Toxicity of Tetracycline and Metronidazole in Chlorella pyrenoidosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drug and Microalgae Culture
2.2. Algal Cell Density and Median Effective Concentration (EC50)
2.3. Combined Toxicity Effect Evaluation
2.4. Toxicity Test
2.4.1. Single Toxicity Assay of TC and MTZ
2.4.2. Combined Toxicity Experiment
2.5. Characterization of Algal Cells and Determination of Extracellular Polymers
2.6. Oxidative Stress Analysis
2.7. Determination of Cell Membrane Permeability
2.8. Data Analysis Method
3. Results and Discussion
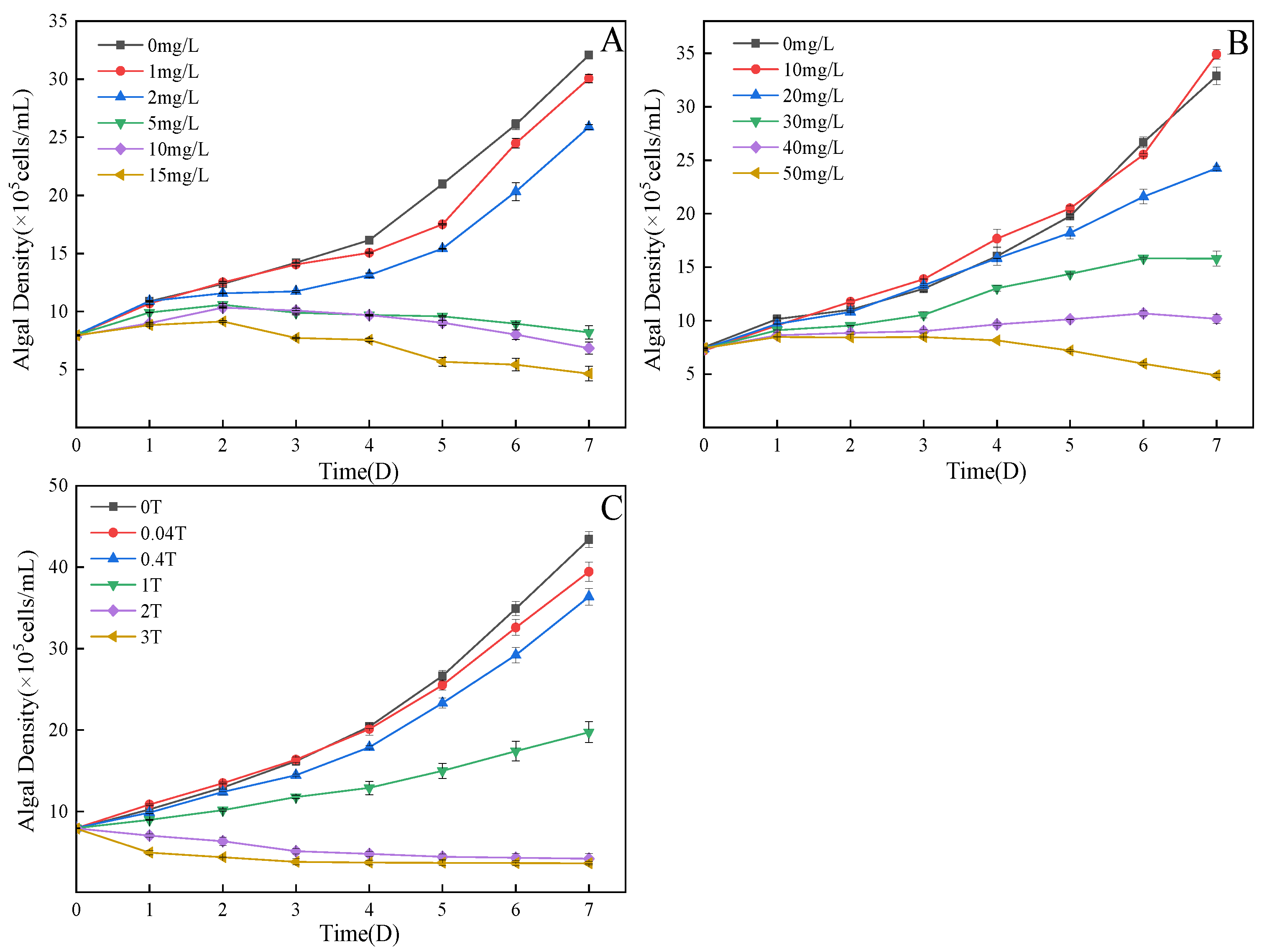
3.1. Influence on the Growth of C. pyrenoidosa
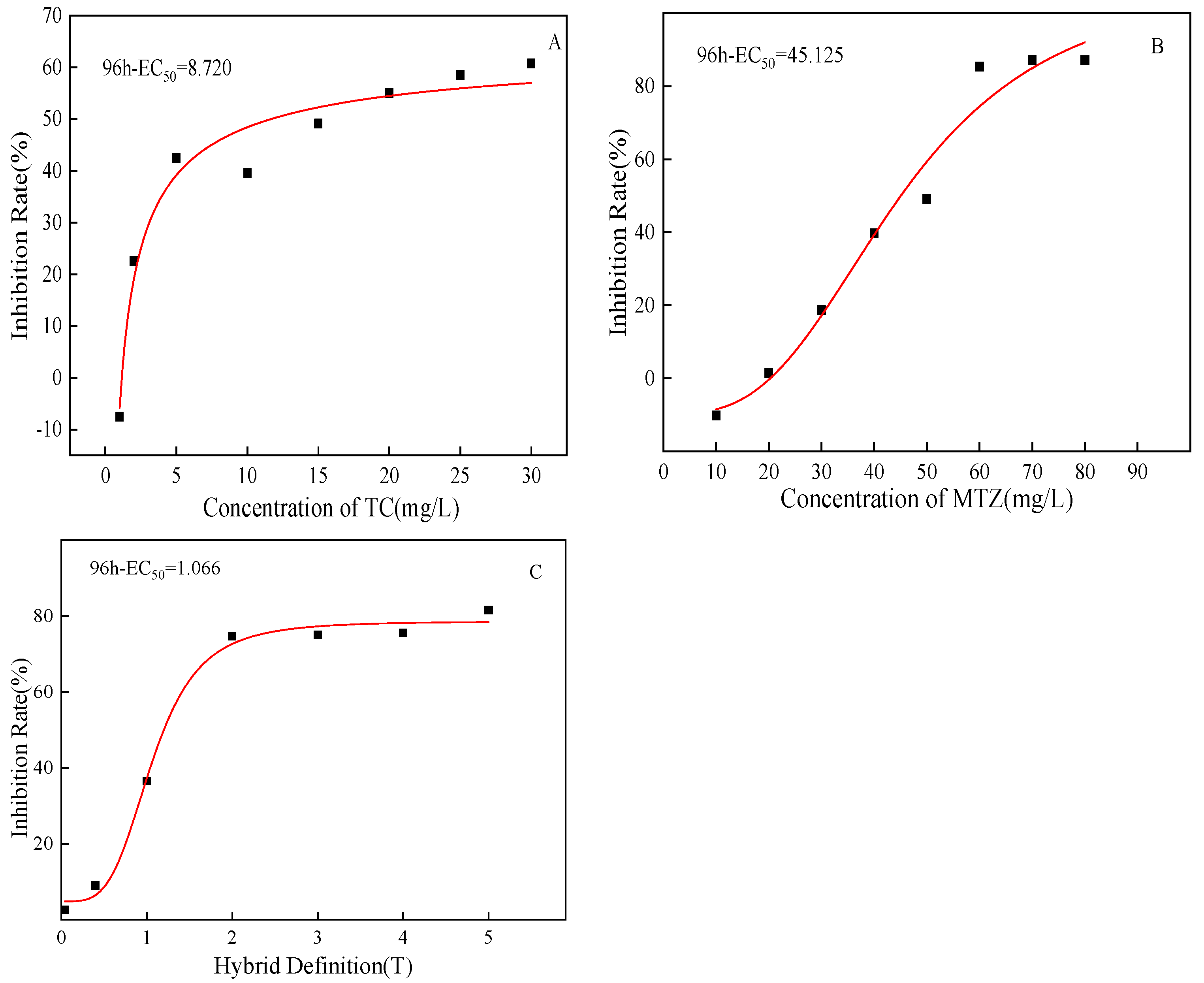
3.2. Evaluation of the Combined Toxic Effect of TC and MTZ on C. pyrenoidosa
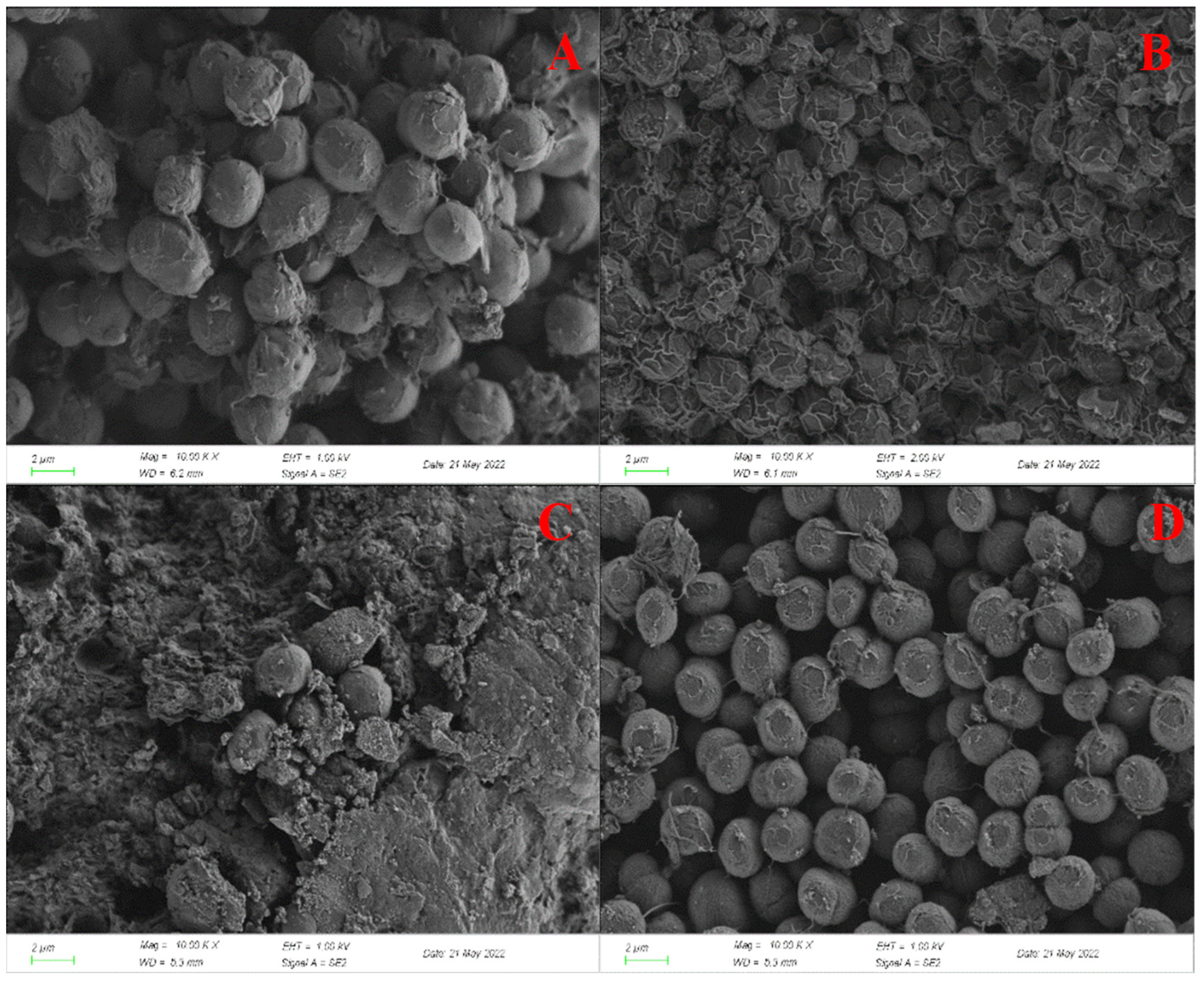
3.3. Effect on Morphology of C. pyrenoidosa
3.4. Effect on the Extracellular Polymer of C. pyrenoidosa
3.5. Effects on the Antioxidant System of C. pyrenoidosa
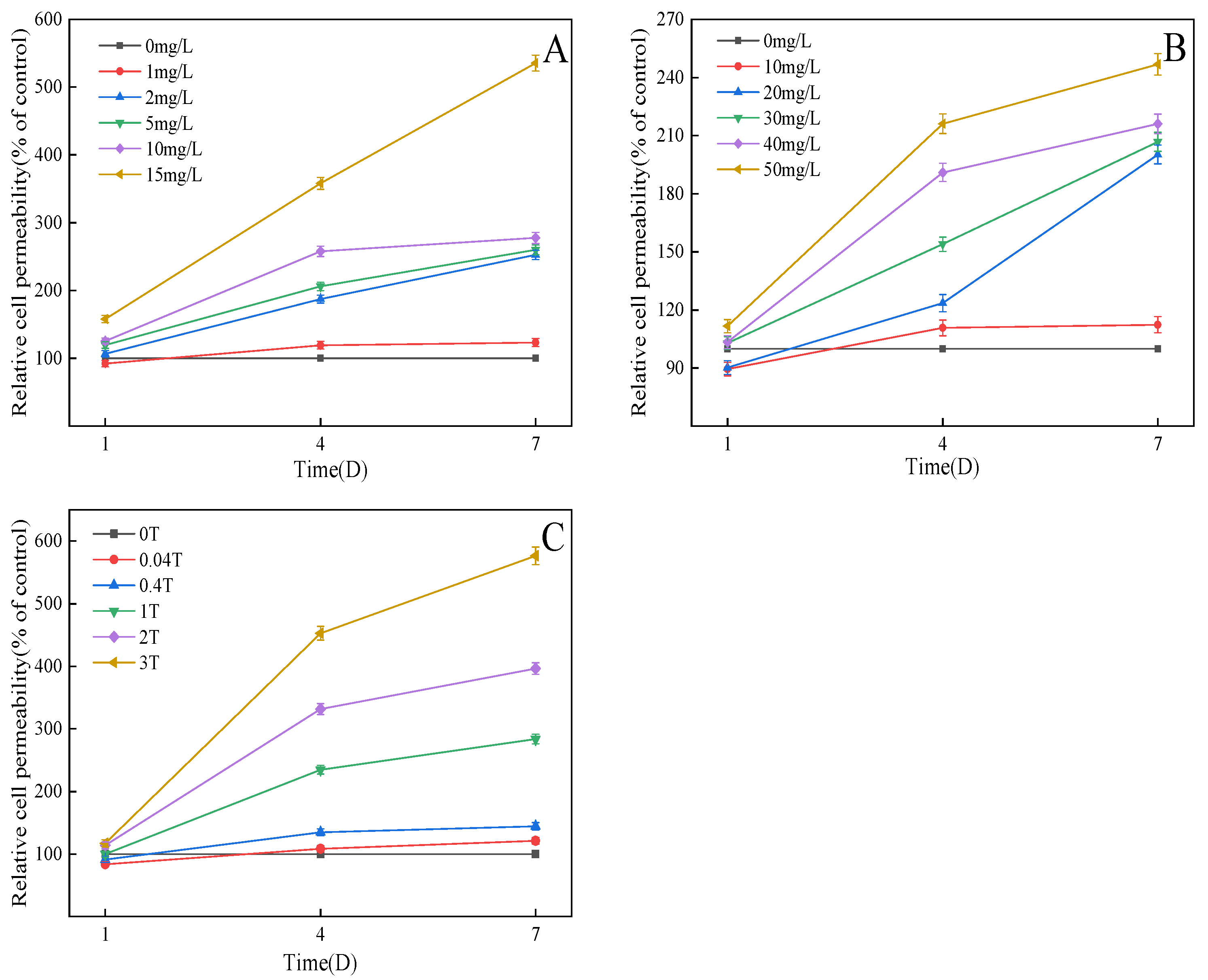
3.6. Effect on Cell Membrane Permeability of C. pyrenoidosa
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Liu, J.; Tian, L.; Li, X.; Zhao, J. Ultrathin carbon-coated Zr3+-ZrO2 nanostructures for efficient visible light photocatalytic antibiotic elimination. Chem. Eng. J. 2021, 412, 128621. [Google Scholar] [CrossRef]
- Li, L.; Shi, L.; Jia, J.; Eltayeb, O.; Shuang, S. Red fluorescent carbon dots for tetracycline antibiotics and pH discrimination from aggregation-induced emission mechanism. Sens. Actuators B Chem. 2021, 332, 129513. [Google Scholar] [CrossRef]
- Ma, Y.P.; Li, M.; Wu, M.M.; Li, Z.; Liu, X. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci. Total Environ. 2015, 518–519, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Sierra, J.C.; Hernández-Ramírez, A.; Zhao, Z.; Martinez-Hernandez, A.; Gracia-Pinilla, M. Construction of direct Z-scheme WO3/ZnS heterojunction to enhance the photocatalytic degradation of tetracycline antibiotic. J. Environ. Chem. Eng. 2021, 9, 105111. [Google Scholar] [CrossRef]
- Rajkumar, R.; Yaakob, Z.; Kamarudin, K.F.; Takriff, M.; Badar, S. The Current Methods for the Biomass Production of the Microalgae from Wastewaters: An Overview. World Appl. Sci. J. 2014, 31, 1744–1758. [Google Scholar]
- Nunes, B.; Antunes, S.C.; Gomes, C.J.C.; Braga, R.M.; Ramos, S.A. Acute Effects of Tetracycline Exposure in the Freshwater Fish Gambusia holbrooki: Antioxidant Effects, Neurotoxicity and Histological Alterations. Arch. Environ. Contam. Toxicol. 2015, 68, 371–381. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, J.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef]
- Klinger, R.E.; Floyd, R.F. Introduction to Freshwater Fish Parasites. Fisheries & Aquatic Sciences. 2009. Available online: https://fisheries.tamu.edu/files/2013/09/Introduction-to-Freshwater-Fish-Parasites.pdf (accessed on 4 January 2023).
- Fernández-Alba, I.M.A.M. Environmental and human health risk assessment of organic micro-pollutants occurring in a Spanish marine fish farm. Environ. Pollut. 2010, 158, 1809–1816. [Google Scholar]
- Olivera, M.E.; Manzo, R.H.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; Dressman, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Ciprofloxacin hydrochloride. J. Pharm. Sci. 2011, 100, 22–33. [Google Scholar] [CrossRef]
- Richardson, M.L.; Bowron, J.M. The fate of pharmaceutical chemicals in the aquatic environment. J. Pharm. Pharmacol. 1985, 37, 1–12. [Google Scholar] [CrossRef]
- Jousimies-Somer, H.; Asikainen, S.; Suomala, P.; Summanen, P. Activity of metronidazole and its hydroxy metabolite against clinical isolates of actinobacillus actinomycetemcomitans. Mol. Oral Microbiol. 1988, 3, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Marta, W.; Joanna, M.; Anna, B.; Magda, C.; Piotr, S. Determination of metronidazole residues in water, sediment and fish tissue samples. Chemosphere 2015, 119, S28–S34. [Google Scholar]
- Perkins, E.J. Aquatic Toxicology and Hazard Evaluation. In Proceedings of the First Annual Symposium on Aquatic Toxicology, Memphis, TN, USA, 25–26 October 1976; Mayer, F.L., Hamelink, J.L., Eds.; ASTM Special Technical Publication 634; ASTM: West Conshohocken, PA, USA, 1979; Volume 10, pp. 31–32. [Google Scholar]
- Marking, L.L. Method for Assessing Additive Toxicity of Chemical Mixtures. In Aquatic Toxicology and Hazard Evaluation; ASTM Special Technical Publications; ASTM: West Conshohocken, PA, USA, 1997. [Google Scholar]
- Mao, Y.F. Toxicological Effects and Aggregation Kinetics of Microplastics on Chlorella Pyrenoidosa. Ph.D. Thesis, Chongqing University, Chongqing, China, 2019. [Google Scholar]
- Gu, S.; Zheng, H.; Xu, Q.; Sun, C.; Shi, M.; Wang, Z.; Li, F. Comparative toxicity of the plasticizer dibutyl phthalate to two freshwater algae. Aquat. Toxicol. 2017, 191, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, W.; Sheng, G. Enantioselective Degradation and Ecotoxicity of the Chiral Herbicide Diclofop in Three Freshwater Alga Cultures. J. Agric. Food Chem. 2008, 56, 2139–2146. [Google Scholar] [CrossRef]
- Wang, W.; Sun, B. VraCP regulates cell wall metabolism and antibiotic resistance in vancomycin-intermediate Staphylococcus aureus strain Mu50. J. Antimicrob. Chemother. 2021, 76, 1712–1723. [Google Scholar] [CrossRef]
- Yin, J.H.; Cheng, D.; Zhu, Y.L.; Liang, Y.Q.; Yu, Z.L. Development of a whole-cell biosensor for detection of antibiotics targeting bacterial cell envelope in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2022, 106, 789–798. [Google Scholar] [CrossRef]
- Xiang, R.; Shi, J.Q.; Yu, Y.; Zhang, H.B.; Dong, C.C.; Yang, Y.J.; Wu, Z.X. The Effect of Bisphenol A on Growth, Morphology, Lipid Peroxidation, Antioxidant Enzyme Activity, and PS II in Cylindrospermopsis raciborskii and Scenedesmus quadricauda. Arch. Environ. Contam. Toxicol. 2018, 74, 515–526. [Google Scholar] [CrossRef]
- Prasun, P.; Shuvrodeb, R.; Sampad, S.; Shouvik, M.; Saheli, P.; Nitai, D.; Arunava, G. Damage of lipopolysaccharides in outer cell membrane and production of ROS-mediated stress within bacteria makes nano zinc oxide a bactericidal agent. Appl. Nanosci. 2015, 5, 857–866. [Google Scholar]
- Zhou, X.D. Effects of Tetracycline and Sulfanilamide Antibiotics on Microcystis aeruginosa and Cyanobacteria microtunica. Master’s Thesis, Northwest A & F University, Xianyang, China, 2017. [Google Scholar]
- Jin, T.; He, Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodbome pathogens. J. Nanoparticle Res. 2011, 12, 6877–6885. [Google Scholar] [CrossRef]
- Zeng, W.M.; Zhang, S.S.; Xia, M.C.; Wu, X.L.; Qiu, G.Z.; Shen, L. Insights into the production of extracellular polymeric substances of Cupriavidus pauculus 1490 under the stimulation of heavy metal ions. RSC Adv. 2020, 34, 20385–20394. [Google Scholar] [CrossRef]
- Zhao, M.M. Effects of Humic Acids on the Physiology of Microcystis and its Competitive Relationship with Spirocera. Master’s Thesis, Northwest A & F University, Xianyang, China, 2019. [Google Scholar]
- Duncan, J.B.; David, C.S. A review of soluble microbial products (SMP) in wastewater treatment systems. Water Res. 1999, 33, 3063–3082. [Google Scholar]
- Matthew, J.D. Stress Resistance and Disease Resistance in Seaweeds: The Role of Reactive Oxygen Metabolism. Adv. Bot. Res. 2005, 43, 175–207. [Google Scholar]
- Kreslavski, V.D.; Carpentier, R.; Klimov, V.V.; Murata, N.; Allakhverdiev, S.I. Molecular mechanisms of stress resistance of the photosynthetic apparatus. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2007, 1, 185–205. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Zink, J.I.; Nel, A.E. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano 2008, 2, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Pakrashi, U.; Dalai, S.; Prathna, T.C.; Trivedi, S.; Myneni, R.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of aluminium oxide nanoparticles towards fresh water algal isolate at low exposure concentration. Aquat. Toxicol. 2013, 132–133, 34–45. [Google Scholar] [CrossRef]
- Morton, L.H.G.; Greenway, D.L.A.; Gaylarde, C.C.; Surman, S.B. Consideration of some implications of the resistance of biofilms to biocides. Int. Biodeterior. Biodegrad. 1988, 41, 247–259. [Google Scholar] [CrossRef]


| Toxicity Ratio (TC:MTZ) | Concentration Combination (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| Toxic | 0 T | 0.04 T | 0.4 T | 1 T | 2 T | 3 T | |
| 1:1 | TC | 0 | 0.1 | 1 | 2.5 | 5 | 7.5 |
| MTZ | 0 | 0.52 | 5.2 | 13 | 26 | 39 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Fan, Z.; Tang, P.; Wu, M.; Xiao, H.; Zeng, Z. Toxicity of Tetracycline and Metronidazole in Chlorella pyrenoidosa. Int. J. Environ. Res. Public Health 2023, 20, 3623. https://doi.org/10.3390/ijerph20043623
Li J, Wang Y, Fan Z, Tang P, Wu M, Xiao H, Zeng Z. Toxicity of Tetracycline and Metronidazole in Chlorella pyrenoidosa. International Journal of Environmental Research and Public Health. 2023; 20(4):3623. https://doi.org/10.3390/ijerph20043623
Chicago/Turabian StyleLi, Junrong, Yingjun Wang, Ziqi Fan, Panyang Tang, Mengting Wu, Hong Xiao, and Zhenxing Zeng. 2023. "Toxicity of Tetracycline and Metronidazole in Chlorella pyrenoidosa" International Journal of Environmental Research and Public Health 20, no. 4: 3623. https://doi.org/10.3390/ijerph20043623
APA StyleLi, J., Wang, Y., Fan, Z., Tang, P., Wu, M., Xiao, H., & Zeng, Z. (2023). Toxicity of Tetracycline and Metronidazole in Chlorella pyrenoidosa. International Journal of Environmental Research and Public Health, 20(4), 3623. https://doi.org/10.3390/ijerph20043623







