2% [
91] and zero in reproductive-age women [
83] were reported in South Africa following folate fortification in 2003. The higher prevalence (6.2%) in this study may be due to inadequate nutrition during illness.
The majority (57.5%) of patients had multiple causes of anaemia compared with 13.6% of elderly inpatients in South Africa [
18] and 32.5% in Italy [
52]. This relates to the high levels of infectious diseases (TB and HIV) in this study population.
Approaches in the evaluation of anaemia are traditional evaluation using the MCV and RPI [
92,
93] and biochemical tests [
16,
23,
40]. South African national guidelines advise categorising anaemia based on MCV and, if microcytic, testing for ID and, if macrocytic, testing for vitamin b12 and folate deficiency [
33,
94]. As shown in this and other studies [
95], this approach will miss many patients with nutrient deficiencies and those with multiple causes. With a substantial prevalence of ID, this study supports the recommendation that iron status needs evaluation in every patient with anaemia [
16,
32] but also adds the evaluation of folate and vitamin B12 deficiencies. In areas of high TB and HIV prevalence, TB (pulmonary and extra-pulmonary) should be excluded as a cause of moderate and severe anaemia [
12,
22]. Overall, a combination of MCV/RPI, biochemical, HIV, and TB testing would seem the optimal approach for moderate and severe anaemia evaluation in the study population.
The strengths of this study are that causes of anaemia were investigated comprehensively, using biochemical criteria and clinical assessment, and in the context of daily practice in primary care and a district hospital setting. An updated pragmatic definition of ID was used compared with previous studies. The cross-sectional study design is suited to estimate the prevalence of anaemia in the study population [
96].
Limitations include missing data due to patient, research assistant, and laboratory factors. Patients had multiple non-interoperable records, making it difficult to obtain historical data. However, there was no indication of a systematic pattern that might invalidate the analysis. Some patients were on drugs associated with anaemia. Due to the cross-sectional study design (with absence of follow-up), it is not possible to state with certainty that any drug was responsible for anaemia. TB was likely responsible for more causes of anaemia, as patients with probable TB on clinical assessment were excluded from analysis. In retrospect, our sample size could have been optimised by using the two-proportions formula with a power of 0.8 instead of using the single-proportion formula, which resulted in only 25.5% of the initial participants being eligible for the final analyses.
The findings are not generalisable but may be transferrable to similar settings.