Trajectories of Seroprevalence and Neutralizing Activity of Antibodies against SARS-CoV-2 in Southern Switzerland between July 2020 and July 2021: An Ongoing, Prospective Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements and Data Collection
2.3. Serological Testing
2.4. Statistical Analysis
2.5. Ethics
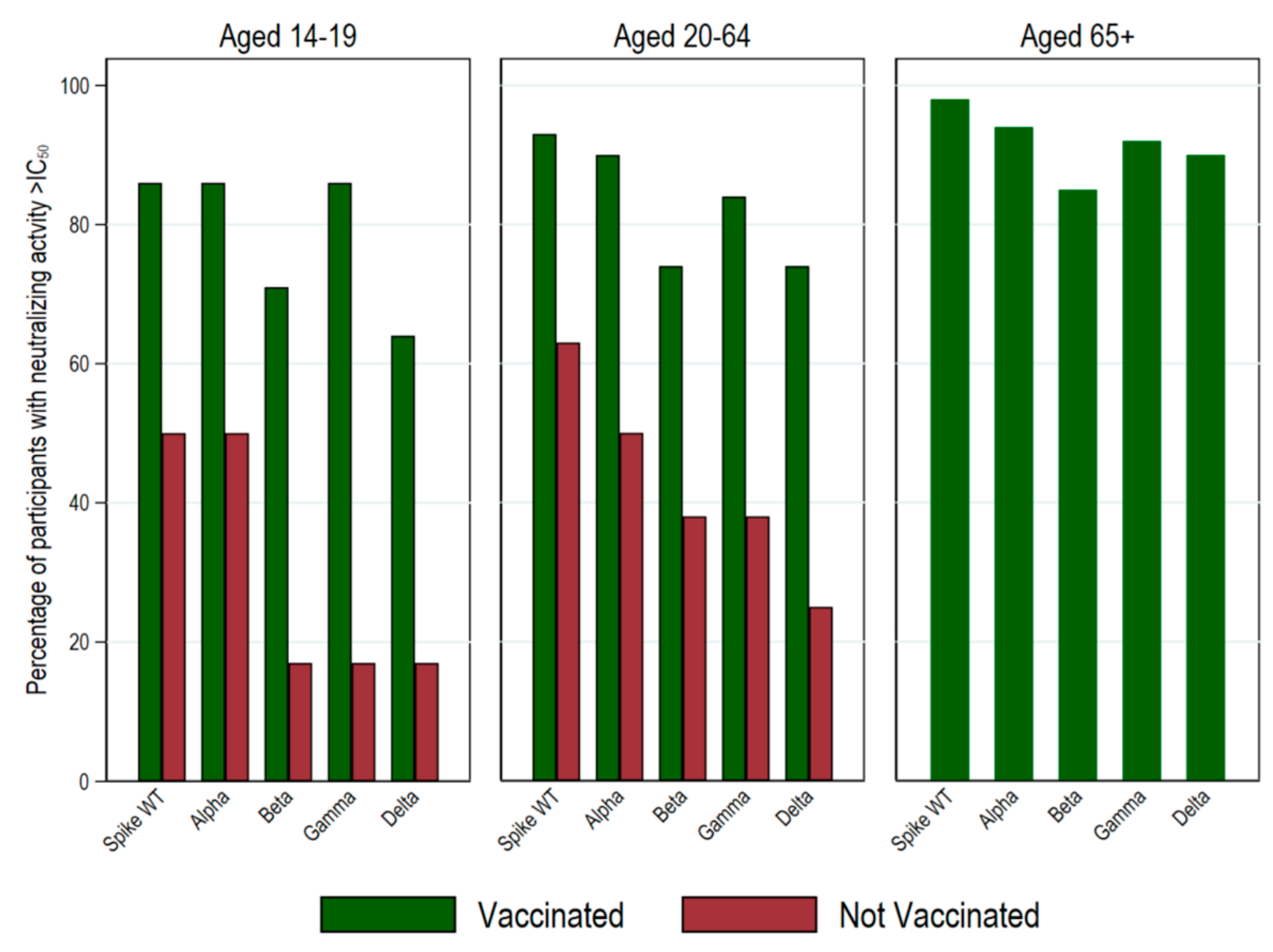
3. Results
Descriptive Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19–11 March 2020. 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 1 November 2023).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 10 February 2023).
- World Health Organization. Population-Based Age-Stratified Seroepidemiological Investigation Protocol for Coronavirus 2019 (COVID-19) Infection, 26 May 2020. World Health Organization, 2020. Report No.: WHO/2019-nCoV/Seroepidemiology/2020.2. Available online: https://apps.who.int/iris/handle/10665/332188 (accessed on 27 October 2022).
- Bobrovitz, N.; Arora, R.K.; Cao, C.; Boucher, E.; Liu, M.; Donnici, C.; Yanes-Lane, M.; Whelan, M.; Perlman-Arrow, S.; Chen, J.; et al. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252617. [Google Scholar] [CrossRef]
- Erikstrup, C.; Laksafoss, A.D.; Gladov, J.; Kaspersen, K.A.; Mikkelsen, S.; Hindhede, L.; Boldsen, J.K.; Jørgensen, S.W.; Ethelberg, S.; Holm, D.K.; et al. Seroprevalence and infection fatality rate of the SARS-CoV-2 Omicron variant in Denmark: A nationwide serosurveillance study. Lancet Reg. Health Eur. 2022, 21, 100479. [Google Scholar] [CrossRef]
- Hoballah, A.; El Haidari, R.; Siblany, G.; Abdel Sater, F.; Mansour, S.; Hassan, H.; Abou-Abbas, L. SARS-CoV-2 antibody seroprevalence in Lebanon: Findings from the first nationwide serosurvey. BMC Infect. Dis. 2022, 22, 42. [Google Scholar] [CrossRef]
- Reicher, S.; Ratzon, R.; Ben-Sahar, S.; Hermoni-Alon, S.; Mossinson, D.; Shenhar, Y.; Friger, M.; Lustig, Y.; Alroy-Preis, S.; Anis, E.; et al. Nationwide seroprevalence of antibodies against SARS-CoV-2 in Israel. Eur. J. Epidemiol. 2021, 36, 727–734. [Google Scholar] [CrossRef]
- Serotracker. Available online: https://serotracker.com/en/Explore (accessed on 10 October 2022).
- Sharma, N.; Sharma, P.; Basu, S.; Saxena, S.; Chawla, R.; Dushyant, K.; Mundeja, N.; Marak, Z.; Singh, S.; Singh, G.; et al. The seroprevalence of severe acute respiratory syndrome coronavirus 2 in Delhi, India: A repeated population-based seroepidemiological study. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 242–251. [Google Scholar] [CrossRef]
- Sharma, P.; Basu, S.; Mishra, S.; Gupta, E.; Agarwal, R.; Kale, P.; Mundeja, N.; Charan, B.; Singh, G.; Singh, M. SARS-CoV-2 Seroprevalence in Delhi, India, During September-October 2021: A Population-Based Seroepidemiological Study. Cureus 2022, 14, e27428. Available online: https://www.cureus.com/articles/106327-sars-cov-2-seroprevalence-in-delhi-india-during-september-october-2021-a-population-based-seroepidemiological-study (accessed on 28 July 2022). [CrossRef]
- Vicentini, C.; Bordino, V.; Cornio, A.R.; Meddis, D.; Marengo, N.; Ditommaso, S.; Giacomuzzi, M.; Memoli, G.; Furfaro, G.; Mengozzi, G.; et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies among health care users of Northern Italy: Results from two serosurveys (October-November 2019 and September-October 2021). Int. J. Infect. Dis. 2022, 124, 49–54. [Google Scholar] [CrossRef]
- Zimmermann, L.; Mukherjee, B. Meta-analysis of nationwide SARS-CoV-2 infection fatality rates in India. PLOS Glob. Public Health 2022, 2, e0000897. [Google Scholar] [CrossRef]
- Barrie, M.B.; Lakoh, S.; Kelly, J.D.; Kanu, J.S.; Squire, J.S.; Koroma, Z.; Bah, S.; Sankoh, O.; Brima, A.; Ansumana, R.; et al. SARS-CoV-2 antibody prevalence in Sierra Leone, March 2021: A cross-sectional, nationally representative, age-stratified serosurvey. BMJ Glob. Health 2021, 6, e007271. [Google Scholar] [CrossRef]
- Murhekar, M.V.; Bhatnagar, T.; Selvaraju, S.; Saravanakumar, V.; Thangaraj, J.W.V.; Shah, N.; Kumar, M.S.; Rade, K.; Sabarinathan, R.; Asthana, S.; et al. SARS-CoV-2 antibody seroprevalence in India, August–September, 2020: Findings from the second nationwide household serosurvey. Lancet Glob. Health 2021, 9, e257–e266. [Google Scholar] [CrossRef]
- Murhekar, M.V.; Bhatnagar, T.; Thangaraj, J.W.V.; Saravanakumar, V.; Santhosh Kumar, M.; Selvaraju, S.; Rade, K.; Kumar, C.P.G.; Sabarinathan, R.; Asthana, S.; et al. Seroprevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in India, June–July 2021: A population-based cross-sectional study. PLoS Med. 2021, 18, e1003877. [Google Scholar] [CrossRef]
- Pathela, P.; Crawley, A.; Weiss, D.; Maldin, B.; Cornell, J.; Purdin, J.; Schumacher, P.K.; Marovich, S.; Li, J.; Daskalakis, D.; et al. Seroprevalence of Severe Acute Respiratory Syndrome Coronavirus 2 Following the Largest Initial Epidemic Wave in the United States: Findings from New York City, 13 May to 21 July 2020. J. Infect. Dis. 2021, 224, 196–206. [Google Scholar] [CrossRef]
- Piler, P.; Thon, V.; Andrýsková, L.; Doležel, K.; Kostka, D.; Pavlík, T.; Dušek, L.; Pikhart, H.; Bobák, M.; Matic, S.; et al. Nationwide increases in anti-SARS-CoV-2 IgG antibodies between October 2020 and March 2021 in the unvaccinated Czech population. Commun. Med. 2022, 2, 19. [Google Scholar] [CrossRef]
- Poljak, M.; Oštrbenk Valenčak, A.; Štrumbelj, E.; Maver Vodičar, P.; Vehovar, V.; Resman Rus, K.; Korva, M.; Knap, N.; Seme, K.; Petrovec, M.; et al. Seroprevalence of severe acute respiratory syndrome coronavirus 2 in Slovenia: Results of two rounds of a nationwide population study on a probability-based sample, challenges and lessons learned. Clin. Microbiol. Infect. 2021, 27, 1039.e1–1039.e7. [Google Scholar] [CrossRef]
- Decru, B.; Van Elslande, J.; Steels, S.; Van Pottelbergh, G.; Godderis, L.; Van Holm, B.; Bossuyt, X.; Van Weyenbergh, J.; Maes, P.; Vermeersch, P. IgG Anti-Spike Antibodies and Surrogate Neutralizing Antibody Levels Decline Faster 3 to 10 Months after BNT162b2 Vaccination Than After SARS-CoV-2 Infection in Healthcare Workers. Front. Immunol. 2022, 13, 909910. [Google Scholar] [CrossRef]
- Herzberg, J.; Vollmer, T.; Fischer, B.; Becher, H.; Becker, A.-K.; Sahly, H.; Honarpisheh, H.; Guraya, S.Y.; Strate, T.; Knabbe, C. Half-Year Longitudinal Seroprevalence of SARS-CoV-2-Antibodies and Rule Compliance in German Hospital Employees. Int. J. Environ. Res. Public. Health 2021, 18, 10972. [Google Scholar] [CrossRef]
- Seery, V.; Raiden, S.; Russo, C.; Borda, M.; Herrera, L.; Uranga, M.; Varese, A.; Marcó del Pont, M.; Chirino, C.; Erramuspe, C.; et al. Antibody response against SARS-CoV-2 variants of concern in children infected with pre-Omicron variants: An observational cohort study. eBioMedicine 2022, 83, 104230. [Google Scholar] [CrossRef]
- Ulyte, A.; Radtke, T.; Abela, I.A.; Haile, S.R.; Berger, C.; Huber, M.; Schanz, M.; Schwarzmueller, M.; Trkola, A.; Fehr, J.; et al. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: Prospective cohort study of 55 schools. BMJ 2021, 372, n616. [Google Scholar] [CrossRef]
- Uyoga, S.; Adetifa, I.M.O.; Karanja, H.K.; Nyagwange, J.; Tuju, J.; Wanjiku, P.; Aman, R.; Mwangangi, M.; Amoth, P.; Kasera, K.; et al. Seroprevalence of anti–SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science 2021, 371, 79–82. [Google Scholar] [CrossRef]
- Gruell, H.; Vanshylla, K.; Tober-Lau, P.; Hillus, D.; Schommers, P.; Lehmann, C.; Kurth, F.; Sander, L.E.; Klein, F. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat. Med. 2022, 28, 477–480. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Long, Q.; Wu, Y.; Hu, X.; He, Y.; Jiang, M.; Li, J.; Zhao, L.; Yang, S.; et al. Comprehensive Humoral and Cellular Immune Responses to SARS-CoV-2 Variants in Diverse Chinese Population. Research 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Rapaka, R.R.; Hammershaimb, E.A.; Neuzil, K.M. Are Some COVID-19 Vaccines Better Than Others? Interpreting and Comparing Estimates of Efficacy in Vaccine Trials. Clin. Infect. Dis. 2022, 74, 352–358. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Soeorg, H.; Jõgi, P.; Naaber, P.; Ottas, A.; Toompere, K.; Lutsar, I. Seroprevalence and levels of IgG antibodies after COVID-19 infection or vaccination. Infect. Dis. 2022, 54, 63–71. [Google Scholar] [CrossRef]
- Böger, B.; Fachi, M.M.; Vilhena, R.O.; Cobre, A.F.; Tonin, F.S.; Pontarolo, R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am. J. Infect. Control 2021, 49, 21–29. [Google Scholar] [CrossRef]
- Mahajan, A.; Manchikanti, L. Value and validity of coronavirus antibody testing. Pain Physician 2020, 23, S381–S390. [Google Scholar] [CrossRef]
- Mohit, E.; Rostami, Z.; Vahidi, H. A comparative review of immunoassays for COVID-19 detection. Expert Rev. Clin. Immunol. 2021, 17, 573–599. [Google Scholar] [CrossRef]
- Borghesi, A.; Golemi, S.; Carapella, N.; Zigliani, A.; Farina, D.; Maroldi, R. Lombardy, Northern Italy: COVID-19 second wave less severe and deadly than the first? A preliminary investigation. Infect. Dis. 2021, 53, 370–375. [Google Scholar] [CrossRef]
- Signorelli, C.; Odone, A.; Gianfredi, V.; Balzarini, F.; Bucci, D.; Croci, R.; Gaetti, G.; Stirparo, G.; Guerra, R. Epidemiological assessment of the first COVID-19 epidemic wave in Lombardy. A systematic review: First COVID-19 epidemic wave in Lombardy. Acta Biomed. Atenei Parm. 2021, 92, e2021462. [Google Scholar] [CrossRef]
- Repubblica e Cantone Ticino Coronavirus: Aggiornamento Sulla Vaccinazione [Coronavirus: Update on Vaccination]. 2021. Available online: https://www4.ti.ch/tich/area-media/comunicati/dettaglio-comunicato?NEWS_ID=189619&cHash=1541af3baf131bf3a6a3b14a14d75636 (accessed on 1 November 2022).
- Fenwick, C.; Turelli, P.; Pellaton, C.; Farina, A.; Campos, J.; Raclot, C.; Pojer, F.; Cagno, V.; Nusslé, S.G.; D’Acremont, V.; et al. A high-throughput cell- and virus-free assay shows reduced neutralization of SARS-CoV-2 variants by COVID-19 convalescent plasma. Sci. Transl. Med. 2021, 13, eabi8452. [Google Scholar] [CrossRef]
- West, E.A.; Anker, D.; Amati, R.; Richard, A.; Wisniak, A.; Butty, A.; Albanese, E.; Bochud, M.; Chiolero, A.; Crivelli, L.; et al. Corona Immunitas: Study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int. J. Public Health 2020, 65, 1529–1548. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Fenwick, C.; Croxatto, A.; Coste, A.T.; Pojer, F.; André, C.; Pellaton, C.; Farina, A.; Campos, J.; Hacker, D.; Lau, K.; et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J. Virol. 2021, 95, e01828-20. [Google Scholar] [CrossRef]
- Federal Office of Public Health FOPH. Coronavirus: Situation in Switzerland. Available online: https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/situation-schweiz-und-international.html (accessed on 27 October 2022).
- Stringhini, S.; Wisniak, A.; Piumatti, G.; Azman, A.S.; Lauer, S.A.; Baysson, H.; De Ridder, D.; Petrovic, D.; Schrempft, S.; Marcus, K.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): A population-based study. Lancet 2020, 396, 313–319. [Google Scholar] [CrossRef]
- Federal Statistical Office. Portraits of the Cantons: Ticino. 2021. Available online: https://www.bfs.admin.ch/bfs/en/home/statistics/regional-statistics/regional-portraits-key-figures/cantons/ticino.html (accessed on 26 September 2022).
- Federal Office of Public Health FOPH. Vaccinazione Anti-COVID-19: Gli Adolescenti dai 12 Anni in su Possono Farsi Vaccinare [Anti-COVID-19 Vaccination: Adolescents 12 Years and Older up can Get Vaccinated]. 2021. Available online: https://www.bag.admin.ch/bag/en/home/das-bag/aktuell/medienmitteilungen.msg-id-84095.html (accessed on 1 November 2022).
- Repubblica e Cantone Ticino Coronavirus—Vaccinazione dei Bambini, Iscrizioni Aperte [Coronavirus—Vaccination of Children, Open Enrollment]. 2022. Available online: https://www4.ti.ch/tich/area-media/comunicati/dettaglio-comunicato?NEWS_ID=199669&cHash=a8cb4d845246c7239e1fdd75c647308e (accessed on 1 November 2022).
- Stringhini, S.; Zaballa, M.-E.; Perez-Saez, J.; Pullen, N.; de Mestral, C.; Picazio, A.; Pennacchio, F.; Wisniak, A.; Richard, A.; Baysson, H.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies after the second pandemic peak. Lancet Infect. Dis. 2021, 21, 600–601. [Google Scholar] [CrossRef]
- Stringhini, S.; Zaballa, M.-E.; Pullen, N.; Perez-Saez, J.; de Mestral, C.; Loizeau, A.J.; Lamour, J.; Pennacchio, F.; Wisniak, A.; Dumont, R.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies 6 months into the vaccination campaign in Geneva, Switzerland, 1 June to 7 July 2021. Eurosurveillance 2021, 26, 2100830. [Google Scholar] [CrossRef]
- Ulyte, A.; Radtke, T.; Abela, I.A.; Haile, S.R.; Ammann, P.; Berger, C.; Trkola, A.; Fehr, J.; Puhan, M.A.; Kriemler, S. Evolution of SARS-CoV-2 Seroprevalence and Clusters in School Children from June 2020 to April 2021 Reflect Community Transmission: Prospective Cohort Study Ciao Corona. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.07.19.21260644v1 (accessed on 27 October 2022).
- Beretta, O.; Pagani, S.C.; Lazzaro, M.; Merlani, G.; Gallacchi, M.B. Seroprevalence of the SARS-CoV-2 Virus in the Population of the Southern Switzerland (Canton Ticino)—Cohort Study, Results at 12 Months. Swiss Med. Wkly. 2021, 151, w30116. Available online: https://smw.ch/article/doi/smw.2021.w30116 (accessed on 27 October 2022). [CrossRef]
- Krutikov, M.; Palmer, T.; Tut, G.; Fuller, C.; Azmi, B.; Giddings, R.; Shrotri, M.; Kaur, N.; Sylla, P.; Lancaster, T.; et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): Prospective cohort study in England. Lancet Healthy Longev. 2022, 3, e13–e21. [Google Scholar] [CrossRef]
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef]
- Van Elslande, J.; Gruwier, L.; Godderis, L.; Vermeersch, P. Estimated Half-Life of SARS-CoV-2 Anti-Spike Antibodies More Than Double the Half-Life of Anti-nucleocapsid Antibodies in Healthcare Workers. Clin. Infect. Dis. 2021, 73, 2366–2368. [Google Scholar] [CrossRef]
- Gornyk, D.; Harries, M.; Glöckner, S.; Strengert, M.; Kerrinnes, T.; Heise, J.-K.; Maaß, H.; Ortmann, J.; Kessel, B.; Kemmling, Y.; et al. SARS-CoV-2 seroprevalence in Germany. Dtsch. Ärztebl. Int. 2021, 118, 824. [Google Scholar] [CrossRef]
- Poustchi, H.; Darvishian, M.; Mohammadi, Z.; Shayanrad, A.; Delavari, A.; Bahadorimonfared, A.; Eslami, S.; Javanmard, S.H.; Shakiba, E.; Somi, M.H.; et al. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: A population-based cross-sectional study. Lancet Infect. Dis. 2021, 21, 473–481. [Google Scholar] [CrossRef]
- Ward, H.; Cooke, G.; Whitaker, M.; Redd, R.; Eales, O.; Brown, J.C.; Collet, K.; Cooper, E.; Daunt, A.; Jones, K.; et al. REACT-2 Round 5: Increasing Prevalence of SARS-CoV-2 Antibodies Demonstrate Impact of the Second Wave and of Vaccine Roll-Out in England. medRxiv 2021. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Hökelekli, O.; Uflacker, L.; Rudolf, H.; Paulussen, M.; Gatermann, S.G. Seroprevalence of SARS-CoV-2 Antibodies in Employees of Three Hospitals of a Secondary Care Hospital Network in Germany and an Associated Fire Brigade: Results of a Repeated Cross-Sectional Surveillance Study Over 1 Year. Int. J. Environ. Res. Public. Health 2022, 19, 2402. [Google Scholar] [CrossRef]
- Stadlbauer, D.; Tan, J.; Jiang, K.; Hernandez, M.M.; Fabre, S.; Amanat, F.; Teo, C.; Arunkumar, G.A.; McMahon, M.; Capuano, C.; et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature 2021, 590, 146–150. [Google Scholar] [CrossRef]
- Amati, R.; Frei, A.; Kaufmann, M.; Sabatini, S.; Pellaton, C.; Fehr, J.; Albanese, E.; Puhan, M.A.; on behalf of the Corona Immunitas Research Group. Functional immunity against SARS-CoV-2 in the general population after a booster campaign and the Delta and Omicron waves, Switzerland, March 2022. Eurosurveillance 2022, 27, 2200561. Available online: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2022.27.31.2200561 (accessed on 11 November 2022). [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Weaver, S.C.; Swanson, K.A.; Cai, H.; Sarkar, R.; et al. BNT162b2-Elicited Neutralization against New SARS-CoV-2 Spike Variants. N. Engl. J. Med. 2021, 385, 472–474. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhu, Y.; Wang, S.; Jia, S.; Gao, Y.; Lu, Y.; Zhou, C.; Liang, R.; Sun, D.; Wang, X.; et al. SARS-CoV-2 immunity and functional recovery of COVID-19 patients 1-year after infection. Signal Transduct. Target. Ther. 2021, 6, 1–12. [Google Scholar] [CrossRef]
- Edara, V.-V.; Lai, L.; Sahoo, M.K.; Floyd, K.; Sibai, M.; Solis, D.; Flowers, M.W.; Hussaini, L.; Ciric, C.R.; Bechnack, S.; et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jahrsdörfer, B.; Proffen, M.; Scholz, J.; Hägele, J.; Ludwig, C.; Vieweg, C.; Grempels, A.; Fabricius, D.; Lotfi, R.; Körper, S.; et al. BNT162b2 Booster Vaccination Elicits Cross-Reactive Immunity Against SARS-CoV-2 Variants B.1.1.529 and B.1.617.2 in Convalescents of All Ages. Front. Immunol. 2022, 13, 920210. [Google Scholar] [CrossRef]
- Lavezzo, E.; Pacenti, M.; Manuto, L.; Boldrin, C.; Cattai, M.; Grazioli, M.; Bianca, F.; Sartori, M.; Caldart, F.; Castelli, G.; et al. Neutralising reactivity against SARS-CoV-2 Delta and Omicron variants by vaccination and infection history. Genome Med. 2022, 14, 61. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.-C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Shahid, Z.; Kalayanamitra, R.; McClafferty, B.; Kepko, D.; Ramgobin, D.; Patel, R.; Aggarwal, C.S.; Vunnam, R.; Sahu, N.; Bhatt, D.; et al. COVID-19 and Older Adults: What We Know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Xie, F.; Ackerson, B.K.; Valluri, S.R.; Jodar, L.; McLaughlin, J.M. Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: A test-negative, case-control study. Lancet Respir. Med. 2023, 11, 176–187. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Fei, Y.; Xu, H.; Zhang, X.; Musa, S.S.; Zhao, S.; He, D. Seroprevalence and infection attack rate of COVID-19 in Indian cities. Infect. Dis. Model. 2022, 7, 25–32. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Arbel, R.; Sergienko, R.; Friger, M.; Peretz, A.; Beckenstein, T.; Yaron, S.; Netzer, D.; Hammerman, A. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat. Med. 2022, 28, 1486–1490. [Google Scholar] [CrossRef]
- Kerr, S.; Vasileiou, E.; Robertson, C.; Sheikh, A. COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes from Delta AY.4.2: Cohort and test-negative study of 5.4 million individuals in Scotland. J. Glob. Health 2022, 12, 05025. [Google Scholar] [CrossRef]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef]
- Saban, M.; Myers, V.; Wilf-Miron, R. Changes in infectivity, severity and vaccine effectiveness against delta COVID-19 variant ten months into the vaccination program: The Israeli case. Prev. Med. 2022, 154, 106890. [Google Scholar] [CrossRef]
- Shoukat, A.; Vilches, T.N.; Moghadas, S.M.; Sah, P.; Schneider, E.C.; Shaff, J.; Ternier, A.; Chokshi, D.A.; Galvani, A.P. Lives saved and hospitalizations averted by COVID-19 vaccination in New York City: A modeling study. Lancet Reg. Health Am. 2022, 5, 100085. [Google Scholar] [CrossRef]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to Covid-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef]
- Nakagama, Y.; Komase, Y.; Kaku, N.; Nitahara, Y.; Tshibangu-Kabamba, E.; Tominaga, T.; Tanaka, H.; Yokoya, T.; Hosokawa, M.; Kido, Y. Detecting Waning Serological Response with Commercial Immunoassays: 18-Month Longitudinal Follow-up of Anti-SARS-CoV-2 Nucleocapsid Antibodies. Microbiol. Spectr. 2022, 10, e00986-22. [Google Scholar] [CrossRef]
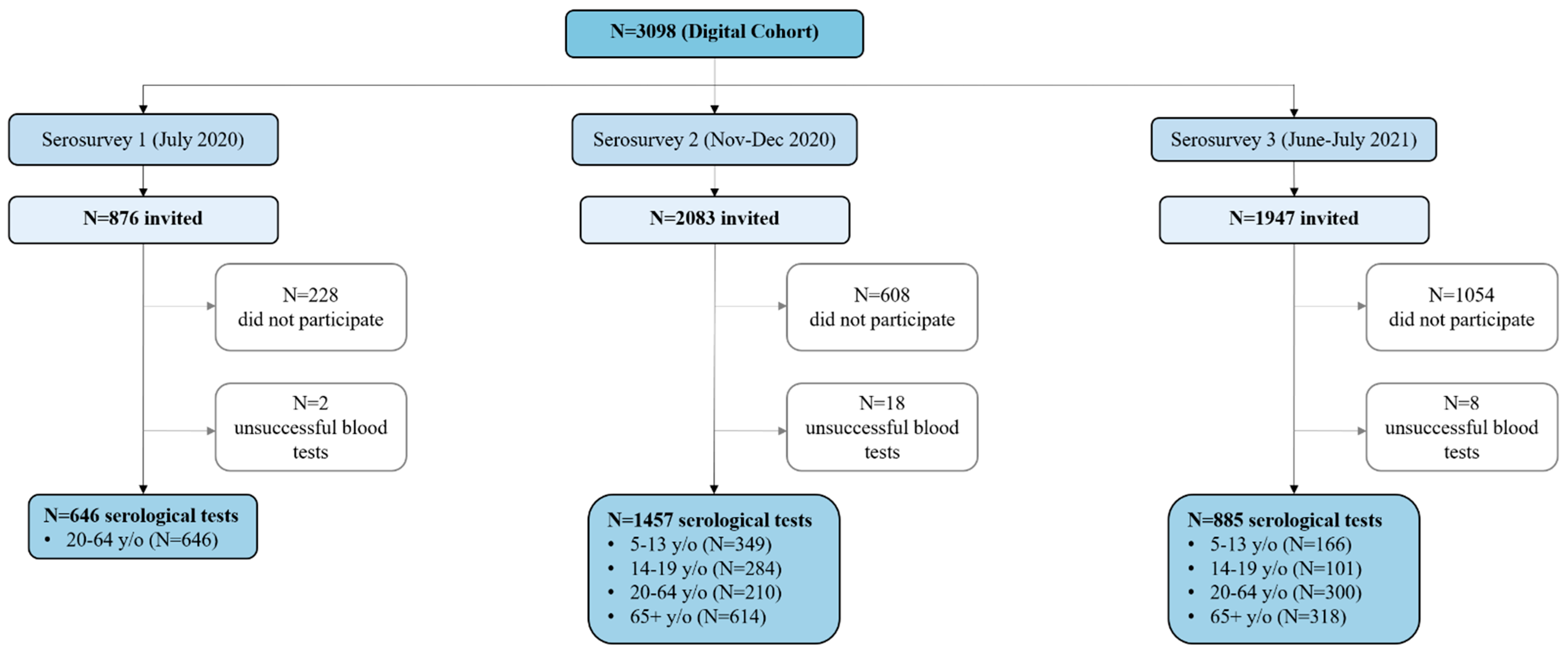
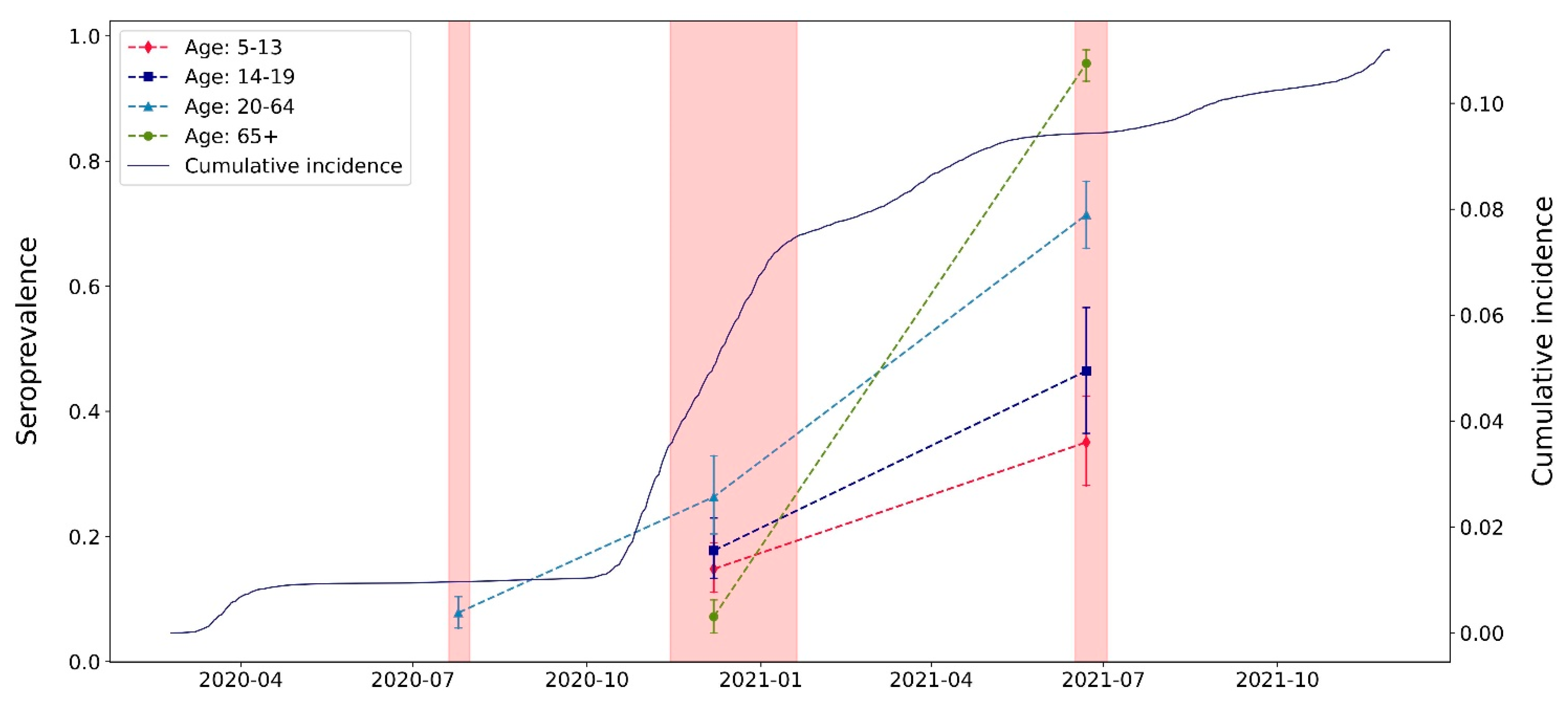
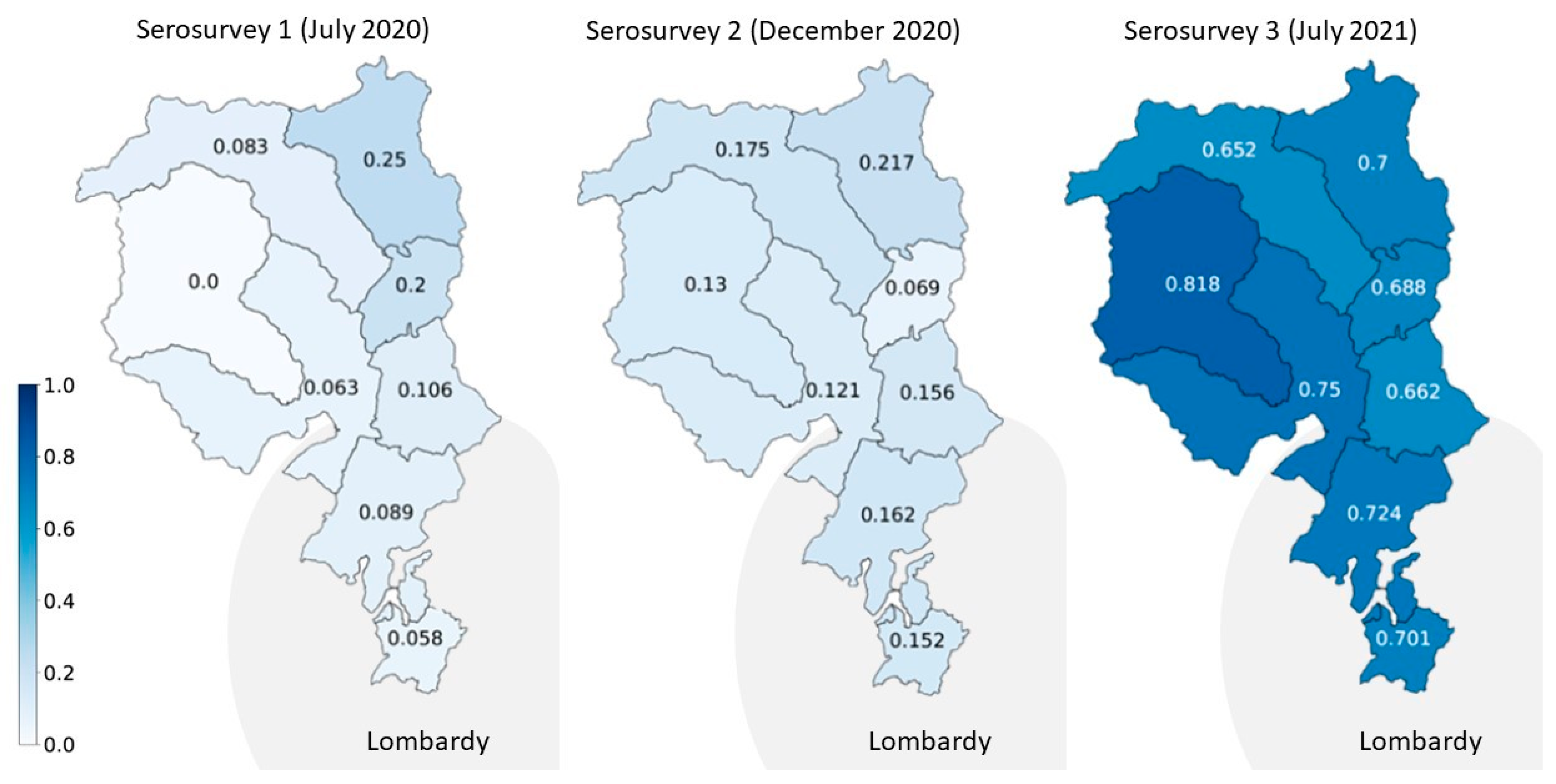
| Serosurvey 1 (July 2020) | Serosurvey 2 (November–December 2020) | Serosurvey 3 (June–July 2021) | ||||
|---|---|---|---|---|---|---|
| Number Tested (Positives) | Seroprevalence (95% CI) | Number Tested (Positives) | Seroprevalence (95% CI) | Number Tested (Positives) | Seroprevalence (95% CI) | |
| Overall | 646 (54) | 7.8% (5.4–10.4) | 1457 (211) | 20.2% (16.4–24.4) | 885 (618) | 72.5% (69.1–76.4) |
| Age group | ||||||
| 5–13 | - | - | 349 (53) | 14.8% (11.0–19.0) | 166 (53) | 35.1% (28.2–42.4) |
| 14–19 | - | - | 284 (52) | 17.7% (13.3–22.9) | 101 (46) | 46.5% (36.5–56.6) |
| 20–64 | 646 (54) | 7.8% (5.4–10.4) | 210 (56) | 26.3% (20.4–33.7) | 300 (214) | 71.4% (66.1–76.7) |
| 65+ | - | - | 614 (50) | 7.2% (4.6–9.9) | 318 (305) | 95.6% (92.8–97.8) |
| Sex | ||||||
| Female | 371 (30) | 7.9% (5.1–11.1) | 787 (104) | 17.8% (14.7–22.3) | 452 (307) | 70.5% (65.9–74.9) |
| Male | 275 (24) | 7.7% (4.5–11.4) | 670 (107) | 22.7% (18.1–28.6) | 433 (311) | 74.5% (70.1–78.8) |
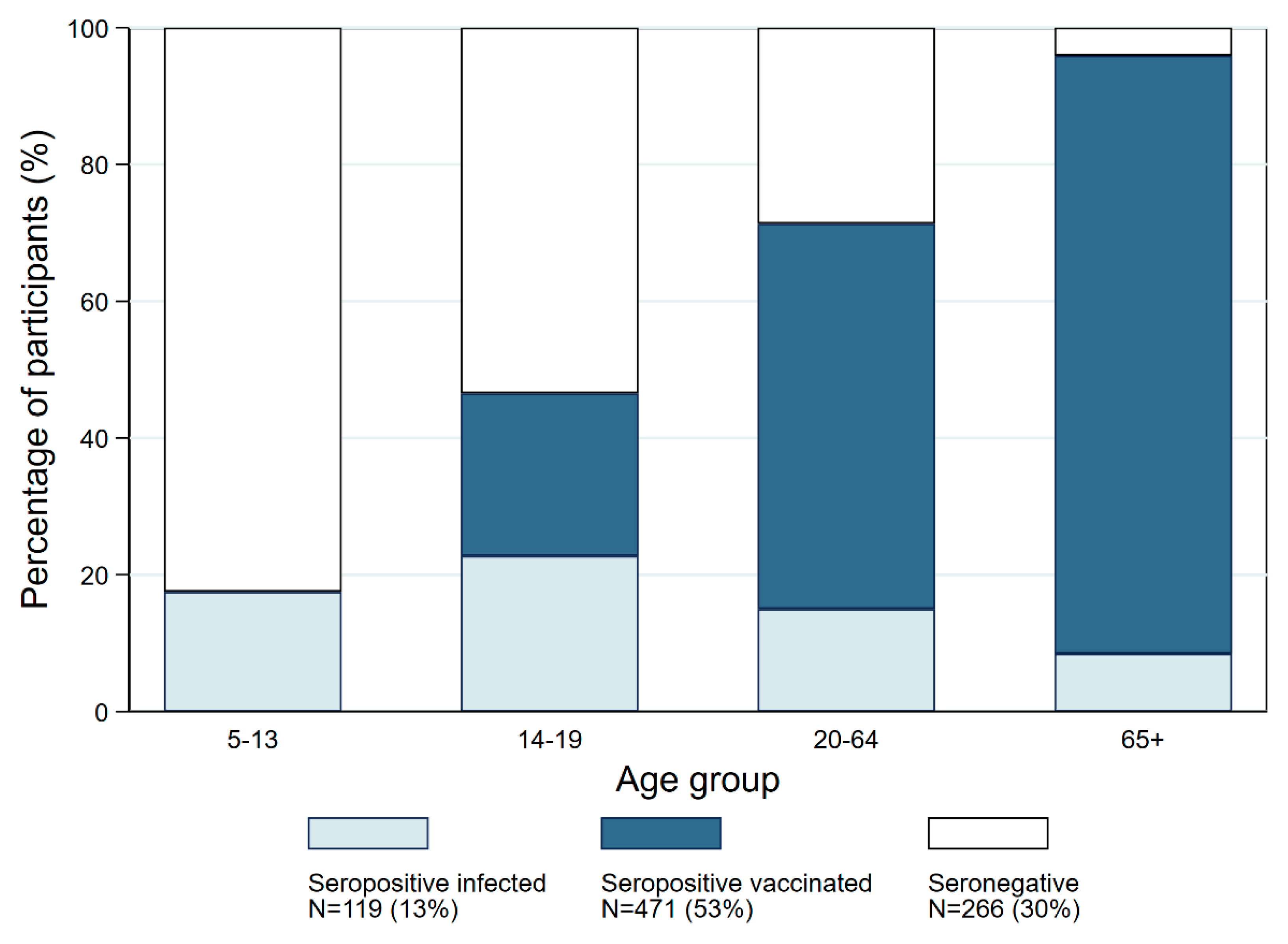
| Age Group | |||||
|---|---|---|---|---|---|
| 5–13 | 14–19 | 20–64 | 65+ | Total | |
| Seronegatives | 113 (82.5) | 54 (53.5) | 86 (28.7) | 13 (4.1) | 266 (31.1) |
| Infection-induced seropositives | 24 (17.5) | 23 (22.8) | 45 (15.0) | 27 (8.5) | 119 (13.9) |
| Vaccine-induced seropositives | 0 (0) | 24 (23.8) | 169 (56.3) | 278 (87.4) | 471 (55.1) |
| Ratio of infection- to vaccine-induced seropositivity | 0.00 | 1.1 | 3.7 | 10.3 | 3.9 |
| TOTAL | 137 | 101 | 300 | 318 | 856 |
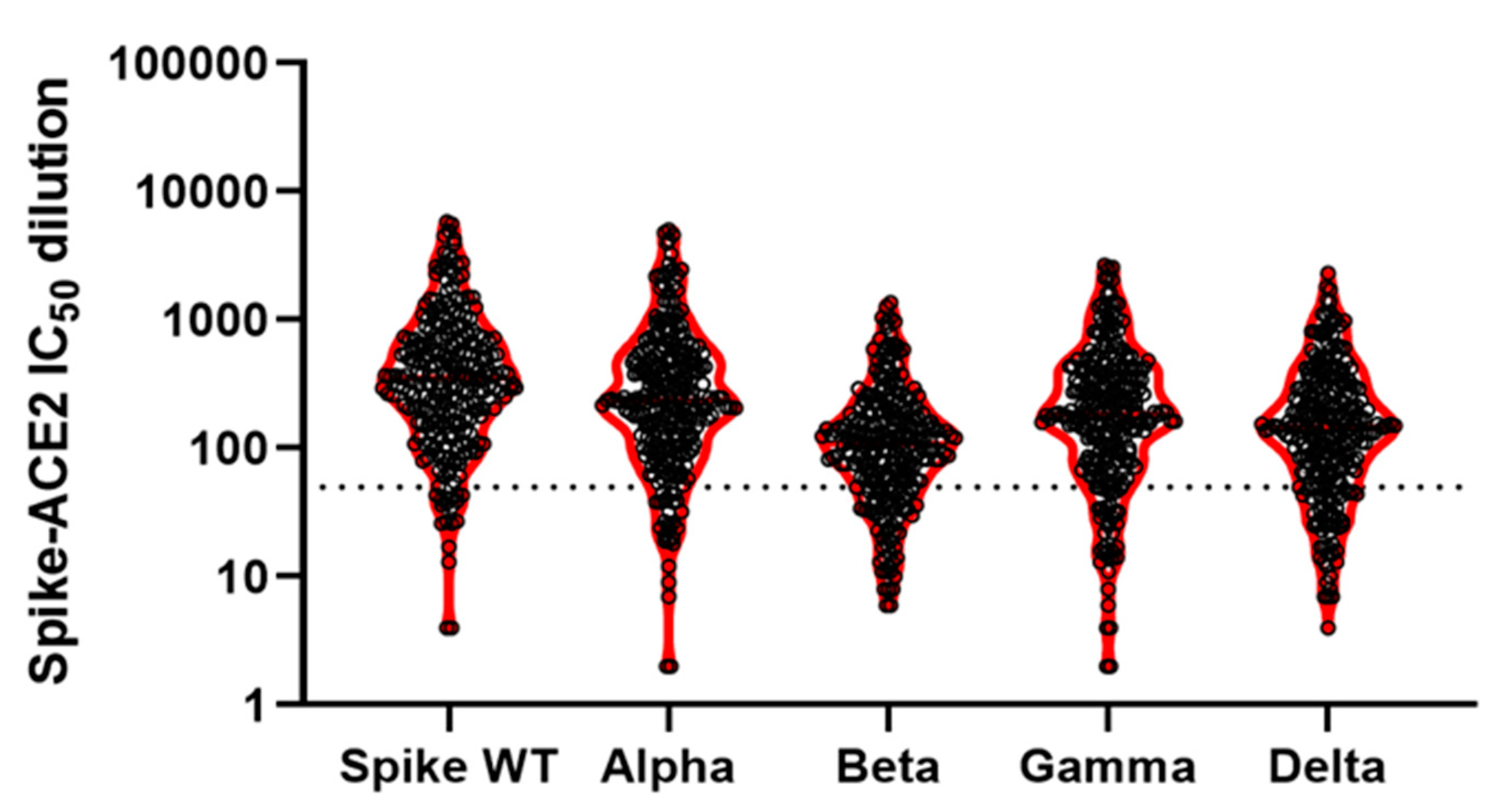
| Anti-Spike Antibodies a | Anti-NuC Antibodies a | Spike Wild Type b | Alpha b | Beta b | Gamma b | Delta b | ||
|---|---|---|---|---|---|---|---|---|
| All | Mean: | 85 | 2 | 651 | 500 | 172 | 339 | 247 |
| N positive: | 250 | 29 | 232 | 223 | 192 | 212 | 196 | |
| % positive: | 100 | 12 | 93 | 89 | 77 | 85 | 78 | |
| N total: | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amati, R.; Piumatti, G.; Franscella, G.; Buttaroni, P.; Camerini, A.-L.; Corna, L.; Levati, S.; Fadda, M.; Fiordelli, M.; Annoni, A.M.; et al. Trajectories of Seroprevalence and Neutralizing Activity of Antibodies against SARS-CoV-2 in Southern Switzerland between July 2020 and July 2021: An Ongoing, Prospective Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 3703. https://doi.org/10.3390/ijerph20043703
Amati R, Piumatti G, Franscella G, Buttaroni P, Camerini A-L, Corna L, Levati S, Fadda M, Fiordelli M, Annoni AM, et al. Trajectories of Seroprevalence and Neutralizing Activity of Antibodies against SARS-CoV-2 in Southern Switzerland between July 2020 and July 2021: An Ongoing, Prospective Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2023; 20(4):3703. https://doi.org/10.3390/ijerph20043703
Chicago/Turabian StyleAmati, Rebecca, Giovanni Piumatti, Giovanni Franscella, Peter Buttaroni, Anne-Linda Camerini, Laurie Corna, Sara Levati, Marta Fadda, Maddalena Fiordelli, Anna Maria Annoni, and et al. 2023. "Trajectories of Seroprevalence and Neutralizing Activity of Antibodies against SARS-CoV-2 in Southern Switzerland between July 2020 and July 2021: An Ongoing, Prospective Population-Based Cohort Study" International Journal of Environmental Research and Public Health 20, no. 4: 3703. https://doi.org/10.3390/ijerph20043703
APA StyleAmati, R., Piumatti, G., Franscella, G., Buttaroni, P., Camerini, A.-L., Corna, L., Levati, S., Fadda, M., Fiordelli, M., Annoni, A. M., Bezani, K., Amendola, A., Fragoso Corti, C., Sabatini, S., Kaufmann, M., Frei, A., Puhan, M. A., Crivelli, L., Albanese, E., & on behalf of the Corona Immunitas Ticino Study Group. (2023). Trajectories of Seroprevalence and Neutralizing Activity of Antibodies against SARS-CoV-2 in Southern Switzerland between July 2020 and July 2021: An Ongoing, Prospective Population-Based Cohort Study. International Journal of Environmental Research and Public Health, 20(4), 3703. https://doi.org/10.3390/ijerph20043703








