Seroprevalence of Anti-SARS-CoV-2 Antibodies Following the Omicron BA.1 Wave
Abstract
1. Introduction
2. Participants and Methods
2.1. Study Participants
2.2. Serologic Testing
2.3. Data Management and Statistics
2.3.1. Sources of Data
2.3.2. Definitions
- Not infected with SARS-CoV-2 before serum sampling: participants without notified infection (no record in the National COVID-19 Database before serum sample collection or in the 14 days afterwards).
- Non-vaccinated: participants who did not have any anti-SARS-CoV-2 vaccine dose recorded before the date of serum sample collection, or had only one dose of a two-dose schedule vaccine (the mRNA vaccines Comirnaty [Pfizer/BioNTech] or Spikevax [Moderna], vector vaccine Vaxzevria [Astra-Zeneca] or protein vaccine Nuvaxovid (Novavax]), or one dose of Jcovden/Janssen (Johnson & Johnson, New Brunswick, NJ, USA) in the 14-day period before the serum sample was taken.
- Partially vaccinated: participants who had received only one dose of a two-dose schedule vaccine (mRNA vaccines Comirnaty [Pfizer/BioNTech] or Spikevax [Moderna], vector vaccine Vaxzevria [Astra-Zeneca] or protein vaccine Nuvaxovid [Novavax]) at least 14 days before serum collection.
- Completely vaccinated: participants who received one dose of the Jcovden/Janssen (Johnson & Johnson) vaccine or both doses of a two-dose schedule vaccine (mRNA vaccines Comirnaty [Pfizer/BioNTech] or Spikevax [Moderna], vector vaccine Vaxzevria [Astra-Zeneca] or protein vaccine Nuvaxovid [Novavax]) at least 14 days before serum collection.
- Vaccinated with additional dose: completely vaccinated participants who received at least one additional dose of an mRNA-based vaccine (Comirnaty [Pfizer/BioNTech] or Spikevax [Moderna]) before serum sampling.
- –
- persons who were not infected with SARS-CoV-2 and had not been vaccinated before sampling (never infected-never vaccinated);
- –
- persons with at least one notified infection at least 14 days before serum sample collection AND never vaccinated (infected-never vaccinated);
- –
- persons not infected with SARS-CoV-2 before sampling AND partially or completely vaccinated, or who had received at least one additional dose (never infected-vaccinated);
- –
- persons with at least one notified infection at least 14 days before serum sample collection AND partially or completely vaccinated, or who had received at least one additional dose (infected-vaccinated).
2.3.3. Statistical Analysis
3. Results
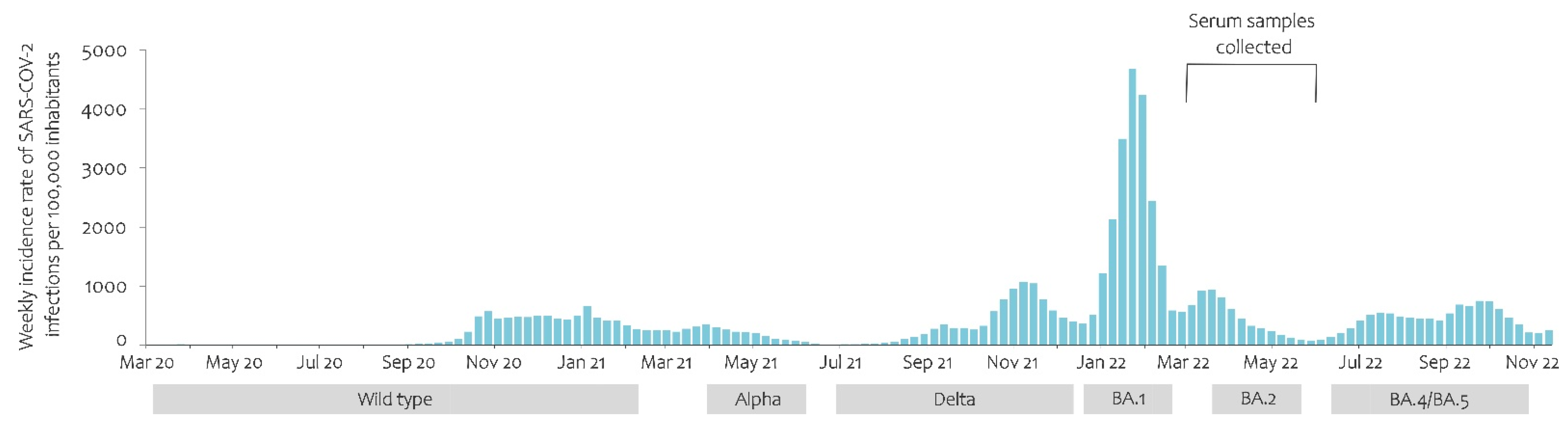
3.1. COVID-19 Pandemic Development and Predominance of the Variants
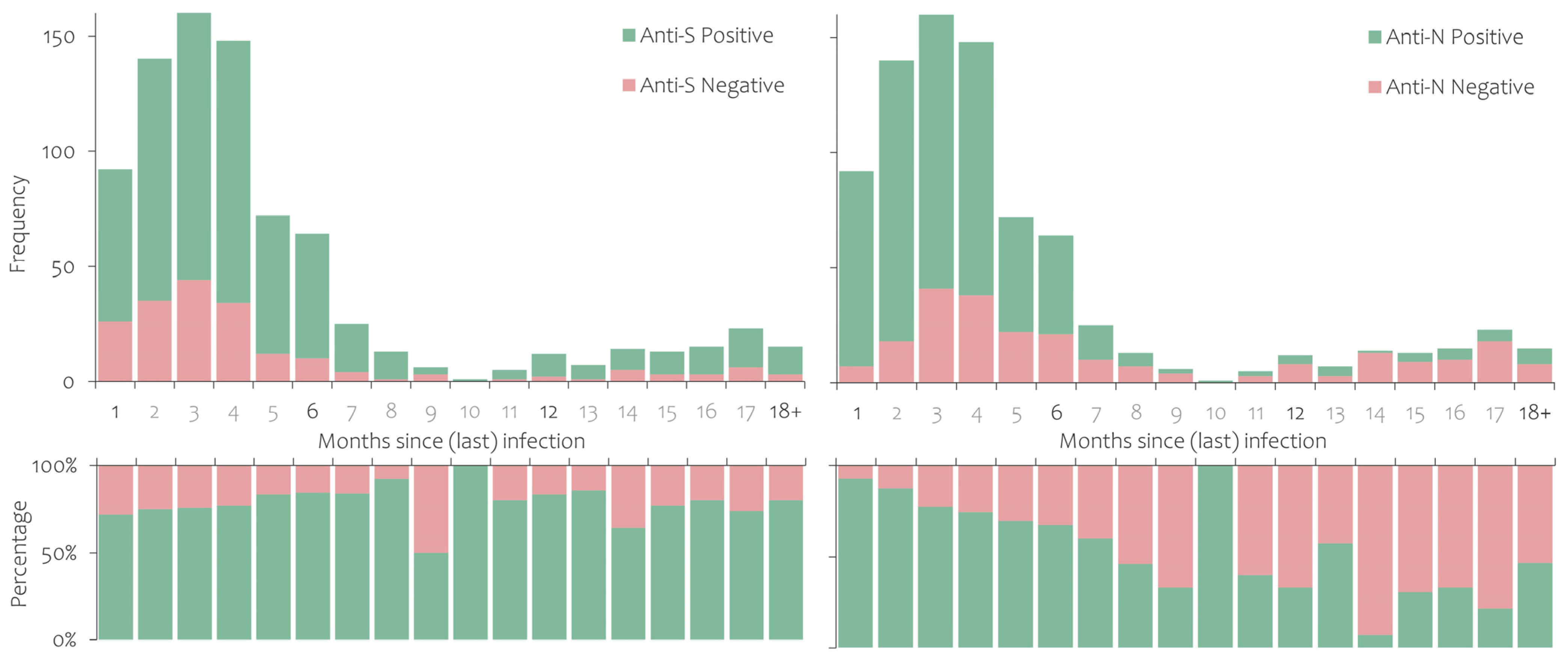
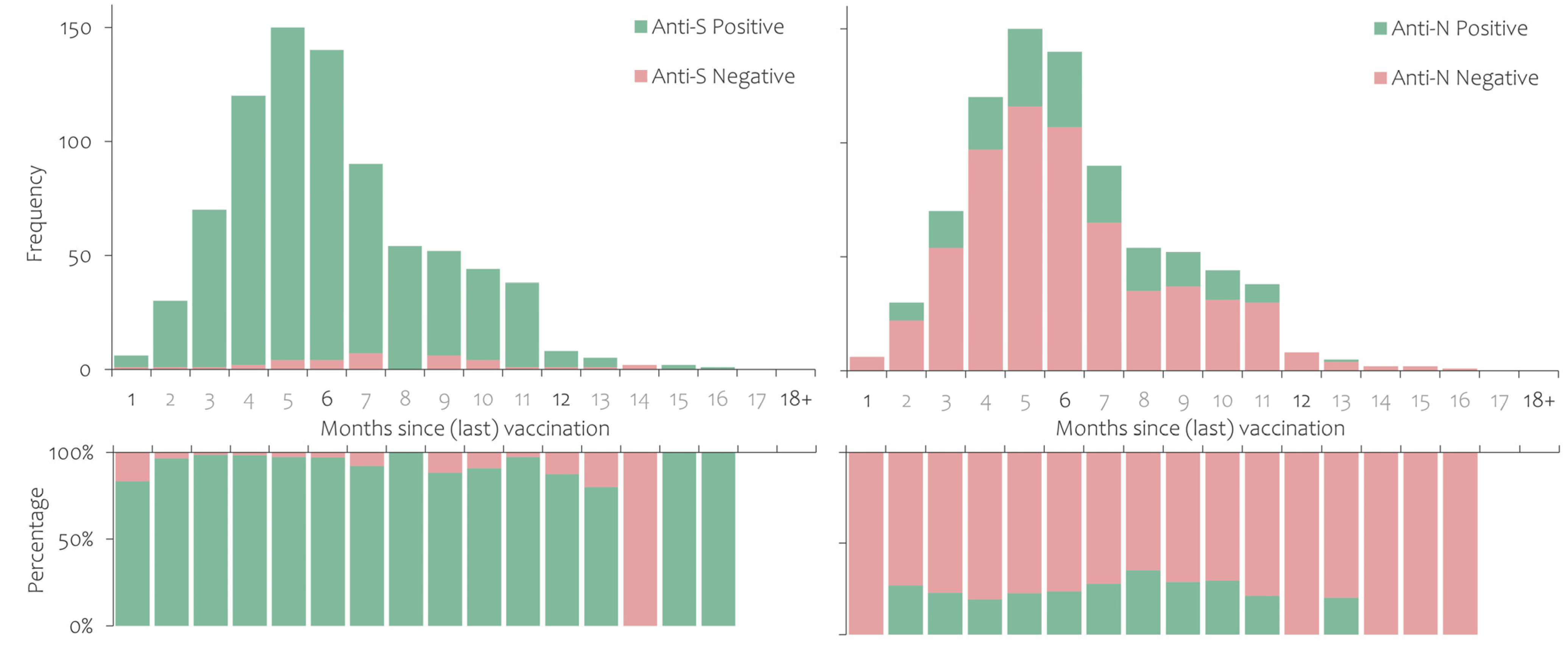
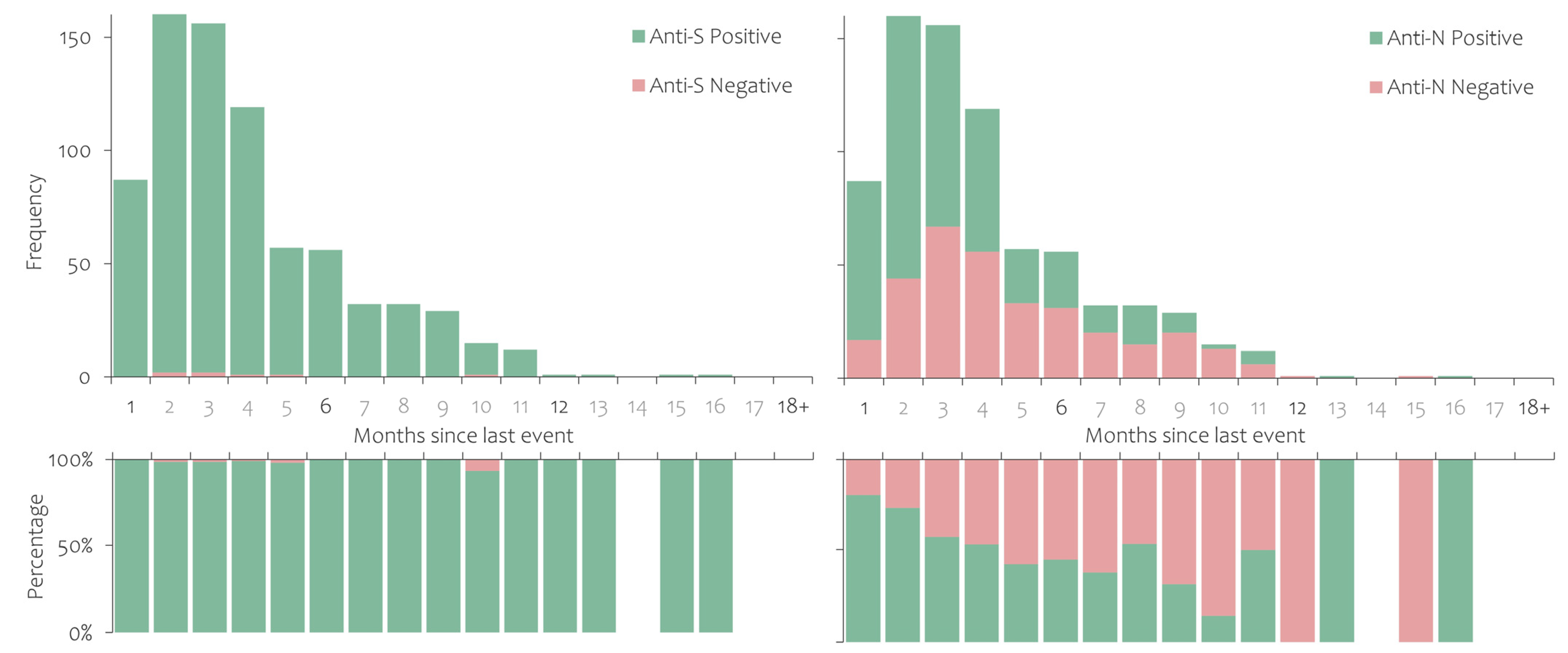
3.2. Prevalence of Antibodies against SARS-CoV-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef] [PubMed]
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the Features and Duration of Antibody Responses to SARS-CoV-2 Infection Associated with Disease Severity and Outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 Immunity: Review and Applications to Phase 3 Vaccine Candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Wedderburn, C.J.; Garcia, P.J.; Boeras, D.; Fongwen, N.; Nkengasong, J.; Sall, A.; Tanuri, A.; Heymann, D.L. Serology Testing in the COVID-19 Pandemic Response. Lancet Infect. Dis. 2020, 20, e245–e249. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin-Bussières, G.; Laumaea, A.; Anand, S.P.; Prévost, J.; Gasser, R.; Goyette, G.; Medjahed, H.; Perreault, J.; Tremblay, T.; Lewin, A.; et al. Decline of Humoral Responses against SARS-CoV-2 Spike in Convalescent Individuals. mBio 2020, 11, e02590-20. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, L.L.; Ransegnola, B.P.; Jin, D.K.; Muecksch, F.; Weisblum, Y.; Bao, W.; George, P.J.; Rodriguez, M.; Tricoche, N.; Schmidt, F.; et al. Serological Assays Estimate Highly Variable SARS-CoV-2 Neutralizing Antibody Activity in Recovered COVID-19 Patients. J. Clin. Microbiol. 2020, 58, e02005-20. [Google Scholar] [CrossRef]
- Muecksch, F.; Wise, H.; Batchelor, B.; Squires, M.; Semple, E.; Richardson, C.; McGuire, J.; Clearly, S.; Furrie, E.; Greig, N.; et al. Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J. Infect. Dis. 2021, 223, 389–398. [Google Scholar] [CrossRef]
- Legros, V.; Denolly, S.; Vogrig, M.; Boson, B.; Siret, E.; Rigaill, J.; Pillet, S.; Grattard, F.; Gonzalo, S.; Verhoeven, P.; et al. A Longitudinal Study of SARS-CoV-2-Infected Patients Reveals a High Correlation between Neutralizing Antibodies and COVID-19 Severity. Cell. Mol. Immunol. 2021, 18, 318–327. [Google Scholar] [CrossRef]
- Johannesen, C.K.; Rezahosseini, O.; Gybel-Brask, M.; Kristensen, J.H.; Hasselbalch, R.B.; Pries-Heje, M.M.; Nielsen, P.B.; Knudsen, A.D.; Fogh, K.; Norsk, J.B.; et al. Risk Factors for Being Seronegative Following SARS-CoV-2 Infection in a Large Cohort of Health Care Workers in Denmark. Microbiol. Spectr. 2021, 9, e00904-21. [Google Scholar] [CrossRef]
- Thiruvengadam, R.; Chattopadhyay, S.; Mehdi, F.; Desiraju, B.K.; Chaudhuri, S.; Singh, S.; Bhartia, V.; Kshetrapal, P.; Natchu, U.C.M.; Wadhwa, N.; et al. Longitudinal Serology of SARS-CoV-2-Infected Individuals in India: A Prospective Cohort Study. Am. J. Trop. Med. Hyg. 2021, 105, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Holmer, H.K.; Mackey, K.; Fiordalisi, C.V.; Helfand, M. Major Update 2: Antibody Response and Risk for Reinfection After SARS-CoV-2 Infection—Final Update of a Living, Rapid Review. Ann. Intern. Med. 2022, 176, 85–91. [Google Scholar] [CrossRef]
- Kirchner, T.; Heinrich, S.; Bonifacius, A.; Engel, B.; Ruhl, L.; Pink, I.; Thomas, N.; Martens, J.; Hoeper, M.M.; Blasczyk, R.; et al. Reduced Humoral but Stable Cellular SARS-CoV-2-Specific Immunity in Liver Transplant Recipients in the First Year after COVID-19. PLoS ONE 2022, 17, e0276929. [Google Scholar] [CrossRef] [PubMed]
- Cantarelli, C.; Angeletti, A.; Perin, L.; Russo, L.S.; Sabiu, G.; Podestà, M.A.; Cravedi, P. Immune Responses to SARS-CoV-2 in Dialysis and Kidney Transplantation. Clin. Kidney J. 2022, 15, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.-J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of Antibody Responses up to 13 Months after SARS-CoV-2 Infection and Risk of Reinfection. eBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef] [PubMed]
- Kučinskaitė-Kodzė, I.; Simanavičius, M.; Šimaitis, A.; Žvirblienė, A. Persistence of SARS-CoV-2-Specific Antibodies for 13 Months after Infection. Viruses 2021, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Beavis, K.G.; Matushek, S.M.; Abeleda, A.P.F.; Bethel, C.; Hunt, C.; Gillen, S.; Moran, A.; Tesic, V. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for Detection of IgA and IgG Antibodies. J. Clin. Virol. 2020, 129, 104468. [Google Scholar] [CrossRef]
- Lassaunière, R.; Frische, A.; Harboe, Z.B.; Nielsen, A.C.Y.; Fomsgaard, A.; Krogfelt, K.A.; Jørgensen, C.S. Evaluation of Nine Commercial SARS-CoV-2 Immunoassays. MedRxiv 2020. [Google Scholar] [CrossRef]
- Ripperger, T.J.; Uhrlaub, J.L.; Watanabe, M.; Wong, R.; Castaneda, Y.; Pizzato, H.A.; Thompson, M.R.; Bradshaw, C.; Weinkauf, C.C.; Bime, C.; et al. Orthogonal SARS-CoV-2 Serological Assays Enable Surveillance of Low-Prevalence Communities and Reveal Durable Humoral Immunity. Immunity 2020, 53, 925–933.e4. [Google Scholar] [CrossRef]
- Van Elslande, J.; Oyaert, M.; Lorent, N.; Vande Weygaerde, Y.; Van Pottelbergh, G.; Godderis, L.; Van Ranst, M.; André, E.; Padalko, E.; Lagrou, K.; et al. Lower Persistence of Anti-Nucleocapsid Compared to Anti-Spike Antibodies up to One Year after SARS-CoV-2 Infection. Diagn. Microbiol. Infect. Dis. 2022, 103, 115659. [Google Scholar] [CrossRef]
- World Health Organization. Population-Based Age-Stratified Seroepidemiological Investigation Protocol for COVID-19 Virus Infection, 17 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Bobrovitz, N.; Arora, R.K.; Cao, C.; Boucher, E.; Liu, M.; Donnici, C.; Yanes-Lane, M.; Whelan, M.; Perlman-Arrow, S.; Chen, J.; et al. Global Seroprevalence of SARS-CoV-2 Antibodies: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0252617. [Google Scholar] [CrossRef]
- Rostami, A.; Sepidarkish, M.; Fazlzadeh, A.; Mokdad, A.H.; Sattarnezhad, A.; Esfandyari, S.; Riahi, S.M.; Mollalo, A.; Dooki, M.E.; Bayani, M.; et al. Update on SARS-CoV-2 Seroprevalence: Regional and Worldwide. Clin. Microbiol. Infect. 2021, 27, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Bergeri, I.; Whelan, M.G.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global SARS-CoV-2 Seroprevalence from January 2020 to April 2022: A Systematic Review and Meta-Analysis of Standardized Population-Based Studies. PLoS Med. 2022, 19, e1004107. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H.C.; Ware, H.; Whelan, M.; Subissi, L.; Li, Z.; Ma, X.; Nardone, A.; Valenciano, M.; Cheng, B.; Noel, K.; et al. SARS-CoV-2 Infection in Africa: A Systematic Review and Meta-Analysis of Standardised Seroprevalence Studies, from January 2020 to December 2021. BMJ Glob. Health 2022, 7, e008793. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Moradi, Y.; Moradkhani, A.; Aghaei, A. SARS-CoV-2 Seroprevalence around the World: An Updated Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2022, 27, 81. [Google Scholar] [CrossRef] [PubMed]
- Anzinger, J.J.; Cameron-McDermott, S.M.; Phillips, Y.Z.R.; Mendoza, L.; Anderson, M.; Cloherty, G.; Strachan-Johnson, S.; Lindo, J.F.; Figueroa, J.P. Prevalence of SARS-CoV-2 Antibodies after the Omicron Surge, Kingston, Jamaica, 2022. J. Clin. Virol. Plus 2022, 2, 100124. [Google Scholar] [CrossRef]
- Zaballa, M.-E.; Perez-Saez, J.; Mestral, C.d.; Pullen, N.; Lamour, J.; Turelli, P.; Raclot, C.; Baysson, H.; Pennacchio, F.; Villers, J.; et al. Seroprevalence of Anti-SARS-CoV-2 Antibodies and Cross-Variant Neutralization Capacity after the Omicron BA.2 Wave in Geneva, Switzerland: A Population-Based Study. Lancet Reg. Health Eur. 2022, 24, 100547. [Google Scholar] [CrossRef]
- O’Brien, S.F.; Caffrey, N.; Yi, Q.-L.; Pambrun, C.; Drews, S.J. SARS-CoV-2 Seroprevalence among Canadian Blood Donors: The Advance of Omicron. Viruses 2022, 14, 2336. [Google Scholar] [CrossRef]
- Wiegand, R.E.; Deng, Y.; Deng, X.; Lee, A.; Meyer, W.A.; Letovsky, S.; Charles, M.D.; Gundlapalli, A.V.; MacNeil, A.; Hall, A.J.; et al. Estimated SARS-CoV-2 Antibody Seroprevalence Trends and Relationship to Reported Case Prevalence from a Repeated, Cross-Sectional Study in the 50 States and the District of Columbia, United States—October 25, 2020–February 26, 2022. Lancet Reg. Health Am. 2023, 18, 100403. [Google Scholar] [CrossRef]
- Castilla, J.; Lecea, Ó.; Salas, C.M.; Quílez, D.; Miqueleiz, A.; Trobajo-Sanmartín, C.; Navascués, A.; Martínez-Baz, I.; Casado, I.; Burgui, C.; et al. Seroprevalence of Antibodies against SARS-CoV-2 and Risk of COVID-19 in Navarre, Spain, May to July 2022. Eurosurveillance 2022, 27, 2200619. [Google Scholar] [CrossRef] [PubMed]
- Amati, R.; Frei, A.; Kaufmann, M.; Sabatini, S.; Pellaton, C.; Fehr, J.; Albanese, E.; Puhan, M.A.; Group, on behalf of the C.I.R. Functional Immunity against SARS-CoV-2 in the General Population after a Booster Campaign and the Delta and Omicron Waves, Switzerland, March 2022. Eurosurveillance 2022, 27, 2200561. [Google Scholar] [CrossRef] [PubMed]
- Ahava, M.J.; Jarva, H.; Jääskeläinen, A.J.; Lappalainen, M.; Vapalahti, O.; Kurkela, S. Rapid Increase in SARS-CoV-2 Seroprevalence during the Emergence of Omicron Variant, Finland. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.E.; Jones, J.M.; Deng, Y.; Nycz, E.; Lee, A.; Iachan, R.; Gundlapalli, A.V.; Hall, A.J.; MacNeil, A. Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies—United States, September 2021–February 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 606. [Google Scholar] [CrossRef]
- Erikstrup, C.; Laksafoss, A.D.; Gladov, J.; Kaspersen, K.A.; Mikkelsen, S.; Hindhede, L.; Boldsen, J.K.; Jørgensen, S.W.; Ethelberg, S.; Holm, D.K.; et al. Seroprevalence and Infection Fatality Rate of the SARS-CoV-2 Omicron Variant in Denmark: A Nationwide Serosurveillance Study. Lancet Reg. Health—Eur. 2022, 21, 100479. [Google Scholar] [CrossRef]
- Ott, R.; Achenbach, P.; Ewald, D.A.; Friedl, N.; Gemulla, G.; Hubmann, M.; Kordonouri, O.; Loff, A.; Marquardt, E.; Sifft, P. SARS-CoV-2 Seroprevalence in Preschool and School-Age Children-Population Screening Findings From January 2020 to June 2022. Dtsch. Arzteblatt Int. 2022, 119, 765–770. [Google Scholar] [CrossRef]
- Oeser, C.; Whitaker, H.; Borrow, R.; Linley, E.; Tonge, S.; Rowe, C.; Otter, A.; Warrener, L.; Campbell, C.N.J.; Ladhani, S.; et al. Following the Omicron Wave, the Majority of Children in England Have Evidence of Previous COVID Infection. J. Infect. 2022. [Google Scholar] [CrossRef]
- Poon, R.W.-S.; Chan, B.P.-C.; Chan, W.-M.; Fong, C.H.-Y.; Zhang, X.; Lu, L.; Chen, L.-L.; Lam, J.-Y.; Cheng, V.C.-C.; Wong, S.S.Y.; et al. SARS-CoV-2 IgG Seropositivity after the Severe Omicron Wave of COVID-19 in Hong Kong. Emerg. Microbes Infect. 2022, 11, 2116–2119. [Google Scholar] [CrossRef]
- Bingham, J.; Cable, R.; Coleman, C.; Glatt, T.N.; Grebe, E.; Mhlanga, L.; Nyano, C.; Pieterson, N.; Swanevelder, R.; Swarts, A.; et al. Estimates of Prevalence of Anti-SARS-CoV-2 Antibodies among Blood Donors in South Africa in March 2022. Res. Sq 2022, rs.3.rs-1687679. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Kaweski, S.E.; Irvine, M.A.; Kim, S.; Chuang, E.S.Y.; Sabaiduc, S.; Fraser, M.; Reyes, R.C.; Henry, B.; Levett, P.N.; et al. Serial Cross-Sectional Estimation of Vaccine-and Infection-Induced SARS-CoV-2 Seroprevalence in British Columbia, Canada. CMAJ 2022, 194, E1599–E1609. [Google Scholar] [CrossRef]
- Doerre, A.; Doblhammer, G. The Influence of Gender on COVID-19 Infections and Mortality in Germany: Insights from Age- and Gender-Specific Modeling of Contact Rates, Infections, and Deaths in the Early Phase of the Pandemic. PLoS ONE 2022, 17, e0268119. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.; Giménez, E.; Albert, E.; Zulaica, J.; Álvarez-Rodríguez, B.; Torres, I.; Rusu, L.; Burgos, J.S.; Peiró, S.; Vanaclocha, H.; et al. Cumulative Incidence of SARS-CoV-2 Infection in the General Population of the Valencian Community (Spain) after the Surge of the Omicron BA.1 Variant. J. Med. Virol. 2023, 95, e28284. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Kong, D.; Shelley, M. Risk Perception, Preventive Behavior, and Medical Care Avoidance among American Older Adults During the COVID-19 Pandemic. J. Aging Health 2021, 33, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Velikonja, N.K.; Erjavec, K.; Verdenik, I.; Hussein, M.; Velikonja, V.G. Association between Preventive Behaviour and Anxiety at the Start of the COVID-19 Pandemic in Slovenia. Slov. J. Public Health 2021, 60, 17–24. [Google Scholar] [CrossRef]
| Participants | Anti-S Positive | Anti-N Positive | |||||
|---|---|---|---|---|---|---|---|
| n | n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Male | 672 | 574 (85.4) | ref | ref | 307 (45.7) | ref | Ref |
| Female | 2227 | 1865 (83.7) | 0.88 (0.69–1.12) | 1.02 (0.75–1.39) | 1102 (49.5) | 1.16 (0.98–1.38) | 1.10 (0.90–1.35) |
| 0–17 | 299 | 233 (77.9) | ref | ref | 183 (61.2) | ref | Ref |
| 18–29 | 748 | 609 (81.4) | 1.24 (0.89–1.73) | 0.48 (0.32–0.71) | 403 (53.9) | 0.74 (0.56–0.97) | 0.60 (0.44–0.82) |
| 30–39 | 757 | 617 (81.5) | 1.25 (0.90–1.74) | 0.40 (0.27–0.60) | 385 (50.9) | 0.66 (0.50–0.86) | 0.52 (0.38–0.71) |
| 40–49 | 306 | 264 (86.3) | 1.78 (1.16–2.72) | 0.35 (0.21–0.58) | 141 (46.1) | 0.54 (0.39–0.75) | 0.48 (0.33–0.70) |
| 50–59 | 327 | 294 (89.9) | 2.52 (1.61–3.97) | 0.46 (0.27–0.78) | 147 (45.0) | 0.52 (0.38–0.71) | 0.60 (0.41–0.86) |
| 60–69 | 251 | 228 (90.8) | 2.81 (1.69–4.67) | 0.47 (0.26–0.85) | 91 (36.3) | 0.36 (0.25–0.51) | 0.53 (0.36–0.79) |
| 70+ | 211 | 194 (91.9) | 3.23 (1.84–5.69) | 0.33 (0.16–0.67) | 59 (28.0) | 0.25 (0.17–0.36) | 0.48 (0.31–0.74) |
| Never infected | 1291 | 1031 (79.9) | ref | ref | 365 (28.3) | ref | ref |
| Infected ≥ 1 | 1608 | 1408 (87.6) | 1.78 (1.45–2.17) | 3.71 (2.92–4.72) | 1044 (64.9) | 4.70 (4.01–5.50) | 4.45 (3.75–5.28) |
| Never vaccinated | 1324 | 906 (68.4) | ref | ref | 775 (58.5) | ref | ref |
| Partially vaccinated | 71 | 70 (98.6) | 32.30 (4.47–233.28) | 28.27 (3.89–205.57) | 34 (47.9) | 0.65 (0.40–1.05) | 0.48 (0.29–0.78) |
| Completely vaccinated | 858 | 824 (96.0) | 11.18 (7.79–16.06) | 15.91 (10.88–23.26) | 410 (47.8) | 0.65 (0.55–0.77) | 0.73 (0.61–0.89) |
| Additional dose | 646 | 639 (98.9) | 42.12 (19.82–89.50) | 91.69 (41.40–203.08) | 190 (29.4) | 0.30 (0.24–0.36) | 0.53 (0.41–0.67) |
| Anti-S | Anti-N | |||
|---|---|---|---|---|
| Negative (%) | Positive (%) | Negative (%) | Positive (%) | |
| Never infected and never vaccinated | 225 (47.0) | 254 (53.0) | 309 (64.5) | 170 (35.5) |
| Infected once, never vaccinated | 188 (27.4) | 499 (72.6) | 230 (33.5) | 457 (66.5) |
| Infected ≥ 2, never vaccinated | 5 (3.2) | 153 (96.8) | 10 (6.3) | 148 (93.7) |
| Total for infected, never vaccinated | 193 (22.8) | 652 (77.2) | 240 (28.4) | 605 (71.6) |
| Never infected, partially vaccinated | 1 (12.5) | 7 (87.5) | 7 (87.5) | 1 (12.5) |
| Never infected, completely vaccinated | 27 (7.8) | 317 (92.2) | 237 (68.9) | 107 (31.1) |
| Never infected, additional dose | 7 (1.5) | 453 (98.5) | 373 (81.1) | 87 (18.9) |
| Total for never infected, vaccinated | 35 (4.3) | 777 (95.7) | 617 (76.0) | 195 (24.0) |
| Infected ≥1, partially vaccinated | 0 (0.0) | 63 (100.0) | 30 (47.6) | 33 (52.4) |
| Infected ≥1, completely vaccinated | 7 (1.4) | 507 (98.6) | 211 (41.1) | 303 (58.9) |
| Infected ≥1, additional dose | 0 (0.0) | 186 (100.0) | 83 (44.6) | 103 (55.4) |
| Total for infected, vaccinated | 7 (0.9) | 756 (99.1) | 324 (42.5) | 439 (57.5) |
| Anti-S Negative and Anti-N Negative | Anti-S Positive and Anti-N Negative | Anti-S Negative and Anti-N Positive | Anti-S Positive and Anti-N Positive | ||
|---|---|---|---|---|---|
| n | 349 | 1141 | 111 | 1298 | |
| Age (years) | median (IQR) | 31.0 (25–40) | 39 (29–59) | 30 (24–39) | 32 (26–46) |
| Gender (male) | n (%) | 73 (20.9) | 292 (25.6) | 25 (22.5) | 282 (21.7) |
| Infected at least once | n (%) | 116 (33.2) | 448 (39.3) | 84 (75.7) | 960 (74.0) |
| Never vaccinated | n (%) | 311 (89.1) | 238 (20.9) | 107 (96.4) | 668 (51.5) |
| mRNA vaccine (1, 2, or 3 doses) | n (%) | 22 (6.3) | 711 (62.3) | 4 (3.6) | 481 (37.1) |
| Vector-based vaccine (1 or 2 doses) | n (%) | 14 (4.0) | 70 (6.1) | 0 (0.0) | 80 (6.2) |
| Combination of different vaccines (2 or 3 doses) | n (%) | 2 (0.6) | 122 (10.7) | 0 (0.0) | 69 (5.3) |
| Participants | Confirmed Cases | Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| n | n (%) | |||
| Anti-S neg | 460 | 109 (23.7) | Ref. | Ref. |
| Anti-S pos | 2439 | 336 (13.8) | 0.51 (0.40–0.66) | 0.66 (0.51–0.85) |
| Anti-N neg | 1490 | 327 (21.9) | Ref. | Ref. |
| Anti-N pos | 1409 | 118 (8.4) | 0.33 (0.26–0.41) | 0.34 (0.27–0.43) |
| Male | 672 | 92 (13.7) | Ref. | Ref. |
| Female | 2227 | 353 (15.9) | 1.19 (0.93–1.52) | 1.21 (0.93–1.59) |
| 0–17 | 299 | 33 (11.0) | Ref. | Ref. |
| 18–29 | 748 | 112 (15.0) | 1.42 (0.94–2.15) | 1.25 (0.80–1.94) |
| 30–39 | 757 | 114 (15.1) | 1.43 (0.95–2.16) | 1.22 (0.78–1.89) |
| 40–49 | 306 | 60 (19.6) | 1.97 (1.24–3.11) | 1.73 (1.08–2.79) |
| 50–59 | 327 | 63 (19.3) | 1.92 (1.22–3.03) | 1.71 (1.07–2.75) |
| 60–69 | 251 | 36 (14.3) | 1.35 (0.81–2.24) | 1.11 (0.66–1.87) |
| 70+ | 211 | 27 (12.8) | 1.18 (0.69–2.03) | 0.89 (0.51–1.56) |
| Never infected | 1291 | 230 (17.8) | Ref. | Excl. |
| Infected ≥ 1 | 1608 | 215 (13.4) | 0.71 (0.58–0.87) | Excl. |
| Never vaccinated | 1324 | 203 (15.3) | Ref. | Excl. |
| Partially vaccinated | 71 | 15 (21.1) | 1.48 (0.82–2.67) | Excl. |
| Completely vaccinated | 858 | 113 (13.2) | 0.84 (0.65–1.07) | Excl. |
| Vaccinated with additional dose | 646 | 114 (17.6) | 1.18 (0.92–1.52) | Excl. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socan, M.; Prosenc, K.; Mrzel, M. Seroprevalence of Anti-SARS-CoV-2 Antibodies Following the Omicron BA.1 Wave. Int. J. Environ. Res. Public Health 2023, 20, 3665. https://doi.org/10.3390/ijerph20043665
Socan M, Prosenc K, Mrzel M. Seroprevalence of Anti-SARS-CoV-2 Antibodies Following the Omicron BA.1 Wave. International Journal of Environmental Research and Public Health. 2023; 20(4):3665. https://doi.org/10.3390/ijerph20043665
Chicago/Turabian StyleSocan, Maja, Katarina Prosenc, and Maja Mrzel. 2023. "Seroprevalence of Anti-SARS-CoV-2 Antibodies Following the Omicron BA.1 Wave" International Journal of Environmental Research and Public Health 20, no. 4: 3665. https://doi.org/10.3390/ijerph20043665
APA StyleSocan, M., Prosenc, K., & Mrzel, M. (2023). Seroprevalence of Anti-SARS-CoV-2 Antibodies Following the Omicron BA.1 Wave. International Journal of Environmental Research and Public Health, 20(4), 3665. https://doi.org/10.3390/ijerph20043665








