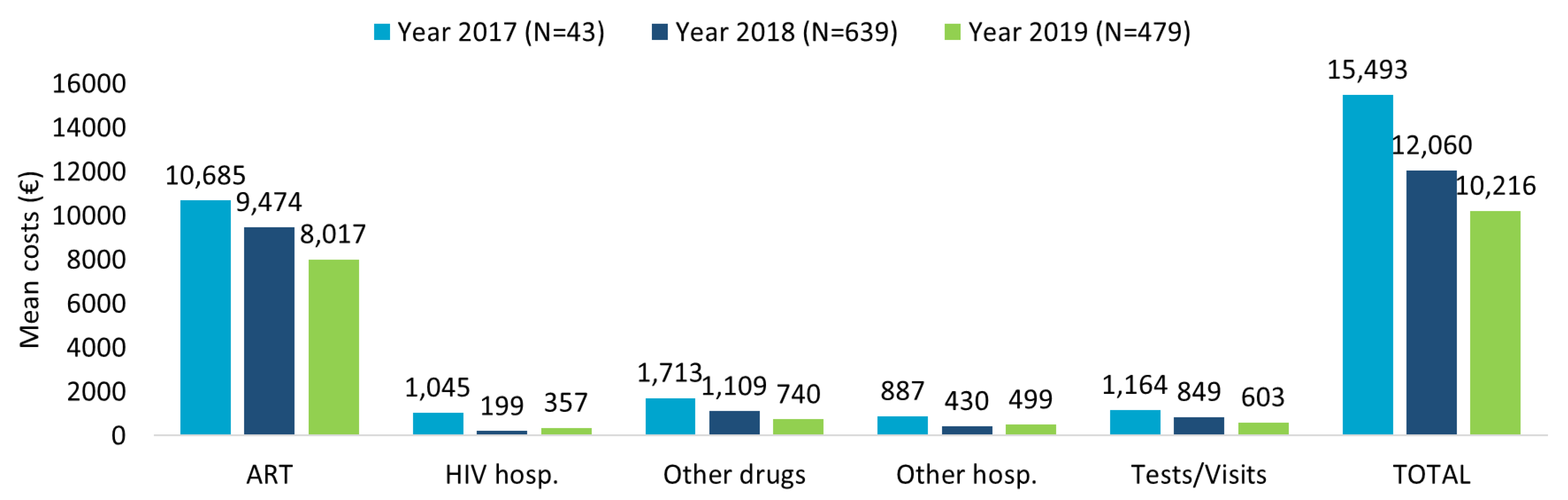Abstract
This real-world analysis conducted on administrative databases of a sample of Italian healthcare entities was aimed at describing the role of therapeutic pathways and drug utilization in terms of adherence, persistence, and therapy discontinuation in HIV-infected patients under antiretroviral therapies (ART) and Tenofovir Alafenamide (TAF)-based regimens on healthcare resource consumption and related direct healthcare costs. Between 2015 and 2019, adults (≥18 years) prescribed with TAF-based therapies were identified and characterized in the year prior to the first prescription (index-date) for TAF-based therapies and followed-up until the end of data availability. Overall, 2658 ART-treated patients were included, 1198 of which were under a TAF-based regimen. TAF-based therapies were associated with elevated percentages of adherence (83.3% patients with proportion of days covered, PDC > 95% and 90.6% with PDC > 85%) and persistence (78.5%). The discontinuation rate was low in TAF-treated patients, ranging from 3.3% in TAF-switchers to 5% in naïve. Persistent patients had lower overall mean annual healthcare expenditures (EUR 11,106 in persistent vs. EUR 12,380 in non-persistent, p = 0.005), and this trend was statistically significant also for costs related to HIV hospitalizations. These findings suggest that a better therapeutic management of HIV infection might result in positive clinical and economic outcomes.
1. Introduction
The development and introduction of antiretroviral therapies (ART) and then the availability of combination ART in the clinical practice has dramatically changed the course of human immunodeficiency virus (HIV) infection from an acute to a chronic disease [1]. Nowadays, life expectancy for people living with HIV (PLWH) is up to that of the general population in those patients who receive a prompt diagnosis and early treatment and achieve adequate viral-load suppression [2]. International guidelines strongly recommend initiating an ART combination soon after diagnosis in accordance with patient needs, attitudes, and readiness [3,4]. The main goal of ART is to inhibit viral replication, to control the progression of HIV-infection into AIDS, to improve the overall clinical outcomes, to prevent the development of antiretroviral resistance, and to reduce HIV transmission [5].
According to the World Health Organization (WHO), adherence is defined as “the extent to which a person’s behaviour—taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider” [6]. Adherence to ART plays a key role in maximizing the viral suppression. Historically, the threshold for adherence was set at ≥95% based on studies conducted on the early era of ART [7,8]. With the second generation of ART, the cut-off level of adherence needed to achieve HIV viral suppression is shifting to lower values such as 80–85% that still allow to achieve a successful immunosuppression [7,9,10,11]. Nevertheless, long-lasting worldwide experience has shown that maintaining an optimal level of adherence to ART is still an unsolved clinical problem [12]. Thus, improving adherence to ART represents an important goal for both patients’ outcomes and healthcare savings in terms of resource utilization and related costs [13]. Among the most widely used ART combinations for PLWH, the Tenofovir Alafenamide (TAF)-based regimen has been shown to achieve a successful viral load suppression, but the results can be influenced by drug administration schedule. Complex regimens often remain among the few therapeutic options for PLWH with previous ART failure or resistance, but they are associated with an increased rate of adverse events and drug–drug interactions, ultimately leading to a higher risk of discontinuation. Conversely, ART simplification could lead to improved adherence levels, lower discontinuation rates, and higher patient satisfaction, feasibly due to the advantage of taking a single pill [14]. Therapeutic persistence, defined as the time that a patient remains on ART before interrupting or switching to another regimen, is also a crucial factor to ensure successful management of HIV, and the discontinuation of therapy has been related not only to lack of sustained viral suppression but also to toxicity or simplification independently from the level of adherence [15,16]. The progress made with HIV therapy is reflected on the shifting paradigm of the economic burden of HIV care, which was previously due mostly to hospitalization and management of opportunistic illness. The introduction of potent combinations of ART that elongated the life expectancy of PLWH made a substantial contribution in decreasing HIV-related hospitalizations and made HIV one of the most expensive chronic diseases, driven mostly by drug costs [17].
This real-world analysis was aimed at describing the demographic and clinical characteristics of patients treated with ART and evaluating therapeutic pathways and drug utilization. A special focus was placed on adherence, persistence, and therapy discontinuation of Tenofovir Alafenamide (TAF)-based regimens either as multi-tablet regimens (MTR) or single-tablet regimens (STR) among naïve patients and those with previous ART who switched for the first time to TAF-based regimens and the resulting impact on healthcare resource consumptions and related direct costs for the Italian National Health Service (INHS).
2. Materials and Methods
2.1. Data Source
The present retrospective observational analysis was based on data collected from the administrative databases of a sample of Italian Local Health Units (LHUs), covering around 5.52 million health-assisted subjects belonging to Apulia, Abruzzo, Molise, Campania, Lazio, Umbria, Liguria, Piedmont, and Veneto regions. The following databases were used for the analysis: (1) demographic database to obtain data on age, gender, and mortality; (2) pharmaceutical database for information related to drug prescriptions, such as anatomical-therapeutic chemical (ATC) code, number of packages, number of units per package, costs, and prescription date; (3) hospitalization database, including all hospitalization data with discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), diagnosis-related group (DRG), and DRG-related charge; and (4) outpatient specialist services database, reporting data on prescriptions, type, description of diagnostic tests, and specialist visits.
To guarantee patients’ privacy, an anonymous univocal numeric code (patient ID) was assigned to each health-assisted subject by the LHUs. The patient ID code allowed the electronic linkage between the databases. The anonymous code of the patient ensures the anonymity of the extracted data in full compliance with UE Data Privacy Regulation 2016/679 (“GDPR”) and Italian D.lgs. n. 196/2003, as amended by D.lgs. n. 101/2018. All the results of the analyses were produced as aggregated summaries, which could not be connected, either directly or indirectly, to individual patients.
2.2. Design of the Analysis
Population Included
All adult (≥18 years old) patients prescribed with ART between January 2015 and December 2019 were screened. Among them, patients with TAF-based therapies were identified during all inclusion period by presence of the following drugs: TAF (ATC code J05AF13); emtricitabine and tenofovir alafenamide (ATC code J05AR17); emtricitabine, tenofovir alafenamide, elvitegravir, and cobicistat (ATC code J05AR18); emtricitabine, tenofovir alafenamide, and rilpivirine (ATC code J05AR19); emtricitabine, tenofovir alafenamide, and bictegravir (ATC code J05AR20); and emtricitabine, tenofovir alafenamide, darunavir, and cobicistat (ATC code J05AR22). Patients with TAF-based therapies were defined as naïve if they did not present any ART prescription in the 12 months before starting TAF-based therapies and as TAF-switchers if they had switched from a non-TAF-based to a TAF-based regimen.
Patients who moved to another LHU during the period considered in the analysis or with just one ART prescription or with prescription gaps greater than 12 months between subsequent prescriptions were excluded from the analysis. The date of the first prescription for TAF detected within the inclusion period was defined as the index date. Patients’ clinical characteristics were evaluated during at least 12 months prior to the index date (characterization period), while patients were followed-up until the end of database availability, which was at least 12 months for each patient.
2.3. Variables Used in the Analysis
At baseline, the following comorbidities were evaluated: depression, respiratory disease, renal failure, alcohol/drugs abuse, cardiovascular disease, neoplastic diseases, diabetes, dyslipidemia, HBV/HCV, and hypertension (codes for identification are reported in Supplementary Table S1). Comorbidity profile was scored using a modified version of the Charlson Comorbidity Index (CCI) not accounting for HIV [18].
Drug utilization of TAF-based regimens was evaluated during follow-up. Adherence was measured as proportion of days covered (PDC), namely the ratio between the number of days of medication supplied and days of therapy, multiplied by 100 [19]. To define adherent patients, two thresholds were selected: the most conservative one, i.e., PDC > 95%, and a PDC > 85%, which represents a newly hypothesized threshold for adherence in view of novel ART entering the market [8]. Persistence to TAF-based regimens was defined as presence of TAF-based regimens prescriptions or any ART in the last quarter of 1-year follow-up. Discontinuation was defined as absence of any ART in the last quarter of follow-up. Switch was identified as a change from TAF-based to a not-TAF-based regimen during follow-up. Changes to a different TAF-based scheme or from TAF-MTR to TAF-STR were not considered a switch of therapy. The analysis of healthcare resource consumptions was reported as mean annual number of the following items for each alive patient: the mean annual number of prescription for HIV-related drugs, mean annual number of other medications, mean annual number of HIV-related hospitalization (DRG 488, 489, 490), mean annual number of hospitalizations for other causes, and mean annual number of outpatient specialist services (visits/diagnostic tests). The related direct healthcare costs in euros (EUR) sustained by INHS were retrieved from the administrative flows of the LHUs involved, which collect data for reimbursement purposes. The mean annual healthcare costs per patient were calculated as overall mean annual costs and divided by cost item, namely drug treatment (HIV-related drugs and other drugs), hospital admissions (HIV-related hospitalization and other hospitalizations), and outpatient specialist services. Healthcare resource consumptions and costs were stratified by persistence and level of adherence. Outliers (patients for whom total cost exceeds the mean cost plus 3-fold standard deviation) were excluded from cost analysis. Drug costs were calculated based on the purchase price by INHS. Hospitalization costs were determined using DRG tariffs. The costs of outpatient services (visits/tests) were defined according to tariffs applied by each region.
2.4. Statistical Analysis
Continuous variables are reported as mean ± standard deviation and categorical variables as numbers and percentages. A generalized linear model (GLM) with gamma distribution and identity link function was developed to assess the relationship between adherence level and non-ART costs. Statistical significance was accepted at p < 0.05. All the analyses were performed using Stata SE version 12.0 (StataCorp, College Station, TX, USA).
3. Results
Overall ART-Treated Population
A total of 2658 adult patients treated with ART were included in the analysis, and their demographic and clinical characteristics in the overall population and by calendar year are shown in Table 1.

Table 1.
Demographic and clinical characteristics of the overall ART-treated patients and divided by calendar year. Continuous variables are reported as mean ± standard deviation and categorical variables as numbers and percentages in brackets.
In the total inclusion period, the mean age was 48.6 years, with the majority of patients in the age range of 36–55 years (61.6%). Male gender was more represented (71.6%). Analyzing the demographic characteristics by each year of inclusion, the male gender remained more frequent (71–73%), and a slight increase in age was noticed over the years. In the overall inclusion period, the most frequent comorbidities were hypertension (19.5%), followed by respiratory disease (15.9%) and dyslipidemia (11.2%) and mild average CCI (0.2) with 87.2% of patients with CCI score 0. The pattern of comorbidity distribution analyzed by each calendar year mirrored that of the total inclusion period.
Patients Treated with TAF-Based Regimens
The analysis focused on 1198 patients prescribed a TAF-based regimen during inclusion period. Table 2 provides the demographic and clinical characteristics of overall TAF-treated patients and in subgroups divided into MTR (N = 514, 43%), STR (N = 684, 57%), naïve (N = 478, 40%), and TAF-switchers (N = 720, 60%).

Table 2.
Demographic and clinical characteristics of patients with TAF-based regimen (overall and stratified by MTF/STR and naïve/switchers). Continuous variables are reported as mean ± standard deviation and categorical variables as numbers and percentages in brackets.
Adherence and persistence in the subgroups and in the total sample population were then investigated. In detail, the proportion of patients adherent to TAF-based regimens was 83.3% with a PDC threshold above >95%; specifically, it was higher among TAF-switchers (85.6%), followed by TAF-STR (84.4%), TAF-MTR (81.9%), and naïve (79.9%). The proportion of adherent patients with PDC > 85% was 90.6% (92.8% among TAF-switchers, 90.9% in TAF-STR, 90.1% in TAF-MTR, and 87.2% in naïve). Data on persistence revealed that the proportion of patients persistent to TAF-based regimens was 78.5% and reached 96% when persistence to any ART was analyzed. Switch to another ART not TAF-based occurred in 18% of patients, and 4% discontinued the therapy. The stratification in subgroups confirmed in general this overall trend, in particular for the rate of discontinuation that ranged between 3.3% (TAF-switchers) and 5% (naïve). Of note, patients under MRT regimen compared to those with STR showed a greater tendency to switch (30.4% vs. 8.8%, respectively) and a lower persistence on TAF-based therapy (66.5% vs. 87.4%, respectively). Overall persistence for TAF naïve patients was 74.3%.
The healthcare resource consumption during first year of follow-up for TAF-based regimes patients evaluated in the last 3 years of inclusion (2017–2018–2019) showed the highest numbers for ART prescriptions and specialistic visits/diagnostic tests, followed by other drug prescriptions, with a decreasing trend over time (Supplementary Table S2A). The related mean annual total costs per patient are consistent with the data of healthcare resource consumption (Figure 1). A decreasing trend over the years was observed, with mean annual total costs per patients of EUR 15,493 for patients included in 2017, EUR 12,060 for those included in 2018, and EUR 10,216 for those included in 2019, mostly burdened by ART expenses.
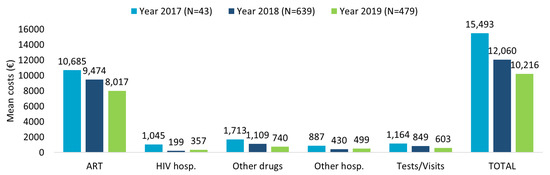
Figure 1.
Mean annual health care costs (EUR) in patients with TAF-based regimen during first year of follow-up for the inclusion years 2017–2018–2019.
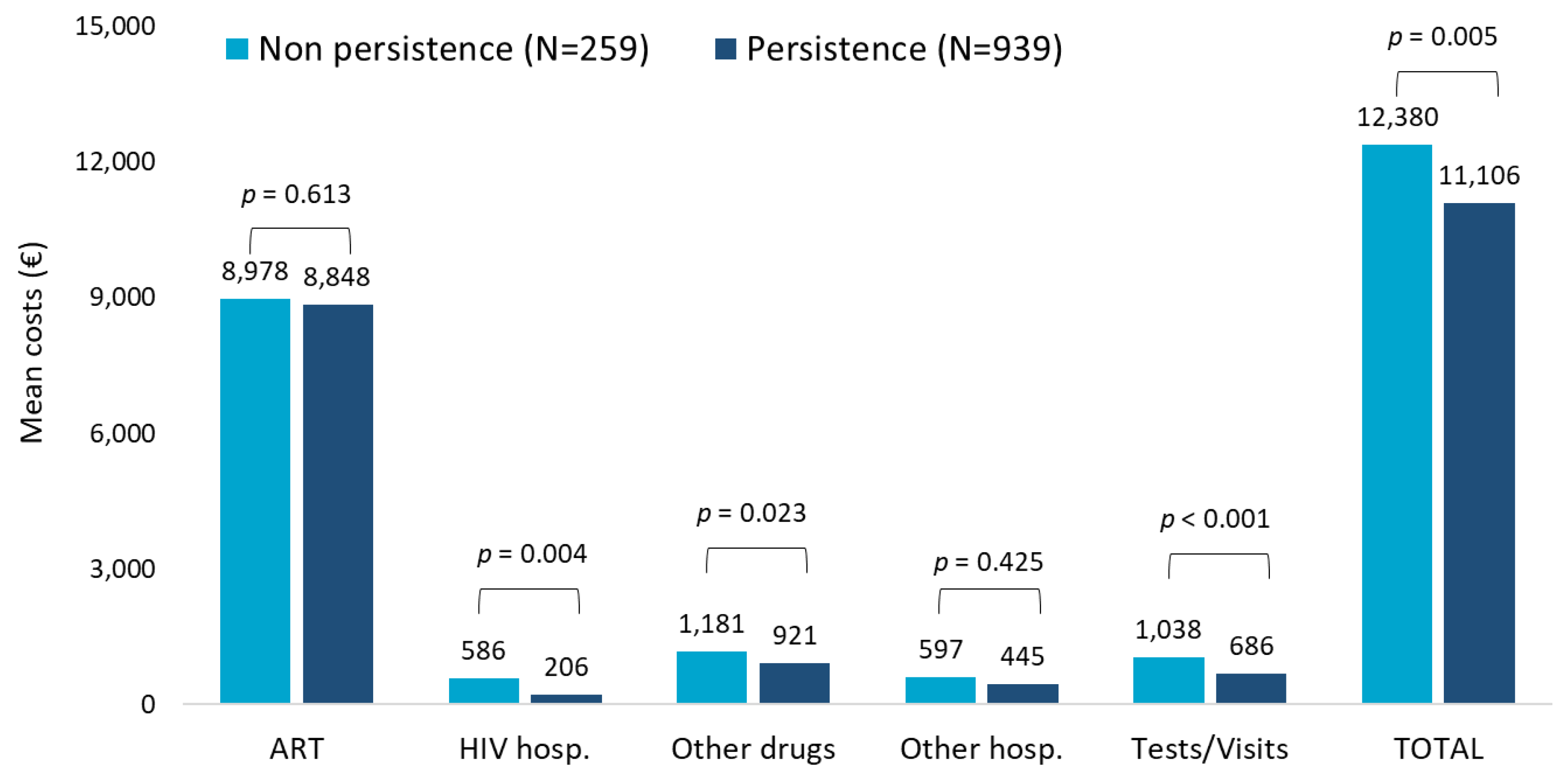
The evaluation of the role of persistence on healthcare resources consumptions during the first year of follow-up in patients with TAF-based regimen revealed that the most relevant consumptions were attributable to ART prescriptions and specialistic visits/diagnostic tests and, lastly, other drug prescriptions to a higher extent in non-persistent patients (Supplementary Table S2B). Regarding the cost analysis shown in Figure 2, patients persistent to TAF-based therapies had significantly lower mean annual costs than the non-persistent ones (EUR 11,106 vs. EUR 12,380, p = 0.005), and this difference was mostly appreciable in the cost item related to HIV hospitalization. Moreover, when the analysis was focused only on patients with HIV-related hospitalization, the mean costs were found to be higher for non-persistent patients (N = 25) compared to persistent ones (N = 79) (EUR 5996 and EUR 2448, respectively; data not shown).

Figure 2.
Mean annual health care costs (EUR) during first year of follow-up for patients with TAF-based regimen based on persistence.
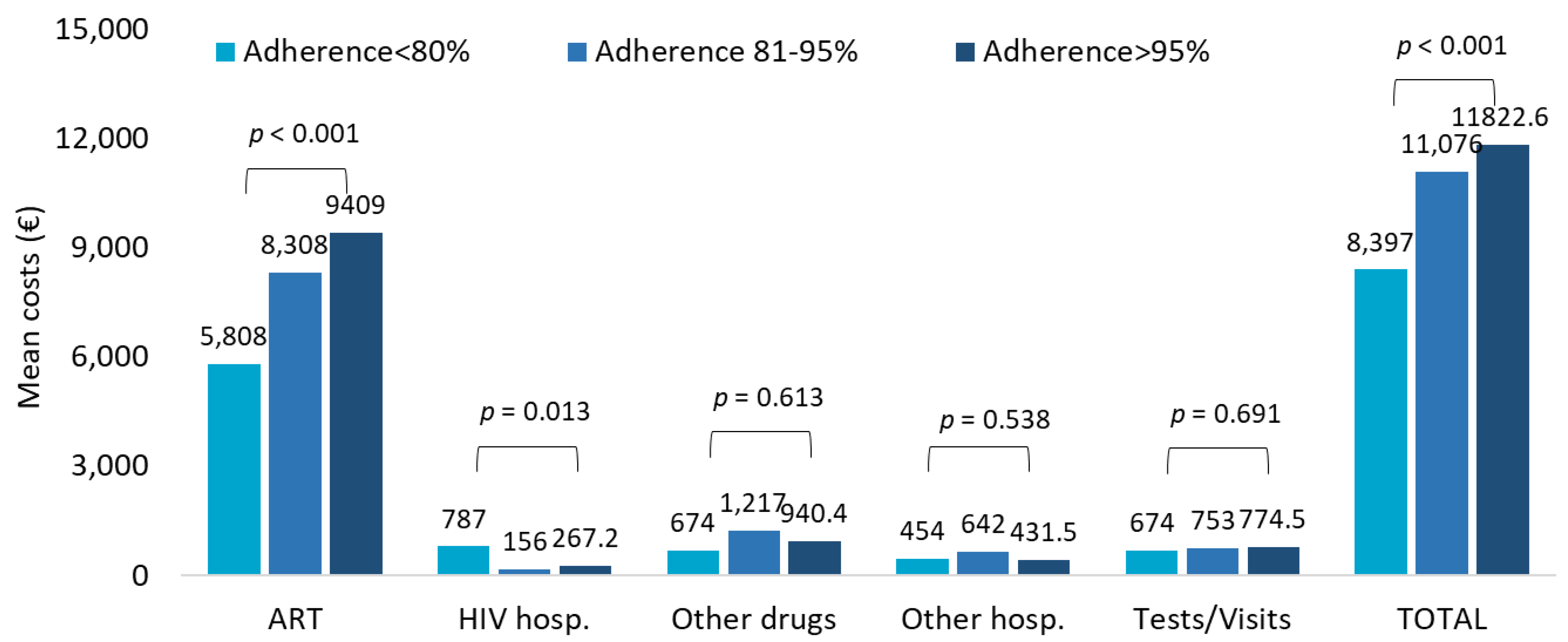
The healthcare resources consumption in TAF-treated patients stratified by adherence <80%, 81–95%, and >95% indicated ART prescriptions and specialistic visits/diagnostic tests as the most impactive items (Supplementary Table S2C). Consistently, during the first year of follow-up, from PDC < 80% to PDC > 95%, the total costs tended to rise, but this effect was mostly due to the higher burden of drug expenses that reflect the growing consumption with increasing adherence (Figure 3).

Figure 3.
Mean annual health care costs (EUR) during first year of follow-up for patients with TAF-based regimen based on adherence.
Healthcare resources consumptions in non-adherent (PDC < 80%) patients treated with ART other than TAF and TAF-based regimens highlighted that even in non-adherent patients, the majority of consumptions were imputable to ART prescriptions and specialistic visits/diagnostic tests although less markedly in the patients receiving a TAF combination (Supplementary Table S2D).
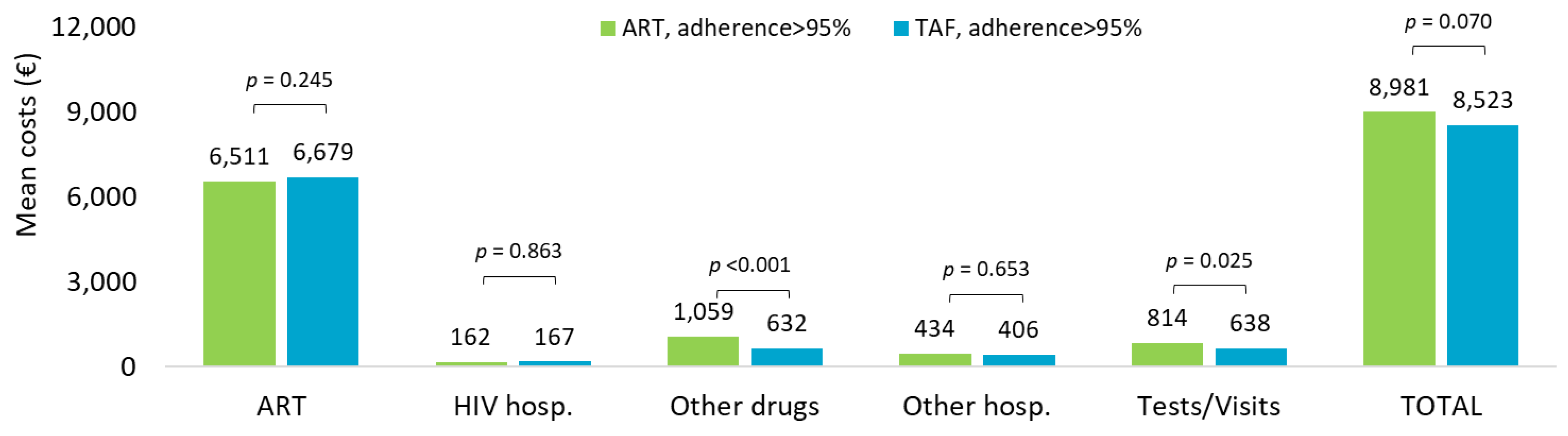
Cost analysis extended to the overall observation period in ART- and TAF-based regimens is shown in Figure 4. The mean annual healthcare direct costs by adherence (PDC > 95%) confirmed that the overall costs tended to be higher in ART-adherent compared to TAF-adherent treatment (EUR 8981 vs. EUR 8523, p = 0.070, nearly significant), mostly driven by the expenses for other drugs (EUR 1059 vs. EUR 632, p < 0.001) and specialistic visits and outpatient services (EUR 814 vs. EUR 638, p = 0.025).
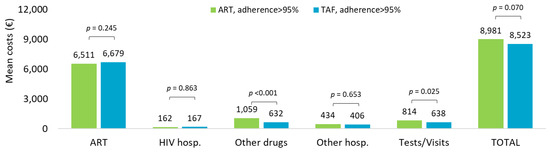
Figure 4.
Mean annual health care costs (EUR) during all available follow-up for patients with ART- and TAF-based regimens and based on adherence (PDC > 95%).
The GLM model showed that the mean yearly non-ART costs increased with older age, specifically by EUR 1364 in patients aged between 51 and 65 years (p < 0.001) and EUR 2265 in those over 65 (nearly significant, p = 0.079). A worse comorbidity profile was also significantly predictive of higher non-ART costs (EUR 1736 for CCI unit increment, p = 0.027) (Supplementary Table S3).
4. Discussion
The current awareness of the management of HIV patients chronically treated with ART and the subsequent rebounds on healthcare resource consumptions and expenditures is highly variable across the different countries [20,21,22].
The present analysis was conducted in a setting of real-life clinical practice in Italy to describe the state-of-art of HIV-infected patients treated with ART and more specifically with TAF-based regimens, evaluate therapeutic pathways and drug utilization, and give special attention to the impact of adherence, persistence, and therapy discontinuation of TAF-based regimens on HIV management and direct healthcare costs.
The demographic characteristics of the overall ART population reported a male predominance and a mean age of around 49 years, which is in line with other Italian real-world studies on HIV [1,23]. Hypertension and dyslipidemia were among the most frequent comorbidities detected; a French study also observed these two conditions to be highly prevalent in PLWH [24]. We then focused on the 1198 patients prescribed with TAF-based regimen during the inclusion period in order to investigate in this specific group the management of treatments in terms of adherence, persistence, and therapy discontinuation and ultimately how their drug utilization could affect healthcare consumptions and costs.
There is large body of evidence from national and international studies that, despite the undeniable progression and growing availability of pharmacological options for HIV patients, the goal of maintaining an adequate therapy adherence in the long term is still far from being reached [25]. This point deserves great efforts from the scientific community since adherence to ART therapy has essential repercussions on viral load control and thus patients’ clinical outcomes but also on the consequent savings in terms of healthcare resource consumptions and expenditures for the national health systems [17]. In our study, a higher proportion of adherent patients was found among patients with STR rather than MTR regimens, which is in line with the evidence reported in the literature on higher adherence to STR than MTR [13,26,27]. Interestingly, the comparison between naïve and TAF-switchers showed greater adherence levels in switchers regardless of the cutoff considered (PDC > 95% or PDC > 85%). This is in line with another real-world study in which patients naïve to ART were found less likely to be adherent [28]. Furthermore, a meta-analysis showed that treatment-experienced patients had the highest pooled odds ratio for optimal adherence vs. suboptimal even though this tendency was not statistically significant [29]. Older studies based on first-generation ART suggested that adherence could be unchanged or decreased after change of therapy and that ART switch should trigger intervention to reassess adherence [30], while most recent studies have observed an increase in the number of patients with improved adherence after switching to a second line [31].
Persistence to TAF-based regimens mirrored the trend observed for adherence, with a high proportion of persistent patients among those with STR or switchers. Sutton et al [32] found in a cohort of U.S veterans a proportion of patients persistent to TAF-based regimens ranging from 64–73% for STR and 46–58% for MTR. These values, however, referred to naïve patients, and this could explain the difference with our findings. Indeed, naïve patients had a trend of less persistence than those who were experienced (i.e., switchers to TAF).
To the best of our knowledge, there are limited data on the actual economic burden for patients with TAF-based regimens in Italy, while the literature reports an estimation of cost-effectiveness or budget-impact analysis on such therapies [33,34]. The latter estimated a direct healthcare cost for year 2018 spanning from EUR 8928 to EUR 11,200, which, although lower than our results, is, however, based in real-life settings [33].
In all the scenarios depicted, cost analysis revealed that during the first year of follow-up, the weightiest item on the annual healthcare direct cost per patient was that for ART, which is consistent with previously published data for European countries [35,36,37].
The downward trend observed over the last 3 years of inclusion was mainly due to a halving in the costs of other drugs, hospitalizations, and outpatients’ services through the years, suggesting that the introduction of innovative ART on the market could influence the costs for the management of patients. Persistence to TAF-based therapies was associated with significantly lower mean annual costs, in particular for the cost item related to HIV hospitalization. Moreover, the comparison of mean annual healthcare direct costs by adherence (PDC > 85% vs. PDC < 85%) in TAF-based regimen revealed that increased costs not related to ART were observed in non-adherent patients; once again, this trend was evident for HIV hospitalizations. In this regard, Toh et al. [38] explored the economic burden of AIDS-defining illnesses, which are still a major threat for PLWH despite the improvement of life expectancy thanks to ART: the authors found that improved level of adherence is related to an increase in the medical costs but could lead to savings from lower incidence rates of AIDS-defining illnesses and the related costs they carry [38].
Considering the mean annual health care resource consumptions and costs during all available follow-up in TAF-treated patients with respect to adherence, we found tendentially increased consumptions and costs with growing adherence levels but this might be explained by the obviously higher burden of drug expenses in adherent patients, which is in line with the literature [17,38]. Multivariate analysis revealed that older age and more complex comorbidity profile were significant predictors of healthcare costs other than ART, adding to the growing body of evidence that aging is one of the upcoming challenges for HIV management and one of the factors that will contribute to increased HIV care costs [39]. Indeed, PLWH age while taking the life-long ART and therefore will have to address issues related to a multi-comorbidity profile such as polypharmacy, polydoctoring, and iatrogenic diseases [40].
These findings should be interpreted in the light of some limitations related to the observational retrospective design of the analysis, which was based on data extracted from administrative databases. Thus, there might be lacking or insufficient clinical information on a number of other potential confounders (i.e., disease severity, comorbidities, previous virological failures/resistance mutations) that may have influenced the results. Secondly, data on the reasons for changing regimens (switch or discontinuation) are not reported within the database as well as the causes of non-adherence since not-measurable variables as patient attitudes towards medication or social status are not present; similarly, adherence and persistence were evaluated based on the drug dispensed; therefore, the actual use by the patient is unknown. Ultimately, indirect costs or out-of-pocket costs could not be evaluated, as administrative databases contain data on healthcare resources reimbursed by INHS.
5. Conclusions
This real-world analysis provided an in-depth characterization of patients prescribed TAF-based regimens in terms of drug utilization and how this could have affected healthcare resource consumptions and costs. Higher levels of adherence and persistence to TAF-based regimens were detected among users of STR rather than MTR and among experienced patients that switched from an ART- to a TAF-based treatment compared to naïve patients.
A declining trend over the years in healthcare resource consumption and the related direct costs could be suggestive of the changing of costs related to the management of HIV patients due to the advent of novel therapeutic options. The total healthcare costs were mainly driven by ART-related costs, which represent almost 70% of the total expenditure. Patients that showed a higher level of TAF persistence and adherence were reported to have a reduced healthcare cost related to HIV hospitalizations compared to those who were non-persistent or not adherent to TAF-based therapies, suggesting that the optimization of the drug utilization may have a positive impact not only from a clinical point of view but from the perspective of the sustainability of the INHS as well.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20053789/s1, Table S1. Codes used for comorbidities identification. Table S2: Healthcare resources consumption in patients with TAF-based regimen during first year of follow-up by (A) year of inclusion (2017–2018–2019), (B) persistence, (C) adherence (PDC > 95%), and (D) non-adherence (PDC < 80% in patients treated with ART- vs. TAF-based regimens). Table S3. Generalized linear model (GLM) for predictors of mean annual non-ART healthcare costs (EUR) during first year of follow-up for patients with TAF-based regimens.
Author Contributions
Conceptualization, V.P., A.R., M.D. and L.D.E.; methodology, D.S.; validation, L.D.E. and F.M.; formal analysis, D.S.; investigation, V.P. and M.D.; resources, M.A., F.B., A.C. (Arturo Cavaliere), A.C. (Andrea Ciaccia), A.C. (Alessandro Chinellato), A.C. (Alberto Costantini), S.D., F.F., S.G., A.L., R.M., E.M., C.P., D.R. and F.S.; data curation, M.A., F.B., A.C. (Arturo Cavaliere), A.C. (Andrea Ciaccia), A.C. (Alessandro Chinellato), A.C. (Alberto Costantini), S.D., F.F., S.G., A.L., R.M., E.M., C.P., D.R. and F.S.; writing—original draft preparation, V.P. and M.D.; writing—review and editing, all authors; visualization, A.R., L.D.E. and F.M.; supervision, L.D.E. All authors have read and agreed to the published version of the manuscript.
Funding
Gilead Sciences S.r.l. purchased the study report that is the basis for this manuscript. This manuscript was developed with Gilead Sciences S.r.l. and CliCon S.r.l. Società Benefit. The views expressed here are those of the authors and not necessarily those of the supporters. The agreement signed by CliCon S.r.l. and Gilead Sciences S.r.l. does not create any entityship, joint venture, or any similar relationship between parties. CliCon S.r.l. is an independent company. Neither CliCon S.r.l. nor any of their representatives are employees of Gilead Sciences S.r.l. for any purpose. The journal fee was funded by Gilead Sciences S.r.l.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee) of Comitato Etico Lazio I (protocol 1080, approval date: 23 September 2020), Comitato Etico Interprovinciale Area I (protocol 140/CE/2020 and 141/CE/2020, approval date: 30 November 2020), Nucleo Ricerca Clinica ULSS Berica (protocol 106370, approval date: 5 November 2020), Comitato Etico Sezione Area Centro Regione Calabria (protocol 212, approval date: 23 June 2020), Comitato Etico Lazio II (protocol 0179046/2020, approval date: 28 October 2020, protocolo 0216084/2020 approval date 16 December 2020, protocol 0031200/2021, approval date 10 February 2021), Comitato Etico Azienda Sanitaria Regionale Molise (approval date 27 October 2020), Comitato Etico Per le Sperimentazioni Cliniche della Provincia di Vicenza (protocol 0036999, approval date 13 April 2021), Comitato Etico per la Sperimentazione Clinica della provincia di Venezia e IRCCS S. Camillo (28 July 2020), Comitato Etico Delle Province di Chieti e Pescara (protocol 7, approval date 18 March 2021), Comitato Etico per le province di L’Aquila e Teramo (protocol number 11, approval date 24 March 2021), Comitato Etico Regionale Umbria (protocol 19414/20/ON, approval date 16 September 2020), Comitato Indipendente di Etica Medica (protocol 48144, approval date 28 May 2021).
Informed Consent Statement
Informed consent was not required since obtaining it is impossible for organizational reasons (pronouncement of the Data Privacy Guarantor Authority, General Authorization for personal data treatment for scientific research purposes—n.9/2014).
Data Availability Statement
All data used for the current study are available upon reasonable request to CliCon S.r.l., which is the body entitled to data treatment and analysis by Local Health Units.
Conflicts of Interest
A.R. is an employee of Gilead Sciences S.r.l.; V.P., M.D., D.S., L.D.E. are employees of CliCon s.r.l. Società Benefit at the time of the analysis. CliCon s.r.l. is an independent company. The agreement signed by Clicon S.r.l. and Gilead Sciences Srl does not create any entity, joint venture or any similar relationship between parties. Neither CliCon S.r.l. nor any of their representatives are employees of Gilead Sciences Srl for any purpose.The remaining authors report no other conflicts of interest in this work.
References
- Seguiti, C.; Salvo, P.F.; Di Stasio, E.; Lamonica, S.; Fedele, A.L.; Manfrida, S.; Ciccarelli, N.; Corvari, B.; De Luca, C.; Tartaglione, L.; et al. Health-related quality of life (HRQoL) from HIV patients’ perspective: Comparison of patient-reported outcome (PRO) measures among people living with hiv (PLWH) and other chronic clinical conditions. J. Patient-Rep. Outcomes 2022, 6, 27. [Google Scholar] [CrossRef]
- Nakagawa, F.; Lodwick, R.K.; Smith, C.J.; Smith, R.; Cambiano, V.; Lundgren, J.D.; Delpech, V.; Phillips, A.N. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS 2012, 26, 335–343. [Google Scholar] [CrossRef]
- European Clinical AIDS Society (EACS). EACS Guidelines Version 11.1. 2022. Available online: https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf (accessed on 28 October 2022).
- Department of Health and Human Services Panel on Antiretroviral. Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv.A (accessed on 28 October 2022).
- Kim, J.; Lee, E.; Park, B.-J.; Bang, J.H.; Lee, J.Y. Adherence to antiretroviral therapy and factors affecting low medication adherence among incident HIV-infected individuals during 2009–2016: A nationwide study. Sci. Rep. 2018, 8, 3133. [Google Scholar] [CrossRef]
- Sabaté, E.; World Health Organization (Eds.) Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-154599-0.
- Castillo-Mancilla, J.R.; Haberer, J.E. Adherence Measurements in HIV: New Advancements in Pharmacologic Methods and Real-Time Monitoring. Curr. HIV/AIDS Rep. 2018, 15, 49–59. [Google Scholar] [CrossRef]
- Byrd, K.K.; Hou, J.G.; Hazen, R.; Kirkham, H.; Suzuki, S.; Clay, P.G.; Bush, T.; Camp, N.M.; Weidle, P.J.; Delpino, A. Antiretroviral Adherence Level Necessary for HIV Viral Suppression Using Real-World Data. J. Acquir. Immune Defic. Syndr. 2019, 82, 245–251. [Google Scholar] [CrossRef]
- O’Halloran Leach, E.; Lu, H.; Caballero, J.; Thomas, J.E.; Spencer, E.C.; Cook, R.L. Defining the optimal cut-point of self-reported ART adherence to achieve viral suppression in the era of contemporary HIV therapy: A cross-sectional study. AIDS Res. Ther. 2021, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Maggiolo, F.; Airoldi, M.; Kleinloog, H.D.; Callegaro, A.; Ravasio, V.; Arici, C.; Bombana, E.; Suter, F. Effect of Adherence to HAART on Virologic Outcome and on the Selection of Resistance-Conferring Mutations in NNRTI- or PI-Treated Patients. HIV Clin. Trials 2007, 8, 282–292. [Google Scholar] [CrossRef]
- Viswanathan, S.; Detels, R.; Mehta, S.H.; Macatangay, B.J.C.; Kirk, G.D.; Jacobson, L.P. Level of Adherence and HIV RNA Suppression in the Current Era of Highly Active Antiretroviral Therapy (HAART). AIDS Behav. 2015, 19, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Ortego, C.; Huedo-Medina, T.B.; Llorca, J.; Sevilla, L.; Santos, P.; Rodríguez, E.; Warren, M.R.; Vejo, J. Adherence to Highly Active Antiretroviral Therapy (HAART): A Meta-Analysis. AIDS Behav. 2011, 15, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Kangethe, A.; Polson, M.; Lord, T.C.; Evangelatos, T.; Oglesby, A. Real-World Health Plan Data Analysis: Key Trends in Medication Adherence and Overall Costs in Patients with HIV. J. Manag. Care Spec. Pharm. 2019, 25, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Cadiñanos, J.; Montejano, R.; de Miguel Buckley, R.; Marcelo, C.; Arribas, J.R. Risks and benefits of reducing the number of drugs to treat HIV-1 infection. Expert Opin. Drug Saf. 2021, 20, 397–409. [Google Scholar] [CrossRef]
- Nyaku, M.; Beer, L.; Shu, F. Non-persistence to antiretroviral therapy among adults receiving HIV medical care in the United States. AIDS Care 2019, 31, 599–608. [Google Scholar] [CrossRef]
- Lewis, J.M.; Smith, C.; Torkington, A.; Davies, C.; Ahmad, S.; Tomkins, A.; Shaw, J.; Kingston, M.; Muqbill, G.; Hay, P.; et al. Real-world persistence with antiretroviral therapy for HIV in the United Kingdom: A multicentre retrospective cohort study. J. Infect. 2017, 74, 401–407. [Google Scholar] [CrossRef]
- Taramasso, L.; Demma, F.; Bitonti, R.; Ferrazin, A.; Giannini, B.; Giacomini, M.; Beltramini, S.; Sasso, E.; Viscoli, C.; Di Biagio, A. How has the cost of antiretroviral therapy changed over the years? A database analysis in Italy. BMC Health Serv. Res. 2018, 18, 691. [Google Scholar] [CrossRef]
- Fraccaro, P.; Kontopantelis, E.; Sperrin, M.; Peek, N.; Mallen, C.; Urban, P.; Buchan, I.E.; Mamas, M.A. Predicting mortality from change-over-time in the Charlson Comorbidity Index: A retrospective cohort study in a data-intensive UK health system. Medicine 2016, 95, e4973. [Google Scholar] [CrossRef]
- Nau, D.P. Proportion of Days Covered (PDC) as a Preferred Method of Measuring Medication Adherence. Springf. VA Pharm. Qual. Alliance 2012, 6, 25. [Google Scholar]
- UNAIDS. Global AIDS Update 2022. Available online: https://www.unaids.org/en/Homepage (accessed on 28 October 2022).
- Chastek, B.; Anderson, A.; Webb, N.; Rock, M.; Gruber, M.J.; Majethia, S.; Zachry, W.; Cohen, J.; Colson, A. ART Regimen Persistence Among Treatment-Experienced Patients With HIV Switching to a MTR or STR Since 2018. Database 2010, 3, 2020. [Google Scholar]
- Brogan, A.; Garris, C.; Priest, J.; Divino, V.; He, J.; Chen, J.; De Koven, M. Single-Tablet Regimens (STR) Offer Better Persistence and Adherence, With Lower Costs by Adherence Status, Than Multiple-Tablet Regimens (MTR) for People Living With HIV (PLWH) Enrolled in Medicaid. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2022. [Google Scholar]
- Perrone, V.; Sangiorgi, D.; Buda, S.; Andretta, M.; Borrè, S.; Cattaruzzi, C.; Gasperini, G.; Lena, F.; Pisterna, A.; Esposti, L.D. Healthcare Resources Use in Patients with Human Immunodeficiency Virus (HIV). Real-World Evidence From Six Italian Local Health Units. Farmeconomia Health Econ. Ther. Pathw. 2018, 19, 37–45. [Google Scholar] [CrossRef]
- Pourcher, V.; Gourmelen, J.; Bureau, I.; Bouee, S. Comorbidities in people living with HIV: An epidemiologic and economic analysis using a claims database in France. PLoS ONE 2020, 15, e0243529. [Google Scholar] [CrossRef]
- King, K.; Horne, R.; Cooper, V.; Glendinning, E.; Michie, S.; Chalder, T. SUPA Group The development of an intervention to support uptake and adherence to antiretroviral therapy in people living with HIV: The SUPA intervention. A brief report. Transl. Behav. Med. 2022, 12, ibab104. [Google Scholar] [CrossRef]
- Cohen, J.; Beaubrun, A.; Bashyal, R.; Huang, A.; Li, J.; Baser, O. Real-world adherence and persistence for newly-prescribed HIV treatment: Single versus multiple tablet regimen comparison among US medicaid beneficiaries. AIDS Res. Ther. 2020, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Scott Sutton, S.; Magagnoli, J.; Hardin, J.W. Impact of Pill Burden on Adherence, Risk of Hospitalization, and Viral Suppression in Patients with HIV Infection and AIDS Receiving Antiretroviral Therapy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Kipsang, J.; Chen, J.; Tang, C.; Li, X.; Wang, H. Self reported adherence to antiretroviral treatment and correlates in Hunan province, the Peoples Republic of China. Int. J. Nurs. Sci. 2018, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Bezabhe, W.M.; Chalmers, L.; Bereznicki, L.R.; Peterson, G.M. Adherence to Antiretroviral Therapy and Virologic Failure. Medicine 2016, 95, e3361. [Google Scholar] [CrossRef]
- Loren, G.M.; Carol, E.G.; Ron, D.H.; Honghu, L.C.; Keith, B.; Andrew, H.K.; Neil, S.W. Impact of Antiretroviral Regimen Switches on Adherence. HIV Clin. Trials 2002, 3, 355–360. [Google Scholar] [CrossRef]
- Moosa, A.; Gengiah, T.N.; Lewis, L.; Naidoo, K. Long-term adherence to antiretroviral therapy in a South African adult patient cohort: A retrospective study. BMC Infect. Dis. 2019, 19, 775. [Google Scholar] [CrossRef]
- Sutton, S.S.; Wang, X.; Diaz-Cuervo, H.; Magagnoli, J. Persistence of antiretroviral therapy regimens among veterans with HIV newly initiating treatment in the US. In Proceedings of the International Congress on Drug Therapy in HIV Infection, Glasgow, UK, 5–8 October 2020; p. 1. Available online: https://www.hivglasgow.org/wp-content/uploads/2020/11/P032_Sutton.pdf (accessed on 31 October 2022).
- Garagiola, E.; Foglia, E.; Ferrario, L.; Cenderello, G.; Di Biagio, A.; Menzaghi, B.; Rizzardini, G.; Croce, D. HTA and HIV: The Case of Dual NRTI Backbones in the Italian Setting. Int. J. Environ. Res. Public. Health 2020, 17, 9010. [Google Scholar] [CrossRef]
- Pradelli, L.; Di Perri, G.; Rizzardini, G.; Martelli, E.; Giardina, S.; Povero, M. A cost-effectiveness analysis of E/C/F/TAF vs three boosted regimens in the Italian context. Farmeconomia Health Econ. Ther. Pathw. 2017, 18, 113–127. [Google Scholar] [CrossRef]
- Valbert, F.; Wolf, E.; Schewe, K.; Klauke, S.; Hanhoff, N.; Hoffmann, C.; Preis, S.; Pahmeier, K.; Wasem, J.; Neumann, A. Cost of Human Immunodeficiency Virus (HIV) and Determinants of Healthcare Costs in HIV-Infected Treatment-Naive Patients Initiated on Antiretroviral Therapy in Germany: Experiences of the PROPHET Study. Value Health 2020, 23, 1324–1331. [Google Scholar] [CrossRef]
- Trapero-Bertran, M.; Oliva-Moreno, J. Economic impact of HIV/AIDS: A systematic review in five European countries. Health Econ. Rev. 2014, 4, 15. [Google Scholar] [CrossRef]
- Tontodonati, M.; Cenderello, G.; Celesia, B.M.; Trezzi, M.; Ursini, T.; Costantini, A.; Marra, D.; Polilli, E.; Catalani, C.; Butini, L.; et al. Cost of HAART in Italy: Multicentric evaluation and determinants from a large HIV outpatient cohort. Clin. Outcomes Res. 2014, 7, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.-S.; Yang, C.-T.; Yang, K.-L.; Ku, H.-C.; Liao, C.-T.; Kuo, S.; Tang, H.-J.; Ko, W.-C.; Ou, H.-T.; Ko, N.-Y. Reduced economic burden of AIDS-defining illnesses associated with adherence to antiretroviral therapy. Int. J. Infect. Dis. 2020, 91, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Krentz, H.; Gill, M. Increased costs of HIV care associated with aging in an HIV-infected population. HIV Med. 2015, 16, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, C.; Cattaneo, D.; Rizzardini, G.; Marcotullio, S.; Maggiolo, F.; Restelli, U.; Croce, D.; Silvola, S. Valutazione di Health Technology Assessment di Dovato (dolutegravir/lamivudina) nel trattamento dei pazienti HIV positivi. Ital. J. Public Health 2020, 9 (Suppl. 5). Available online: https://www.ijph.it/hta-dovato-dolutegravir-lamivudina (accessed on 31 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).