Multilevel Toxicity Evaluations of Polyethylene Microplastics in Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Polyethylene Microplastics (PE-MP)
2.2. Zebrafish (Danio rerio) as Test Organism
2.3. Fish Embryo Toxicity Test (FET)
2.4. Juveniles Test
2.5. Adult Toxicity Test: Histology, Micronucleus, Comet Assay and Biochemical Biomarkers
2.5.1. Histology
2.5.2. Genotoxicity and Cytotoxicity
2.5.3. Biochemical Biomarkers
2.6. Statistical Analysis
3. Results
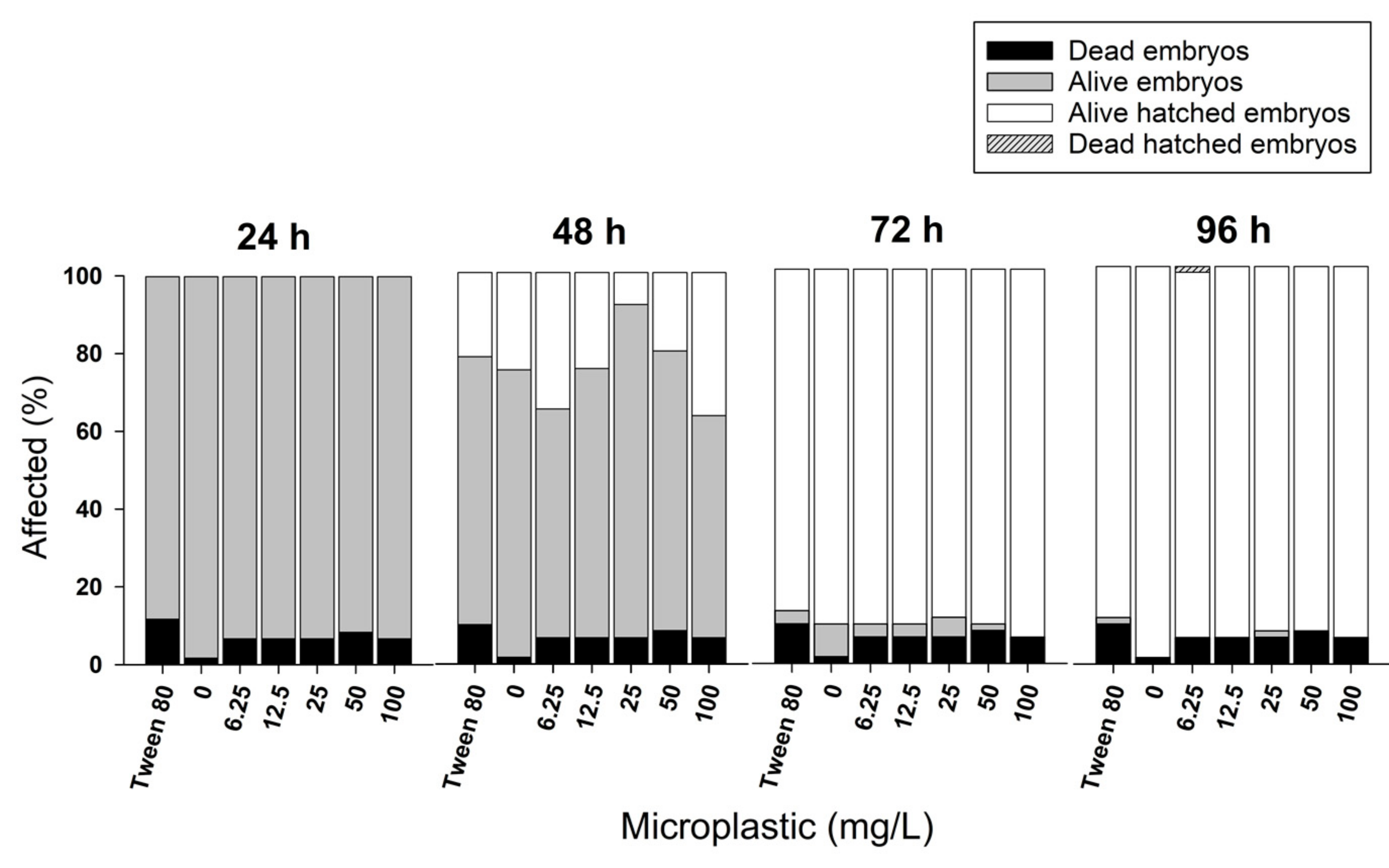
3.1. Fish Embryo Toxicity Test (FET)
3.2. Juveniles Test
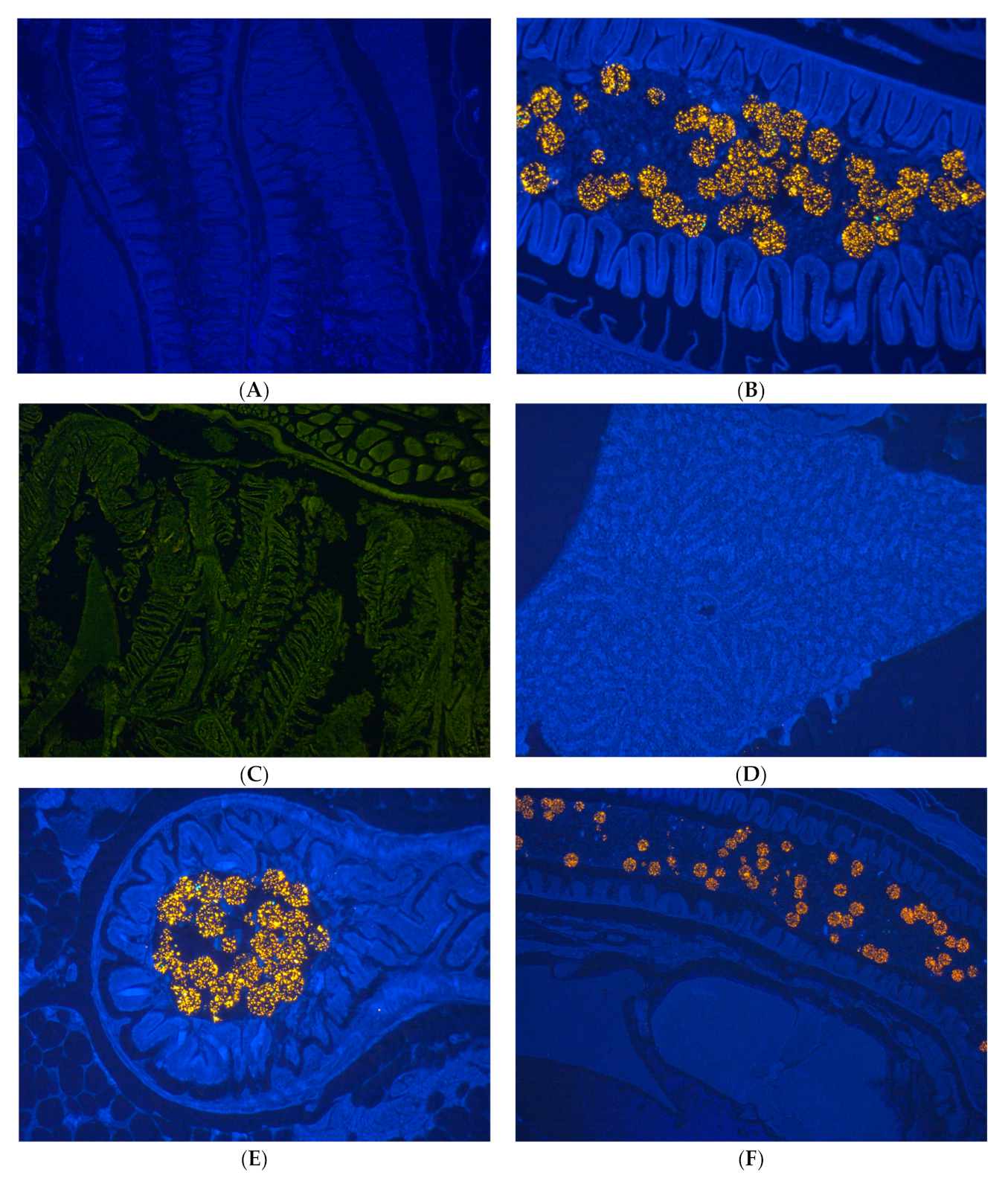
3.3. Histology
3.4. Genotoxicity and Cytotoxicity
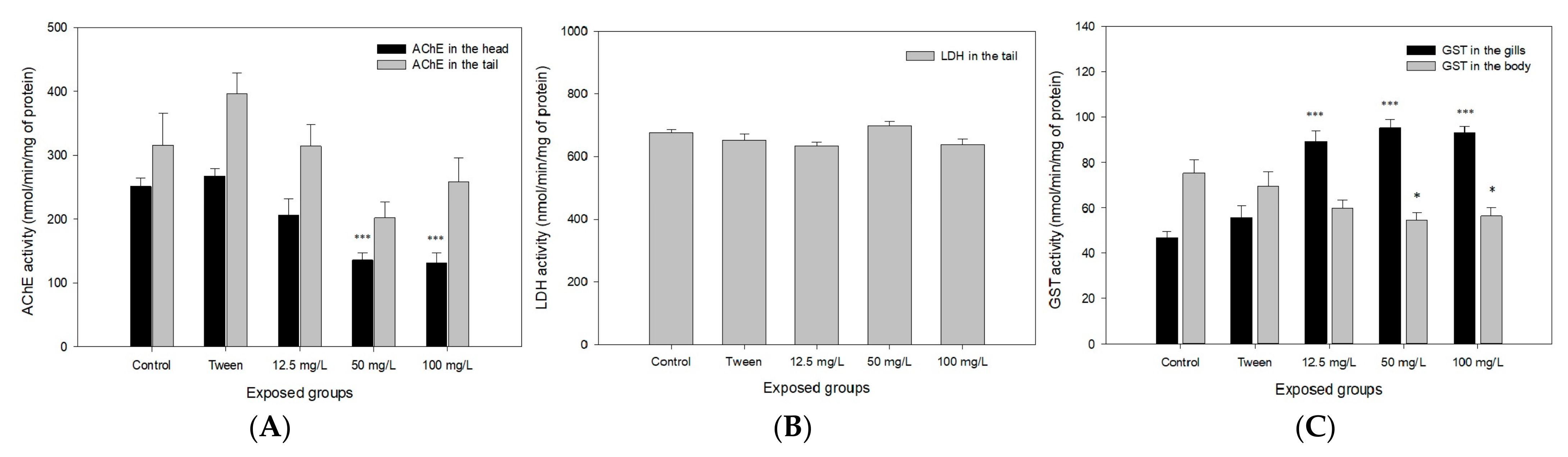
3.5. Biochemical Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, A.P.C.; Rocha, T.L.; Melo e Silva, D.M.; Malafaia, G. Micro(nano)plastics as an emerging risk factor to the health of amphibian: A scientometric and systematic review. Chemosphere 2021, 283, 131090. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastic as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.R.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Meaza, I.; Toyoda, J.H.; Wise Sr, J.P. Microplastics in sea turtles, marine mammals and humans: A one environmental health perspective. front. Environ. Sci. 2020, 8, 575614. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.-J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef]
- BRASIL. Lei nº 12.305, de 2 de Agosto de 2010. Institui a Política Nacional de Resíduos Sólidos. Available online: https://www.planalto.gov.br/ccivil_03/_ato2007-2010/2010/lei/l12305.htm (accessed on 12 December 2022).
- Anagnosti, L.; Varvaresou, A.; Pavlou, P.; Protopapa, E.; Carayanni, V. Worldwide actions against plastic pollution from microbeads and microplastics in cosmetics focusing on european policies. Has the issue been handled effectively? Mar. Pollut. Bull. 2021, 162, 111883. [Google Scholar] [CrossRef]
- ABIPLAST. Perfil 2020. A Indústria de Transformação e Reciclagem de Plástico no Brasil. Available online: http://www.abiplast.org.br/wp-content/uploads/2021/08/Perfil2020_abiplast.pdf (accessed on 1 June 2022).
- Camargo, A.L.G.; Girard, P.; Sanz-Lazaro, C.; Silva, A.C.M.; Faria, E.; Figueiredo, B.R.S.; Caixeta, D.S.; Bletter, M.C.M. Microplastics in sediments of the Pantanal Wetlands, Brazil. Front. Environ. Sci. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Silva, P.H.S.; Sousa, F.D.B. Microplastic pollution of Pato Lagoon, south of Brazil. Environ. Chall. 2021, 4, 100076. [Google Scholar] [CrossRef]
- Drabinski, T.L.; Carvalho, D.G.; Gaylarde, C.C.; Lourenço, M.F.P.; Machado, W.R.T.V.; Fonseca, E.M.; Silva, A.L.C.; Baptista Neto, J.A. Microplastics in freshwater river in Rio de Janeiro and its role as a source of microplastic pollution oin Guananbara Bay, SE Brazil. Micro 2023, 3, 208–223. [Google Scholar] [CrossRef]
- Rani-Borges, B.; Martins, T.F.G.; Pompêo, M. Status of Brazilian Research on Microplastics Present in Aquatic Ecosystems: Freshwater. PANAMJAS 2021, 16, 106–117. Available online: http://panamjas.org/pdf_artigos/PANAMJAS_16(1)_106-117.pdf. (accessed on 13 February 2023).
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastic and microplastic in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Magyary, I. Recent advances and future trends in zebrafish bioassays for aquatic ecotoxicology. Ecocycles 2018, 4, 12–18. [Google Scholar] [CrossRef]
- OECD. Test. No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD iLibrary: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Parichy, D.M.; Elizondo, M.R.; Mills, M.G.; Gordon, T.N.; Engeszer, R.E. Norma table of post-embryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev. Dyn. 2011, 238, 2975–3015. [Google Scholar] [CrossRef]
- OECD. Test No. 203: Fish, Acute Toxicity Test; OECD iLibrary: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of micro- and nanoplastic in Daphnia magna—Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Déniel, M.; Nicolai, T.; Chassenieux, C.; Lagarde, F. Towards more realistic reference microplastics a nanoplastics: Preparation of polyethylene micro/nanoparticles with a biosurfactant. Environ. Sci. Nano 2019, 6, 315–324. [Google Scholar] [CrossRef]
- Castania, V.A.; Silveira, J.W.S.; Issy, A.C.; Pitol, D.L.; Castania, M.L.; Dorigan Neto, A.; Del Bel, E.A.; Defino, H.L.A. Advantages of a combined method of decalcification compared to EDTA. Microsc. Res. Tech. 2015, 78, 111–118. [Google Scholar] [CrossRef]
- Gonçalves, C.; Martins, M.; Costa, M.H.; Costa, P.M. Development of a method for the detection of polystyrene microplastics in paraffin-embedded histological sections. Histochem. Cell Biol. 2018, 149, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. The in vitro micronucleus techique. Mutat. Res. 2000, 455, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.D.S.; Fontanetti, C.S. Micronucleus test and observation of nuclear alterations in erythrocytes of Nile tilapia exposed to waters affected by refinery effluent. Mutat. Res. 2006, 605, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCOY, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Kujawski, M.; Czub-Świerczek, M.; Markowska, J.; Szyfter, K. Bleomycin-induced DNA damage and its removal in lymphocytes of breast cancer patients studied by comet assay. Mutat. Res. 1997, 385, 223–233. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalhgo, A.P.; Soares, A.M. Inhibition of acetylcholinesterase activity as effect criterion in acute test with juvenile Daphnia magna. Chemosphere 1996, 32, 727–738. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres Jr., V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Vassault, A. Lactate dehydrogenase. UV-method with pyruvate and NADH. Methods Enzym. Anal. 1983, 3, 118–126. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-s-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Wibowo, A.T.; Nugrahapraja, H.; Wahyuono, R.A.; Islami, I.; Haekal, M.H.; Fardiansyah, Y.; Sugiyo, P.W.; Putro, Y.K.; Fauzia, F.N.; Santoso, H.; et al. Microplastic contamination in the human gastrointestinal tract and daily consumables associated with an Indonesian farming community. Sustainability 2021, 13, 12840. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Ribeiro Júnior, G.; Galvão, L.S.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Malafaia, G.; Souza, A.M.; Pereira, A.C.; Gonçalves, S.; Araújo, A.P.C.; Ribeiro, R.X.; Rocha, T.L. Developmental toxicity in zebrafish exposed to polyethylene microplastics under static and semi-static aquatic systems. Sci. Total Environ. 2020, 700, 134867. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Martin, D.M.; Hardy, J.D. Acipenseride through Ictaluridae. In Development of Fishes of the Mid-Atlantic Bight: An Atlas of Egg, Larval, and Juvenile Stages; Fish and Wildlife Services: Washington, DC, USA, 1978. [Google Scholar]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish embryos and larvae as alternative animal models for toxicity testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef]
- Manuel, P.; Almeida, M.; Martins, M.; Oliveira, M. Effects of nanoplastics on zebrafish embryo-larval stages: A case study with polystyrene (PS) and polymethylmethacrylate (PMMA) particles. Environ. Res. 2022, 213, 113584. [Google Scholar] [CrossRef]
- Harder, W. Anatomy of Fishes, 2nd ed.; E. Schweizerbart: Stuttgart, Germany, 1975. [Google Scholar]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adults zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Bhuyan, S. Effects of microplastics on fish and in human health. Front. Environ. Sci. 2022, 10, 1–17. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and frag-mentation of plastic debris in global environments. Philos. Trans. Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Vendel, A.; Bessa, F.; Alves, V.E.N.; Amorim, A.L.A.; Patrício, J.; Palma, A.R.T. Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar. Pollut. Bull. 2017, 117, 448–455. [Google Scholar] [CrossRef]
- Frenzilli, G.; Nigro, M.; Lyons, B.P. The comet assay for the evaluation of genotoxic impact in aquatic environments. Mutat. Res. 2009, 681, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.P.C.; Luz, T.M.; Rocha, T.L.; Ahmed, M.A.I.; Melo e Silva, D.; Rahman, M.M. Toxicity evaluation of the combination of emerging pollutants with polyethylene microplastics in zebrafish: Perspective study of genotoxicity, mutagenicity, and, redox unbalance. J. Hazard. Mat. 2022, 432, 128691. [Google Scholar] [CrossRef] [PubMed]
- Piaui, T.B.; Fascineli, M.L.; Moura, D.S.; Albernaz, L.C.; Espindola, L.S.; Grisologia, C.K. In the screening of alternative insecticides to control Aedes aegytpti larvae 2-methylanthraquinone showed no genotoxicity and low toxicity to zebrafish (Danio rerio). Genet. Mol. Biol. 2022, 45, 1. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687–46710. [Google Scholar] [CrossRef]
- Glisic, B.; Mihaljevic, I.; Popovic, M.; Zaja, R.; Loncar, J.; Fent, K.; Kovacevic, R.; Smital, T. Characterization of glutathione-S-transferases in zebrafish (Danio rerio). Aquat. Toxicol. 2015, 158, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Lettieri, T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Prüstt, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Freire, I.; Fascineli, M.L.; Piau, T.B.; Pereira, B.F.; Grisolia, C.K. Multilevel Toxicity Evaluations of Polyethylene Microplastics in Zebrafish (Danio rerio). Int. J. Environ. Res. Public Health 2023, 20, 3617. https://doi.org/10.3390/ijerph20043617
de Souza Freire I, Fascineli ML, Piau TB, Pereira BF, Grisolia CK. Multilevel Toxicity Evaluations of Polyethylene Microplastics in Zebrafish (Danio rerio). International Journal of Environmental Research and Public Health. 2023; 20(4):3617. https://doi.org/10.3390/ijerph20043617
Chicago/Turabian Stylede Souza Freire, Ingrid, Maria Luiza Fascineli, Tathyana Benetis Piau, Bruno Fiorelini Pereira, and Cesar Koppe Grisolia. 2023. "Multilevel Toxicity Evaluations of Polyethylene Microplastics in Zebrafish (Danio rerio)" International Journal of Environmental Research and Public Health 20, no. 4: 3617. https://doi.org/10.3390/ijerph20043617
APA Stylede Souza Freire, I., Fascineli, M. L., Piau, T. B., Pereira, B. F., & Grisolia, C. K. (2023). Multilevel Toxicity Evaluations of Polyethylene Microplastics in Zebrafish (Danio rerio). International Journal of Environmental Research and Public Health, 20(4), 3617. https://doi.org/10.3390/ijerph20043617





