Abortion and Female Cancer Risks among Women Aged 20 to 45 Years: A 10-Year Longitudinal Population-Based Cohort Study in Taiwan
Abstract
1. Introduction
2. Methods
2.1. Ethical Aspects
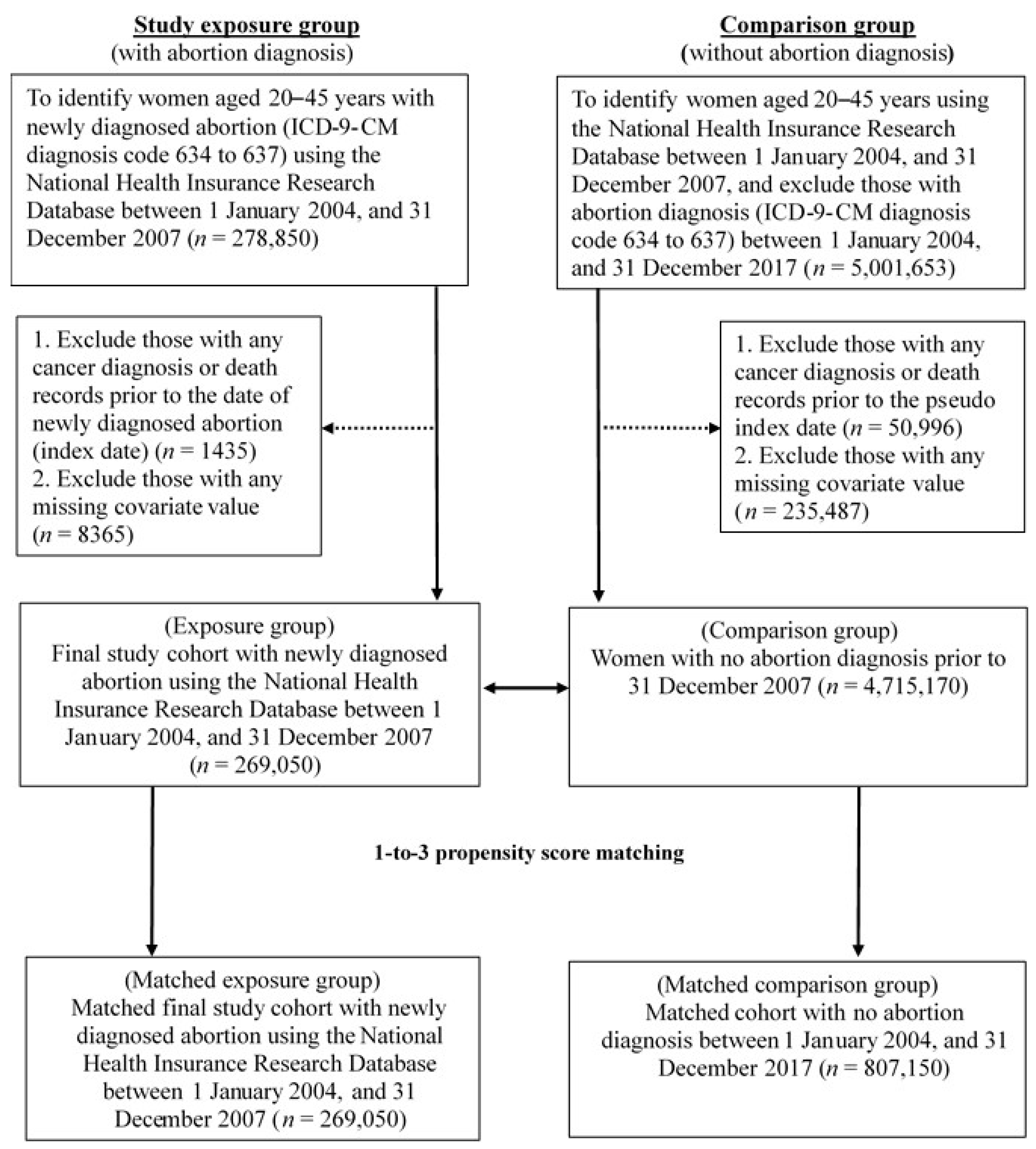
2.2. Study Population
2.3. Variable Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variables | ICD-9-CM | ICD-10-CM | Period |
|---|---|---|---|
| Dependent Variables | |||
| Breast cancer | 174 | C50 | From index date to the study end date |
| Cervical cancer | 180 | C53 | |
| Uterine cancer | 182 | C54 | |
| Ovarian cancer | 1830 | C56 | |
| Key Independent Variable | |||
| Abortion | 634, 635, 636, 637 | The first date of abortion was defined as the index date among women aged 20–45 years between 1 January 2004 and 31 December 2007 | |
| Covariates | |||
| Fertility | administrative billing codes: 81017C, 81018C, 81019C, 97004C, 97005D, 81024C, 81025C, 81026C, 97934C, 81034C, 57114C, 97001A, 97001K, 97002A, 97003B, 97931K, 97932A, 97933B, P3504C, 81004C, 81028C, 97009C, 81005C, 81029C, 97014C, 57115C, 81005C, 81011C, 97006K, 97007A, 97008B, 98001K, 98002A, 98003B, 98004C | From index date to the study end date | |
| Diabetes mellitus | 250 | Derived from one year before and after index date | |
| Polycystic ovarian syndrome | 2564 | ||
| Endometrial hyperplasia | 6213 | ||
| Endometriosis | 617 | ||
| Hormone-related drugs | ATC codes: G01AE, G01AX, G03AC03, G03CA03, G03CA04, G03CA07, G03CA53, G03CA57, G03DA, G03DA02, G03DA03, G03DA04, G03DB01, G03DB03, G03DB08, G03DC, G03DC01, G03DC02, G03DC04, G03EB, G03EA01, G03EA02, G03FA01, G03FA02, G03FA03, G03FA04, G03FA10, G03FA12, G03FB01, G03FB05, G03FB06, G03HB01, L02AA01, L02AB01, L02AB02 | ||
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Registry Annual Report, 2019 Taiwan. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=14913 (accessed on 15 September 2022).
- Cancer Risk Factor. Available online: https://www.cancer.org/cancer.html (accessed on 16 September 2022).
- Melbye, M.; Wohlfahrt, J.; Olsen, J.H.; Frisch, M.; Westergaard, T.; Helweg-Larsen, K.; Andersen, P.K. Induced abortion and the risk of breast cancer. N. Engl. J. Med. 1997, 336, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B.; Xue, F.; Colditz, G.A.; Willett, W.C. Induced and spontaneous abortion and incidence of breast cancer among young women: A prospective cohort study. Arch. Intern. Med. 2007, 167, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, K.A.; Gao, D.L.; Ray, R.M.; Rowland, M.R.; Nelson, Z.C.; Wernli, K.J.; Li, W.; Thomas, D.B. Induced abortions and the risk of all cancers combined and site-specific cancers in Shanghai. Cancer Causes Control 2006, 17, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Abortion and Breast Cancer Risk. Available online: https://www.cancer.org/healthy/cancer-causes/medical-treatments/abortion-and-breast-cancer-risk.html (accessed on 16 September 2022).
- Howe, H.L.; Senie, R.T.; Bzduch, H.; Herzfeld, P. Early abortion and breast cancer risk among women under age 40. Int. J. Epidemiol. 1989, 18, 300–304. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Li, W.; Song, F.; Dai, H.; Wang, J.; Gao, Y.; Liu, X.; Chen, C.; Yan, Y.; et al. A meta-analysis of the association between induced abortion and breast cancer risk among Chinese females. Cancer Causes Control 2014, 25, 227–236. [Google Scholar] [CrossRef]
- Yuan, X.; Yi, F.; Hou, C.; Lee, H.; Zhong, X.; Tao, P.; Li, H.; Xu, Z.; Li, J. Induced abortion, Birth control methods, and breast cancer risk: A case-control study in China. J. Epidemiol. 2019, 29, 173–179. [Google Scholar] [CrossRef]
- Husby, A.; Wohlfahrt, J.; Melbye, M. Pregnancy duration and endometrial cancer risk: Nationwide cohort study. BMJ 2019, 366, l4693. [Google Scholar] [CrossRef]
- Riman, T.; Dickman, P.W.; Nilsson, S.; Correia, N.; Nordlinder, H.; Magnusson, C.M.; Persson, I.R. Risk factors for invasive epithelial ovarian cancer: Results from a Swedish case-control study. Am. J. Epidemiol. 2002, 156, 363–373. [Google Scholar] [CrossRef]
- Zhang, M.; Lee, A.H.; Binns, C.W. Reproductive and dietary risk factors for epithelial ovarian cancer in China. Gynecol. Oncol. 2004, 92, 320–326. [Google Scholar] [CrossRef]
- Dick, M.L.; Siskind, V.; Purdie, D.M.; Green, A.C. Incomplete pregnancy and risk of ovarian cancer: Results from two Australian case-control studies and systematic review. Cancer Causes Control 2009, 20, 1571–1585. [Google Scholar] [CrossRef]
- Wang, P.D.; Lin, R.S. Induced abortion in Taiwan. J. R. Soc. Health 1995, 115, 100–108. [Google Scholar] [CrossRef]
- Austin, P.C. The performance of different propensity-score methods for estimating relative risks. J. Clin. Epidemiol. 2008, 61, 537–545. [Google Scholar] [CrossRef]
- Dehejia, R.H.; Wahba, S. Propensity Score-matching methods for nonexperimental causal studies. Rev. Econ. Stat. 2002, 84, 151–161. [Google Scholar] [CrossRef]
- Sedgwick, P. Incidence rate ratio. BMJ 2010, 344, e1589. [Google Scholar] [CrossRef] [PubMed]
- Beral, V.; Bull, D.; Doll, R.; Peto, R.; Reeves, G. Breast cancer and abortion: Collaborative reanalysis of data from 53 epidemiological studies, including 83?000 women with breast cancer from 16 countries. Lancet 2004, 363, 1007–1016. [Google Scholar] [PubMed]
- Guo, J.; Huang, Y.; Yang, L.; Xie, Z.; Song, S.; Yin, J.; Kuang, L.; Qin, W. Association between abortion and breast cancer: An updated systematic review and meta-analysis based on prospective studies. Cancer Causes Control 2015, 26, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Mahue-Giangreco, M.; Ursin, G.; Sullivan-Halley, J.; Bernstein, L. Induced abortion, miscarriage, and breast cancer risk of young women. Cancer Epidemiol. Biomark. Prev. 2003, 12, 209–214. [Google Scholar]
- Tong, H.; Wu, Y.; Yan, Y.; Dong, Y.; Guan, X.; Liu, Y.; Lu, Z. No association between abortion and risk of breast cancer among nulliparous women: Evidence from a meta-analysis. Medicine 2020, 99, e20251. [Google Scholar] [CrossRef]
- Xu, W.H.; Xiang, Y.B.; Ruan, Z.X.; Zheng, W.; Cheng, J.R.; Dai, Q.; Gao, Y.T.; Shu, X.O. Menstrual and reproductive factors and endometrial cancer risk: Results from a population-based case-control study in urban Shanghai. Int. J. Cancer 2004, 108, 613–619. [Google Scholar] [CrossRef]
- Parslov, M.; Lidegaard, O.; Klintorp, S.; Pedersen, B.; Jønsson, L.; Eriksen, P.S.; Ottesen, B. Risk factors among young women with endometrial cancer: A Danish case-control study. Am. J. Obstet. Gynecol. 2000, 182 Pt 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.J.; Na, R.; Weiderpass, E.; Adami, H.O.; Anderson, K.E.; van den Brandt, P.A.; Brinton, L.A.; Chen, C.; Cook, L.S.; Doherty, J.A.; et al. Pregnancy outcomes and risk of endometrial cancer: A pooled analysis of individual participant data in the Epidemiology of Endometrial Cancer Consortium. Int. J. Cancer 2021, 148, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.W.; Allen, J.C., Jr.; Lek, S.M.; Chia, M.L.; Tan, N.S.; Tan, T.C. Serum progesterone distribution in normal pregnancies compared to pregnancies complicated by threatened miscarriage from 5 to 13 weeks gestation: A prospective cohort study. BMC Pregnancy Childbirth 2018, 18, 360. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.; Rosenzweig, S.; Wiensch, A.; Ramus, S.J.; Menon, U.; Gentry-Maharaj, A.; Ziogas, A.; Anton-Culver, H.; Whittemore, A.S.; Sieh, W.; et al. Expanding our understanding of ovarian cancer risk: The role of incomplete pregnancies. J. Natl. Cancer Inst. 2021, 113, 301–308. [Google Scholar] [CrossRef]
- Mikkelsen, A.P.; Egerup, P.; Ebert, J.F.M.; Kolte, A.M.; Nielsen, H.S.; Lidegaard, Ø. Pregnancy loss and cancer risk: A nationwide observational study. EClinicalMedicine 2019, 15, 80–88. [Google Scholar] [CrossRef]
- Gierach, G.L.; Modugno, F.; Ness, R.B. Relations of gestational length and timing and type of incomplete pregnancy to ovarian cancer risk. Am. J. Epidemiol. 2005, 161, 452–461. [Google Scholar] [CrossRef]
- Braem, M.G.; Onland-Moret, N.C.; Schouten, L.J.; Kruitwagen, R.F.; Lukanova, A.; Allen, N.E.; Wark, P.A.; Tjønneland, A.; Hansen, L.; Braüner, C.M.; et al. Multiple miscarriages are associated with the risk of ovarian cancer: Results from the European Prospective Investigation into Cancer and Nutrition. PLoS ONE 2012, 7, e37141. [Google Scholar] [CrossRef]
- Troisi, R.; Bjørge, T.; Gissler, M.; Grotmol, T.; Kitahara, C.M.; Myrtveit Saether, S.M.; Ording, A.G.; Sköld, C.; Sørensen, H.T.; Trabert, B.; et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: A review of the evidence. J. Intern. Med. 2018, 283, 430–445. [Google Scholar] [CrossRef]
- Sung, H.K.; Ma, S.H.; Choi, J.-Y.; Hwang, Y.; Ahn, C.; Kim, B.-G.; Kim, Y.-M.; Kim, J.W.; Kang, S.; Kim, J.; et al. The effect of breastfeeding duration and parity on the risk of epithelial ovarian cancer: A systematic review and meta-analysis. J. Prev. Med. Public Health 2016, 49, 349–366. [Google Scholar] [CrossRef]
- Roura, E.; Travier, N.; Waterboer, T.; de Sanjosé, S.; Bosch, F.X.; Pawlita, M.; Pala, V.; Weiderpass, E.; Margall, N.; Dillner, J.; et al. The Influence of hormonal factors on the risk of developing cervical cancer and pre-cancer: Results from the EPIC cohort. PLoS ONE 2016, 11, e0147029. [Google Scholar]
| Before PSM Matching | p-Value | After PSM Matching | p-Value | Standardized Difference | |||
|---|---|---|---|---|---|---|---|
| Abortion | Non-Abortion | Abortion | Non-Abortion | ||||
| N | 269,050 | 4,715,170 | 269,050 | 807,150 | |||
| Age (in years) (Mean±SD) | 28.73 (±6.28) | 31.45 (±8.02) | <0.001 | 28.73 (±6.28) | 28.75 (±6.46) | 0.128 | 0.3% |
| Age categories (N, %)# | <0.001 | 1.000 | |||||
| 20–24 | 81,246 (30.20%) | 1,238,399 (26.26%) | 81,246 (30.20%) | 243,740 (30.20%) | 0.0% | ||
| 25–29 | 73,636 (27.37%) | 848,943 (18.00%) | 73,636 (27.37%) | 220,938 (27.37%) | 0.0% | ||
| 30–34 | 59,609 (22.16%) | 783,941 (16.63%) | 59,609 (22.16%) | 178,819 (22.15%) | 0.0% | ||
| 35–39 | 38,481 (14.30%) | 824,727 (17.49%) | 38,481 (14.3%) | 115,446 (14.3%) | 0.0% | ||
| 40+ | 16,078 (5.98%) | 1,019,160 (21.61%) | 16,078 (5.98%) | 48,207 (5.97%) | 0.0% | ||
| Average monthly payroll (NTD, Mean ± SD) | 30,621 (±22905) | 32,951 (±23776) | <0.001 | 30,621 (±22905) | 30,925 (±23022) | <0.001 | 1.3% |
| Average monthly payroll group (N, %) # | <0.001 | <0.001 | |||||
| Dependent | 29,818 (11.08%) | 509,710 (10.81%) | 29,818 (11.08%) | 87,691 (10.86%) | 0.7% | ||
| Less than NTD 20,000 | 31,390 (11.67%) | 536,115 (11.37%) | 31,390 (11.67%) | 92,337 (11.44%) | 0.7% | ||
| NTD 20,000–NTD 40,000 | 153,828 (57.17%) | 2,698,492 (57.23%) | 153,828 (57.17%) | 463,190 (57.39%) | 0.4% | ||
| NTD 40,001+ | 54,014 (20.08%) | 970,853 (20.59%) | 54,014 (20.08%) | 163,932 (20.31%) | 0.6% | ||
| Fertility (N, %) # | <0.001 | 0.980 | |||||
| Nulliparous | 123,082 (45.75%) | 3,404,682 (72.21%) | 123,082 (45.75%) | 369,269 (45.75%) | 0.0% | ||
| Parous | 145,968 (54.25%) | 1,310,488 (27.79%) | 145,968 (54.25%) | 437,881 (54.25%) | 0.0% | ||
| Diabetes mellitus (N, %) # | <0.001 | 0.961 | |||||
| No | 261,105 (97.05%) | 4,548,294 (96.46%) | 261,105 (97.05%) | 783,330 (97.05%) | 0.0% | ||
| Yes | 7945 (2.95%) | 166,876 (3.54%) | 7945 (2.95%) | 23,820 (2.95%) | 0.0% | ||
| Polycystic ovarian syndrome (N, %) # | <0.001 | 0.849 | |||||
| No | 259,636 (96.50%) | 4,610,982 (97.79%) | 259,636 (96.50%) | 778,971 (96.51%) | 0.1% | ||
| Yes | 9414 (3.5%) | 104,188 (2.21%) | 9414 (3.50%) | 28,179 (3.49%) | 0.1% | ||
| Endometrial hyperplasia (N, %) # | <0.001 | 0.854 | |||||
| No | 264,657 (98.37%) | 4,653,461 (98.69%) | 264,657 (98.37%) | 794,013 (98.37%) | 0.0% | ||
| Yes | 4393 (1.63%) | 61,709 (1.31%) | 4393 (1.63%) | 13,137 (1.63%) | 0.0% | ||
| Endometriosis (N, %)# | <0.001 | 0.975 | |||||
| No | 254,523 (94.60%) | 4,487,591 (95.17%) | 254,523 (94.60%) | 763,582 (94.60%) | 0.0% | ||
| Yes | 14,527 (5.40%) | 227,579 (4.83%) | 14,527 (5.40%) | 43,568 (5.40%) | 0.0% | ||
| Hormone-related drugs (N, %) # | <0.001 | 0.996 | |||||
| No | 75,149 (27.93%) | 2,880,486 (61.09%) | 75,149 (27.93%) | 225,451 (27.93%) | 0.0% | ||
| Yes | 193,901 (72.07%) | 1,834,684 (38.91%) | 193,901 (72.07%) | 581,699 (72.07%) | 0.0% | ||
| Charlson comorbidity index (Mean ± SD) | 0.51 (±0.86) | 0.50 (±0.89) | 0.001 | 0.51 (±0.86) | 0.51 (±0.86) | 0.601 | 0.0% |
| Charlson comorbidity index categories (N, %) # | <0.001 | 0.998 | |||||
| 0 score | 174,904 (65.01%) | 3,148,004 (66.76%) | 174,904 (65.01%) | 524,763 (65.02%) | 0.0% | ||
| 1 score | 64,880 (24.11%) | 1,043,789 (22.14%) | 64,880 (24.11%) | 194,613 (24.11%) | 0.0% | ||
| ≧2 scores | 29,266 (10.88%) | 523,377 (11.10%) | 29,266 (10.88%) | 87,774 (10.87%) | 0.0% | ||
| Total Person-Years | Cancer Events N (%) | Incidence Rate (per 100,000 Person-Years) | Incidence Rate Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abortion | Non-Abortion | Abortion | Non-Abortion | p-Value | Abortion | Non-Abortion | IRR | 95% CI | p-Value | |
| Breast cancer | ||||||||||
| Yes | 22,790.78 | 66,951.09 | 3006(1.12%) | 8869(1.10%) | 0.790 | 92.00 | 90.45 | 1.02 | 0.98–1.06 | 0.414 |
| No | 3,244,557.51 | 9,737,942.55 | 266,044(98.88%) | 98,281(98.90%) | ||||||
| Cervical cancer | ||||||||||
| Yes | 3898.80 | 10,657.30 | 654(0.24%) | 1798(0.22%) | 0.056 | 19.95 | 18.28 | 1.09 | 1.00–1.19 | 0.055 |
| No | 3,273,542.84 | 9,825,290.13 | 268,396(99.76%) | 805,352(99.78%) | ||||||
| Uterine cancer | ||||||||||
| Yes | 3876.90 | 15,939.70 | 564(0.21%) | 2171(0.27%) | <0.001 | 17.20 | 22.07 | 0.78 | 0.71–0.86 | <0.001 |
| No | 3,275,442.46 | 9,822,676.97 | 68,486(99.79%) | 804,979(99.73%) | ||||||
| Ovarian cancer | ||||||||||
| Yes | 4767.54 | 17,966.91 | 802(0.30%) | 2966(0.37%) | <0.001 | 24.48 | 30.18 | 0.81 | 0.75–0.88 | <0.001 |
| No | 3,270,992.30 | 9,808,883.83 | 268,248(99.70%) | 804,184(99.63%) | ||||||
| Breast Cancer | Cervical Cancer | Uterine Cancer | Ovarian Cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aHR | 95% CI | p-Value | aHR | 95% CI | p-Value | aHR | 95% CI | p-Value | aHR | 95% CI | p-Value | |
| Abortion | ||||||||||||
| Non (Ref) | ||||||||||||
| Yes | 1.01 | 0.97–1.05 | 0.719 | 1.09 | 0.99–1.19 | 0.072 | 0.77 | 0.70–0.85 | <0.001 | 0.81 | 0.75–0.88 | <0.001 |
| Age categories | ||||||||||||
| 20–24 (Ref) | ||||||||||||
| 25–29 | 2.51 | 2.32–2.70 | <0.001 | 1.14 | 1.02–1.28 | 0.026 | 2.28 | 1.96–2.65 | <0.001 | 1.15 | 1.05–1.26 | 0.002 |
| 30–34 | 4.69 | 4.36–5.05 | <0.001 | 1.40 | 1.24–1.58 | <0.001 | 3.48 | 3.01–4.03 | <0.001 | 1.10 | 1.00–1.21 | 0.047 |
| 35–39 | 7.17 | 6.64–7.74 | <0.001 | 1.55 | 1.36–1.77 | <0.001 | 4.30 | 3.69–5.02 | <0.001 | 1.11 | 1.00–1.24 | 0.056 |
| 40+ | 7.75 | 7.11–8.45 | <0.001 | 1.54 | 1.30–1.83 | <0.001 | 4.89 | 4.12–5.80 | <0.001 | 0.92 | 0.79–1.07 | 0.261 |
| Average monthly payroll group | ||||||||||||
| Less than NTD 20,000 (Ref) | ||||||||||||
| Dependent | 1.19 | 1.10–1.29 | <0.001 | 0.71 | 0.61–0.82 | <0.001 | 1.12 | 0.96–1.31 | 0.142 | 1.12 | 0.98–1.29 | 0.107 |
| NTD 20,001–NTD 40,000 | 1.07 | 1.01–1.15 | 0.030 | 0.72 | 0.65–0.81 | <0.001 | 0.95 | 0.84–1.08 | 0.471 | 1.14 | 1.02–1.27 | 0.020 |
| NTD 40,001+ | 1.32 | 1.23–1.42 | <0.001 | 0.45 | 0.38–0.52 | <0.001 | 1.13 | 0.98–1.30 | 0.093 | 1.16 | 1.02–1.31 | 0.020 |
| Fertility | ||||||||||||
| Nulliparous (Ref) | ||||||||||||
| Parous | 1.02 | 0.98–1.07 | 0.272 | 0.80 | 0.73–0.88 | <0.001 | 0.63 | 0.58–0.69 | <0.001 | 0.86 | 0.80–0.93 | <0.001 |
| Diabetes mellitus | ||||||||||||
| No (Ref) | ||||||||||||
| Yes | 0.63 | 0.57–0.69 | <0.001 | 0.52 | 0.42–0.64 | <0.001 | 0.84 | 0.70–1.00 | 0.047 | 0.43 | 0.36–0.51 | <0.001 |
| Polycystic ovarian syndrome | ||||||||||||
| No (Ref) | ||||||||||||
| Yes | 0.93 | 0.84–1.03 | 0.172 | 0.99 | 0.80–1.23 | 0.929 | 1.83 | 1.57–2.14 | <0.001 | 1.55 | 1.37–1.77 | <0.001 |
| Endometrial hyperplasia | ||||||||||||
| No (Ref) | ||||||||||||
| Yes | 1.33 | 1.20–1.49 | <0.001 | 1.53 | 1.22–1.92 | <0.001 | 3.95 | 3.46–4.52 | <0.001 | 1.10 | 0.91–1.34 | 0.317 |
| Endometriosis | ||||||||||||
| No (Ref) | ||||||||||||
| Yes | 1.08 | 1.01–1.16 | 0.030 | 0.83 | 0.71–0.99 | 0.034 | 1.61 | 1.43–1.80 | <0.001 | 3.67 | 3.38–3.98 | <0.001 |
| Hormone-related drugs | ||||||||||||
| No (Ref) | ||||||||||||
| Yes | 0.91 | 0.87–0.94 | <0.001 | 1.34 | 1.22–1.48 | <0.001 | 1.17 | 1.07–1.28 | <0.001 | 1.11 | 1.02–1.20 | 0.012 |
| Charlson comorbidity index categories | ||||||||||||
| 0 score (Ref) | ||||||||||||
| 1 score | 1.10 | 1.05–1.15 | <0.001 | 0.93 | 0.83–1.04 | 0.196 | 1.08 | 0.98–1.19 | 0.122 | 1.05 | 0.96–1.15 | 0.318 |
| ≧2 scores | 2.67 | 2.55–2.79 | <0.001 | 5.03 | 4.59–5.50 | <0.001 | 2.49 | 2.27–2.74 | <0.001 | 4.91 | 4.56–5.28 | <0.001 |
| Breast Cancer | Cervical Cancer | Uterine Cancer | Ovarian Cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age categories | ||||||||||||
| 20–24 | 1.05 | 0.90–1.21 | 0.555 | 1.17 | 0.97-1.41 | 0.095 | 0.90 | 0.67-1.21 | 0.472 | 0.83 | 0.71-0.97 | 0.020 |
| 25–29 | 0.97 | 0.88–1.07 | 0.503 | 1.19 | 0.99–1.42 | 0.064 | 0.73 | 0.59–0.90 | 0.003 | 0.74 | 0.63–0.86 | <0.001 |
| 30–34 | 1.03 | 0.96–1.12 | 0.352 | 1.16 | 0.97–1.38 | 0.107 | 0.82 | 0.70–0.98 | 0.025 | 0.84 | 0.72–0.99 | 0.034 |
| 35–39 | 0.98 | 0.91–1.06 | 0.633 | 0.87 | 0.70–1.08 | 0.204 | 0.71 | 0.59–0.85 | <0.001 | 0.88 | 0.73–1.05 | 0.149 |
| 40+ | 1.05 | 0.95–1.17 | 0.343 | 0.98 | 0.73–1.33 | 0.909 | 0.79 | 0.62–1.00 | 0.049 | 0.78 | 0.58–1.05 | 0.097 |
| Average monthly payroll group | ||||||||||||
| Dependent | 0.94 | 0.84–1.06 | 0.306 | 0.94 | 0.72–1.24 | 0.666 | 0.73 | 0.57–0.95 | 0.019 | 0.88 | 0.70–1.10 | 0.258 |
| Less than NTD 20,000 | 0.98 | 0.85–1.12 | 0.723 | 1.21 | 0.98–1.50 | 0.073 | 0.66 | 0.49–0.89 | 0.006 | 0.82 | 0.64–1.04 | 0.097 |
| NTD 20,001–NTD 40,000 | 1.06 | 1.00–1.12 | 0.052 | 1.06 | 0.95–1.20 | 0.300 | 0.77 | 0.68–0.88 | <0.001 | 0.80 | 0.72–0.89 | <0.001 |
| NTD 40,001+ | 1.00 | 0.92–1.08 | 0.923 | 1.18 | 0.93–1.51 | 0.183 | 0.89 | 0.74–1.06 | 0.190 | 0.81 | 0.69–0.97 | 0.018 |
| Fertility | ||||||||||||
| Nulliparous | 1.02 | 0.97–1.08 | 0.466 | 1.01 | 0.90–1.14 | 0.855 | 0.67 | 0.60–0.75 | <0.001 | 0.76 | 0.68–0.85 | <0.001 |
| Parous | 1.01 | 0.94–1.08 | 0.820 | 1.20 | 1.05–1.37 | 0.007 | 1.02 | 0.88–1.19 | 0.759 | 0.87 | 0.78–0.97 | 0.013 |
| Diabetes mellitus | ||||||||||||
| No | 1.02 | 0.97–1.06 | 0.496 | 1.11 | 1.02–1.22 | 0.023 | 0.78 | 0.71–0.86 | <0.001 | 0.81 | 0.75–0.88 | <0.001 |
| Yes | 1.08 | 0.88–1.32 | 0.469 | 0.68 | 0.42–1.13 | 0.135 | 0.76 | 0.51–1.14 | 0.189 | 0.91 | 0.60–1.36 | 0.630 |
| Polycystic ovarian syndrome | ||||||||||||
| No | 1.02 | 0.98–1.06 | 0.406 | 1.10 | 1.01–1.21 | 0.035 | 0.79 | 0.72–0.87 | <0.001 | 0.83 | 0.77–0.90 | <0.001 |
| Yes | 1.00 | 0.78–1.27 | 0.980 | 0.82 | 0.49–1.36 | 0.445 | 0.64 | 0.43–0.94 | 0.024 | 0.57 | 0.41–0.79 | 0.001 |
| Endometrial hyperplasia | ||||||||||||
| No | 1.02 | 0.98–1.07 | 0.336 | 1.09 | 1.00–1.20 | 0.054 | 0.82 | 0.74–0.90 | <0.001 | 0.81 | 0.75–0.88 | <0.001 |
| Yes | 0.91 | 0.70–1.16 | 0.437 | 1.03 | 0.62–1.72 | 0.898 | 0.44 | 0.30–0.64 | <0.001 | 0.91 | 0.58–1.43 | 0.691 |
| Endometriosis | ||||||||||||
| No | 1.01 | 0.97–1.06 | 0.586 | 1.10 | 1.01–1.21 | 0.037 | 0.75 | 0.67–0.82 | <0.001 | 0.83 | 0.76–0.91 | <0.001 |
| Yes | 1.08 | 0.94–1.25 | 0.286 | 0.92 | 0.63–1.35 | 0.676 | 1.03 | 0.81–1.31 | 0.794 | 0.74 | 0.62–0.88 | 0.001 |
| Hormone-related drugs | ||||||||||||
| No | 0.99 | 0.92–1.06 | 0.753 | 1.21 | 1.01–1.46 | 0.043 | 0.87 | 0.73–1.04 | 0.119 | 0.79 | 0.67–0.93 | 0.004 |
| Yes | 1.03 | 0.98–1.09 | 0.223 | 1.06 | 0.96–1.17 | 0.265 | 0.75 | 0.67–0.84 | <0.001 | 0.82 | 0.75–0.90 | <0.001 |
| Charlson comorbidity index categories | ||||||||||||
| 0 score | 1.00 | 0.94–1.06 | 0.967 | 1.10 | 0.97–1.26 | 0.143 | 0.72 | 0.63–0.83 | <0.001 | 0.79 | 0.71–0.89 | <0.001 |
| 1 score | 1.05 | 0.96–1.14 | 0.320 | 1.16 | 0.93–1.45 | 0.190 | 0.82 | 0.67–0.99 | 0.042 | 0.97 | 0.81–1.16 | 0.731 |
| ≧2 scores | 1.03 | 0.95–1.12 | 0.472 | 1.05 | 0.91–1.21 | 0.510 | 0.86 | 0.72–1.02 | 0.080 | 0.76 | 0.67–0.87 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-T.; Tai, S.-Y.; Tsao, Y.-H.; Chen, F.-M.; Hsieh, H.-M. Abortion and Female Cancer Risks among Women Aged 20 to 45 Years: A 10-Year Longitudinal Population-Based Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2023, 20, 3682. https://doi.org/10.3390/ijerph20043682
Shen C-T, Tai S-Y, Tsao Y-H, Chen F-M, Hsieh H-M. Abortion and Female Cancer Risks among Women Aged 20 to 45 Years: A 10-Year Longitudinal Population-Based Cohort Study in Taiwan. International Journal of Environmental Research and Public Health. 2023; 20(4):3682. https://doi.org/10.3390/ijerph20043682
Chicago/Turabian StyleShen, Cheng-Ting, Shu-Yu Tai, Yu-Hsiang Tsao, Fang-Ming Chen, and Hui-Min Hsieh. 2023. "Abortion and Female Cancer Risks among Women Aged 20 to 45 Years: A 10-Year Longitudinal Population-Based Cohort Study in Taiwan" International Journal of Environmental Research and Public Health 20, no. 4: 3682. https://doi.org/10.3390/ijerph20043682
APA StyleShen, C.-T., Tai, S.-Y., Tsao, Y.-H., Chen, F.-M., & Hsieh, H.-M. (2023). Abortion and Female Cancer Risks among Women Aged 20 to 45 Years: A 10-Year Longitudinal Population-Based Cohort Study in Taiwan. International Journal of Environmental Research and Public Health, 20(4), 3682. https://doi.org/10.3390/ijerph20043682






