Development of a Person-Centred Integrated Care Approach for Chronic Disease Management in Dutch Primary Care: A Mixed-Method Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Scoping Review and Document Analysis (Phase 1)
2.3. Online Surveys with Healthcare Professionals (Phase 2)
2.4. Individual Interviews with Patients (Phase 3)
2.5. Finalization of PC-IC Approach (Phase 4)
3. Results
3.1. Scoping Review and Document Analysis (Phase 1)
3.1.1. Scoping Review
Assessment of Multiple Domains—Integral Health Status
| Authors | Country | Type of Publication | Title |
|---|---|---|---|
| AHRQ—American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity 2012 [23] | United States | Consensus document | Guiding Principles for the Care of Older Adults with Multimorbidity: An Approach for Clinicians |
| Boehmer & Abu Dabrh et al., 2018 [39] | United States | Systematic review | Does the chronic care model meet the emerging needs of people living with multimorbidity? A systematic review and thematic synthesis |
| Boehmer & Guerton et al., 2019 [42] | United States | Descriptive article | Capacity Coaching: A New Strategy for Coaching Patients Living with Multimorbidity and Organizing Their Care |
| Boyd & Fortin 2010 [40] | United States | Review | Future of Multimorbidity Research: How Should Understanding of Multimorbidity Inform Health System Design? |
| Culpepper 2012 [43] | United States | Review | Does Screening for Depression in Primary Care Improve Outcome? |
| Engamba & Steel et al., 2019 [44] | United Kingdom | Analysis | Tackling multimorbidity in primary care: is relational continuity the missing ingredient? |
| Fortin & Hudon et al., 2007 [45] | Canada | Review | Caring for Body and Soul: the Importance of Recognizing and Managing Psychological Distress in Persons with Multimorbidity |
| Hopman & de Bruin et al., 2016 [38] | Netherlands | Systematic literature review | Effectiveness of comprehensive care programs for patients with multiple chronic conditions or frailty: A systematic literature review |
| Lenzen & Daniëls et al., 2015 [46] | Netherlands | Background paper | Setting goals in chronic care: Shared decision-making as self-management support by the family physician |
| Marengoni & Angleman et al., 2011 [5] | Sweden | Systematic literature review | Aging with multimorbidity: A systematic review of the literature |
| Muth & van den Akker et al., 2014 [47] | Germany | Original study | The Ariadne principles: how to handle multimorbidity in primary care consultations |
| Muth & Blom et al., 2019 [48] | Germany/United Kingdom | Systematic guideline review & expert consensus | Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: a systematic guideline review and expert consensus |
| NICE—National Institute for Health + Care Excellence 2016 [24] | United Kingdom | Guideline | Multimorbidity: clinical assessment and management |
| Poitras & Maltais et al., 2018 [41] | Canada | Scoping review | What are the effective elements in patient-centred and multimorbidity care? A scoping review |
| Ricci-Cabello & Violan et al., 2015 [49] | United Kingdom | Scoping review | Impact of multi-morbidity on quality of healthcare and its implications for health policy, research, and clinical practice. A scoping review |
| Smith & Wallace et al., 2016 [50] | Ireland | Systematic review | Interventions for improving outcomes in patients with multimorbidity in primary care and community settings |
| Stokes & Man et al., 2017 [51] | United Kingdom | Review | The Foundations Framework for Developing and Reporting New Models of Care for Multimorbidity |
| Wallace & Salisbury et al., 2015 [19] | United Kingdom | Review | Managing patients with multimorbidity in primary care |
Case Management
Clinical Assessment
Patient Preferences and Priorities
Care Plan
3.1.2. Document Analysis
3.2. Online Qualitative Surveys with Healthcare Professionals (Phase 2)
3.2.1. General Comments
3.2.2. Assessment of Integral Health Status
3.2.3. Setting Personal Health Goals
3.2.4. Choosing Interventions
3.2.5. Individual Care Plan
3.2.6. Evaluation
3.3. Individual Interviews with Patients (Phase 3)
3.3.1. Personalized Care
3.3.2. Co-Operation
3.3.3. Role of the Patient
3.3.4. Role of the Practice Nurse
3.4. Finalization of Recommended PC-IC Approach (Phase 4)
3.4.1. Health Insurers
3.4.2. Dutch Centre of Expertise on Health Disparities (Pharos)
3.4.3. Finalization of the PC-IC Approach
4. Discussion
4.1. Summary of Results
4.2. Comparison to Existing Literature & Interpretation
4.3. Strengths & Limitations
4.4. Implications
4.4.1. Recommendations for Future Research
4.4.2. Recommendations for Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategies
- PubMed
- (“Primary Health Care”[Mesh:NoExp] OR “Primary care”[tiab] OR “Primary health care”[tiab] OR “Family practice”[Mesh] OR “Family care”[tiab] OR “General Practice”[Mesh] OR “General Practice”[tiab]) AND (“Multiple Chronic Conditions”[Mesh] OR “Multimorbidity”[Mesh] OR “Multi-morbidity”[tiab] OR “Multimorbidity”[tiab])
- Limit: review or systematic review.
- Embase
- (exp primary health care/OR general practice/OR (primary care OR primary health care OR family care OR general Practice).ti,ab,kw.)
- AND
- (Multiple chronic conditions/OR (multimorbidity OR multi-morbidity).ti,ab,kw.)
- Limit: reviews.
- Cochrane
- Multimorbidity
- TRIP
- (“Primary care” OR “Primary health care” OR “Family practice” OR “Family care” OR “General Practice”) AND (“Multiple Chronic Conditions” OR “Multimorbidity”)
- Limit: guidelines.
- GIN
- Multimorbidity
Appendix B. Interview Guide for Individual Interviews with Patients
|
Appendix C. Categories of Unique Key Interventions for Disease-Specific and Holistic Care Resulting from the Document Analysis
| Category | Item | Factors |
|---|---|---|
| Physiological functioning | ||
| Medical parameters | Treatment of risk factors CVD | Treat risk factors CVD according to CVD guideline |
| Optimize cardiovascular risk profile | ||
| Treatment of elevated cholesterol | Reduce elevated cholesterol | |
| Treatment of elevated blood glucose | Treatment of elevated blood glucose/reach target value blood glucose | |
| Reach target value blood glucose with education and information | ||
| Education on influence of physical activity on blood glucose | ||
| Improve self-management by education on self-check of blood glucose | ||
| Treatment of elevated blood pressure | Reduce elevated blood pressure | |
| Lifestyle | Promote healthy body weight | Advise balance for persons with healthy body weight |
| Promote reduction of waist circumference for overweight or obese persons | ||
| Promote weight loss for overweight or obese persons | ||
| Reduce body weight | ||
| Improve self-management with healthy diet and physical activity | ||
| Promote healthy diet | History taking diet | |
| Promote healthy alcohol use | ||
| Promote healthy diet | ||
| Give dietary advice | ||
| Promote physical activity | Attention for inactivity | |
| Advise to prevent excessive sitting | ||
| Advise strengthening exercises for muscles and bones | ||
| Promote physical activity | ||
| Coaching on physical activity | ||
| Improve endurance | ||
| Promote quitting tobacco use | Advise or give coaching to quit tobacco use | |
| Advise strongly to quit tobacco use | ||
| Advise to prevent inhaling smoke from others | ||
| Treatment to improve motivation to quit tobacco use | ||
| Medication | Promote correct use of medication | Pay attention to correct use of medication |
| Counselling for oxygen therapy | ||
| Evaluate use of inhalation medication | ||
| Deliver adequate pharmaceutical care through patient counselling | ||
| Detect suboptimal medication use | ||
| Education on goal and pharmacology of medication | ||
| Education on use of inhalation medication aids | ||
| Education on oxygen therapy | ||
| Improve self-management by education on correct use of medication | ||
| Improve therapy compliance | Discuss therapy compliance | |
| Improve medication safety | Deliver adequate pharmaceutical care by reviewing pharmacotherapy | |
| Deliver adequate pharmaceutical care by medication surveillance | ||
| Discuss medication safety | ||
| Medication review when prescribing new medication | ||
| Periodical medication review by pharmacy and physician | ||
| Physical functioning | ||
| Prevention | Attention for oral care | Attention for oral care |
| Attention for foot care | Advise patients on foot care and shoes | |
| Improve self-management with foot checks | ||
| Attention for flu vaccinations | Yearly invitation for flu vaccination | |
| Education on flu vaccinations | ||
| Actual complaints | Reduce burden of cough | Reduce burden of cough by advising physical activity |
| Reduce burden of cough by education on clearing sputum | ||
| Reduce burden of cough by breathing and relaxation exercises | ||
| Reduce dyspnoea | Reduce fear for dyspnoea by psychological counselling | |
| Reduce dyspnoea by breathing and relaxation exercises | ||
| Reduce dyspnoea by adapting pace | ||
| Reduce dyspnoea with medication | ||
| Reduce dyspnoea by improving respiratory muscles | ||
| Attention for self-management of exacerbations | Attention for exacerbation management | |
| Reduce disease burden in future: prevent exacerbations | ||
| Education on reducing chances of exacerbations | ||
| Education on early detection of exacerbations | ||
| Improve self-management by composing an action plan for exacerbations | ||
| Functional (dis)abilities | ||
| Mobility | Attention for mobility | Education on possible limitations of fitness to drive |
| Functioning at home | Functioning at home | Advise aids |
| Quality of Life | ||
| Treatment burden | Reduce treatment burden | Reduce treatment burden |
| Identify treatments with limited effects | ||
| Identify medication with elevated chance of side effects | ||
| Identify non-pharmaceutical interventions as alternatives to medication | ||
| Identify alternative planning of appointments | ||
| Identify ways to maximalise effect of current treatments | ||
| Identify causes of high treatment burden | ||
| Emotional well-being | Learn coping with condition through psychological counselling | Advise contact with peers |
| Treatment of adaptation problems | ||
| Use psychosocial interventions (training for coping skills, relaxation exercises, stress-management) | ||
| Psychosocial counselling to learn coping strategies | ||
| Education on the condition | ||
| Education on coping with the (consequences of) chronic condition | ||
| Pay attention to psychosocial factors (fear of dyspnoea, shame, sexual problems, social isolation, depression) A | ||
| Support for psychological problems | Support for psychological problems | |
| Reduce stress, fear, sadness, and depression | ||
| Social functioning | ||
| Social relationships | Attention for social relationships | Discuss important themes in partner relationship |
| Discuss communication to others | ||
| Pay attention to social context of patient | ||
| Labour participation | Improve labour participation | Advise occupation physician for work related problems |
| Discuss communication at work | ||
| Support for reintegration: reduce burden, improve capacity | ||
| Reduce disease burden in future: prevent invalidity and incapacity for work | ||
| Education on the possible limitations for work | ||
References
- World Health Organization. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; pp. 1–162.
- Hasselaar, J. Nivel Zorgregistraties Eerste Lijn. 2022. Available online: https://www.nivel.nl/nl/nivel-zorgregistraties-eerste-lijn/nivel-zorgregistraties-eerste-lijn (accessed on 6 November 2022).
- Johnston, M.C.; Crilly, M.; Black, C.; Prescott, G.J.; Mercer, S.W. Defining and measuring multimorbidity: A systematic review of systematic reviews. Eur. J. Public Health 2019, 29, 182–189. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Public Health and the Environment. Number of People with Chronic Conditions Known at the General Practice. 2019. Available online: https://www.volksgezondheidenzorg.info/onderwerp/chronische-ziekten-en-multimorbiditeit/cijfers-context/huidige-situatie#node-aantal-mensen-met-chronische-aandoening-bekend-bij-de-huisarts (accessed on 27 August 2019).
- Marengoni, A.; Angleman, S.; Melis, R.; Mangialasche, F.; Karp, A.; Garmen, A.; Meinow, B.; Fratiglioni, L. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 2011, 10, 430–439. [Google Scholar] [CrossRef]
- Mujica-Mota, R.E.; Roberts, M.; Abel, G.; Elliott, M.; Lyratzopoulos, G.; Roland, M.; Campbell, J. Common patterns of morbidity and multi-morbidity and their impact on health-related quality of life: Evidence from a national survey. Qual. Life Res. 2015, 24, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Fortin, M.; Dubois, M.-F.; Hudon, C.; Soubhi, H.; Almirall, J. Multimorbidity and quality of life: A closer look. Health Qual. Life Outcomes 2007, 5, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijken, M.; Van Kerkhof, M.; Dekker, J.; Schellevis, F.G. Comorbidity of chronic diseases: Effects of disease pairs on physical and mental functioning. Qual. Life Res. 2005, 14, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Grumbach, K.; Bodenheimer, T. A primary care home for Americans: Putting the house in order. JAMA 2002, 288, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Bodenheimer, T.; Wagner, E.H.; Grumbach, K. Improving primary care for patients with chronic illness. JAMA 2002, 288, 1775–1779. [Google Scholar] [CrossRef]
- Wagner, E.H.; Austin, B.T.; Davis, C.; Hindmarsh, M.; Schaefer, J.; Bonomi, A.; Stuart, B.; Mahler, E.; Koomson, P.; Adler-Milstein, J.; et al. Improving chronic illness care: Translating evidence into action. Health Aff. 2001, 20, 64–78. [Google Scholar] [CrossRef] [Green Version]
- NDF Zorgstandaard Diabetes Type 2. 2015. Available online: https://www.zorgstandaarddiabetes.nl/type-2/ (accessed on 27 August 2019).
- Platform Vitale Vaten. Zorgstandaard Cardiovasculair Risicomanagement 2013; Platform Vitale Vaten: Den Haag, The Netherlands.
- Long Alliantie Nederland. Zorgstandaard COPD. 2016. Available online: http://www.longalliantie.nl/zorgstandaard-copd/ (accessed on 27 August 2019).
- Cramm, J.M.; Nieboer, A.P. In the Netherlands, rich interaction among professionals conducting disease management led to better chronic care. Health Aff. 2012, 31, 2493–2500. [Google Scholar] [CrossRef] [Green Version]
- Cramm, J.M.; Rutten-Van Molken, M.P.; Nieboer, A.P. The potential for integrated care programmes to improve quality of care as assessed by patients with COPD: Early results from a real-world implementation study in The Netherlands. Int. J. Integr. Care 2012, 12, e191. [Google Scholar] [CrossRef] [Green Version]
- Cramm, J.M.; Adams, S.A.; Walters, B.H.; Tsiachristas, A.; Bal, R.; Huijsman, R.; Mölken, M.P.R.-V.; Nieboer, A.P. The role of disease management programs in the health behavior of chronically ill patients. Patient Educ. Couns. 2014, 95, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Tsiachristas, A.; Cramm, J.M.; Nieboer, A.P.; Mölken, M.P.R.-V. Changes in costs and effects after the implementation of disease management programs in the Netherlands: Variability and determinants. Cost Eff. Resour. Alloc. 2014, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Wallace, E.; Salisbury, C.; Guthrie, B.; Lewis, C.; Fahey, T.; Smith, S.M. Managing patients with multimorbidity in primary care. BMJ 2015, 350, h176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, C.M.; Darer, J.; Boult, C.; Fried, L.P.; Boult, L.; Wu, A.W. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA 2005, 294, 716–724. [Google Scholar] [CrossRef]
- van Weel, C.; Carelli, F.; Gerada, C. Reforming primary care: Innovation or destruction? Br. J. Gen. Pract. 2012, 62, 43–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J. The European Definition of General Practice; WHO Europe Office: Copenhagen, Denmark, 2005.
- AHRQ–Agency for Healthcare Research + Quality. Guiding Principles for the Care of Older Adults with Multimorbidity: An Approach for Clinicians. American Geriatrics Society. NGC:009523. Guideline Clearing Report. 2012. Available online: http://www.guideline.gov/content.aspx?id=39322 (accessed on 27 August 2019).
- NICE–National Institute for Health + Care Excellence. Multimorbidity: Clinical Assessment and Management (NG56). Guideline. 2016. Available online: https://www.nice.org.uk/guidance/ng56 (accessed on 27 August 2019).
- WHO. People-Centred and Integrated Health Services: An Overview of the Evidence; Interim Report; World Health Organization: Geneva, Switzerland, 2015.
- Coulter, A.; Entwistle, V.A.; Eccles, A.; Ryan, S.; Shepperd, S.; Perera, R. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst. Rev. 2015, 2015, Cd010523. [Google Scholar] [CrossRef] [Green Version]
- Reuben, D.B.; Tinetti, M.E. Goal-oriented patient care—An alternative health outcomes paradigm. N. Engl. J. Med. 2012, 366, 777–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikka, R.; Morath, J.M.; Leape, L. The Quadruple Aim: Care, health, cost and meaning in work. BMJ Qual. Saf. 2015, 24, 608–610. [Google Scholar] [CrossRef] [Green Version]
- Bodenheimer, T.; Sinsky, C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann. Fam. Med. 2014, 12, 573–576. [Google Scholar] [CrossRef] [Green Version]
- de Bruin, S.R.; Versnel, N.; Lemmens, L.C.; Molema, C.C.; Schellevis, F.G.; Nijpels, G.; Baan, C.A. Comprehensive care programs for patients with multiple chronic conditions: A systematic literature review. Health Policy 2012, 107, 108–145. [Google Scholar] [CrossRef] [Green Version]
- Salisbury, C.; Man, M.-S.; Bower, P.; Guthrie, B.; Chaplin, K.; Gaunt, D.M.; Brookes, S.; Fitzpatrick, B.; Gardner, C.; Hollinghurst, S.; et al. Management of multimorbidity using a patient-centred care model: A pragmatic cluster-randomised trial of the 3D approach. Lancet 2018, 392, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Möhler, R.; Köpke, S.; Meyer, G. Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: Revised guideline (CReDECI 2). Trials 2015, 16, 204. [Google Scholar] [CrossRef] [Green Version]
- Skivington, K.; Matthews, L.; Simpson, S.; Craig, P.; Baird, J.; Blazeby, J.; Boyd, K.; Craig, N.; French, D.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for reporting qualitative research: A synthesis of recommendations. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef]
- Barents, E.S.E.; Bilo, H.J.G.; Bouma, M.; Dankers, M.; De Rooij, A.; Hart, H.E.; Houweling, S.T.; IJzerman, R.G.; Janssen, P.G.H.; Kerssen, A.; et al. NHG-Standaard Diabetes Mellitus Type 2 (Vierde (Partiële) Herziening). 2018. Available online: https://www.nhg.org/standaarden/volledig/nhg-standaard-diabetes-mellitus-type-2 (accessed on 27 August 2019).
- Bischoff, E.; Bouma, M.; Broekhuizen, L.; Donkers, J.; Hallensleben, C.; De Jong, J.; Snoeck-Stroband, J.; In’t Veen, J.C.; Van Vugt, S.; Wagenaar, M. NHG-Standaard COPD (Derde Herziening). 2015. Available online: https://www.nhg.org/standaarden/volledig/nhg-standaard-copd (accessed on 27 August 2019).
- Nederlands Huisartsen Genootschap. NHG-Standaard Cardiovasculair Risicomanagement (Tweede Herziening). 2019. Available online: https://www.nhg.org/standaarden/volledig/cardiovasculair-risicomanagement (accessed on 27 August 2019).
- Hopman, P.; de Bruin, S.R.; Forjaz, M.J.; Rodriguez-Blazquez, C.; Tonnara, G.; Lemmens, L.C.; Onder, G.; Baan, C.A.; Rijken, M. Effectiveness of comprehensive care programs for patients with multiple chronic conditions or frailty: A systematic literature review. Health Policy 2016, 120, 818–832. [Google Scholar] [CrossRef] [Green Version]
- Boehmer, K.R.; Abu Dabrh, A.M.; Gionfriddo, M.R.; Erwin, P.; Montori, V.M. Does the chronic care model meet the emerging needs of people living with multimorbidity? A systematic review and thematic synthesis. PLoS ONE 2018, 13, e0190852. [Google Scholar] [CrossRef] [Green Version]
- Boyd, C.M.; Fortin, M. Future of multimorbidity research: How should understanding of multimorbidity inform health system design? Public Health Rev. 2010, 32, 451–474. [Google Scholar] [CrossRef] [Green Version]
- Poitras, M.-E.; Maltais, M.-E.; Bestard-Denommé, L.; Stewart, M.; Fortin, M. What are the effective elements in patient-centered and multimorbidity care? A scoping review. BMC Health Serv. Res. 2018, 18, 446. [Google Scholar] [CrossRef] [Green Version]
- Boehmer, K.R.; Guerton, N.; Soyring, J.; Hargraves, I.; Dick, S.; Montori, V.M. Capacity Coaching: A New Strategy for Coaching Patients Living with Multimorbidity and Organizing Their Care. Mayo Clin. Proc. 2019, 94, 278–286. [Google Scholar] [CrossRef]
- Culpepper, L. Does screening for depression in primary care improve outcome? Curr. Psychiatry Rep. 2012, 14, 345–352. [Google Scholar] [CrossRef]
- Engamba, S.A.; Steel, N.; Howe, A.; Bachman, M. Tackling multimorbidity in primary care: Is relational continuity the missing ingredient? Br. J. Gen. Pract. 2019, 69, 92–93. [Google Scholar] [CrossRef] [Green Version]
- Fortin, M.; Hudon, C.; Bayliss, E.A.; Soubhi, H.; Lapointe, L. Caring for body and soul: The importance of recognizing and managing psychological distress in persons with multimorbidity. Int. J. Psychiatry Med. 2007, 37, 1–9. [Google Scholar] [CrossRef]
- Lenzen, S.A.; Daniëls, R.; Van Bokhoven, M.A.; Van Der Weijden, T.; Beurskens, A. Setting goals in chronic care: Shared decision making as self-management support by the family physician. Eur. J. Gen. Pract. 2014, 21, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muth, C.; van den Akker, M.; Blom, J.W.; Mallen, C.D.; Rochon, J.; Schellevis, F.G.; Becker, A.; Beyer, M.; Gensichen, J.; Kirchner, H.; et al. The Ariadne principles: How to handle multimorbidity in primary care consultations. BMC Med. 2014, 12, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muth, C.; Blom, J.W.; Smith, S.M.; Johnell, K.; Gonzalez-Gonzalez, A.I.; Nguyen, T.S.; Brueckle, M.-S.; Cesari, M.; Tinetti, M.E.; Valderas, J.M. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: A systematic guideline review and expert consensus. J. Intern. Med. 2019, 285, 272–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci-Cabello, I.; Violán, C.; Foguet-Boreu, Q.; Mounce, L.T.; Valderas, J.M. Impact of multi-morbidity on quality of healthcare and its implications for health policy, research and clinical practice. A scoping review. Eur. J. Gen. Pract. 2015, 21, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Wallace, E.; O’Dowd, T.; Fortin, M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst. Rev. 2016, 3, Cd006560. [Google Scholar] [CrossRef]
- Stokes, J.; Man, M.-S.; Guthrie, B.; Mercer, S.W.; Salisbury, C.; Bower, P. The Foundations Framework for Developing and Reporting New Models of Care for Multimorbidity. Ann. Fam. Med. 2017, 15, 570–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, J.B.; Daudey, L.; Heijdra, Y.F.; Molema, J.; Dekhuijzen, P.N.R.; Vercoulen, J.H. Development of a battery of instruments for detailed measurement of health status in patients with COPD in routine care: The Nijmegen Clinical Screening Instrument. Qual. Life Res. 2009, 18, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Palmer, K.; Marengoni, A.; Forjaz, M.J.; Jureviciene, E.; Laatikainen, T.; Mammarella, F.; Muth, C.; Navickas, R.; Prados-Torres, A.; Rijken, M.; et al. Multimorbidity care model: Recommendations from the consensus meeting of the Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS). Health Policy 2018, 122, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Smeets, R.G.M.; Kroese, M.E.; Ruwaard, D.; Hameleers, N.; Elissen, A.M. Person-centred and efficient care delivery for high-need, high-cost patients: Primary care professionals’ experiences. BMC Fam. Pract. 2020, 21, 106. [Google Scholar]
- Smeets, R.G.M.; Hertroijs, D.F.L.; Mukumbang, F.C.; Kroese, M.E.A.L.; Ruwaard, D.; Elissen, A.M.J. First Things First: How to Elicit the Initial Program Theory for a Realist Evaluation of Complex Integrated Care Programs. Milbank Q. 2022, 100, 151–189. [Google Scholar]
- Hameleers, N. Op Weg Naar Een Integraal Programma Chronische Zorg; Care and Public Health Research Institute (CAPHRI), Faculty of Health, Medicine and Life Sciences, Maastricht University: Maastricht, The Netherlands, 2017. [Google Scholar]
- Rimmelzwaan, L.M.; Bogerd, M.J.L.; Schumacher, B.M.A.; Slottje, P.; Van Hout, H.P.J.; Reinders, M.E. Multimorbidity in General Practice: Unmet Care Needs From a Patient Perspective. Front. Med. 2020, 7, 530085. [Google Scholar] [CrossRef]
- Rijken, M.; Stüssgen, R.; Leemrijse, C.; Bogerd, M.J.L.; Korevaar, J.C. Priorities and preferences for care of people with multiple chronic conditions. Health Expect. 2021, 24, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Leijten, F.R.; Struckmann, V.; van Ginneken, E.; Czypionka, T.; Kraus, M.; Reiss, M.; Tsiachristas, A.; Boland, M.; de Bont, A.; Bal, R.; et al. The SELFIE framework for integrated care for multi-morbidity: Development and description. Health Policy 2018, 122, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bour, S.S.; Raaijmakers, L.H.A.; Bischoff, E.W.M.A.; Goossens, L.M.A.; Mölken, M.P.M.H.R.-V. How Can a Bundled Payment Model Incentivize the Transition from Single-Disease Management to Person-Centred and Integrated Care for Chronic Diseases in the Netherlands? Int. J. Environ. Res. Public Health 2023, 20, 3857. [Google Scholar] [CrossRef]
- Bampton, R.; Cowton, C.J. The E-Interview. Forum Qual. Soc. Res. 2002, 3. [Google Scholar] [CrossRef]
- Burgers, J.S.; van der Weijden, T.; Bischoff, E. Challenges of Research on Person-Centered Care in General Practice: A Scoping Review. Front. Med. 2021, 8, 669491. [Google Scholar]



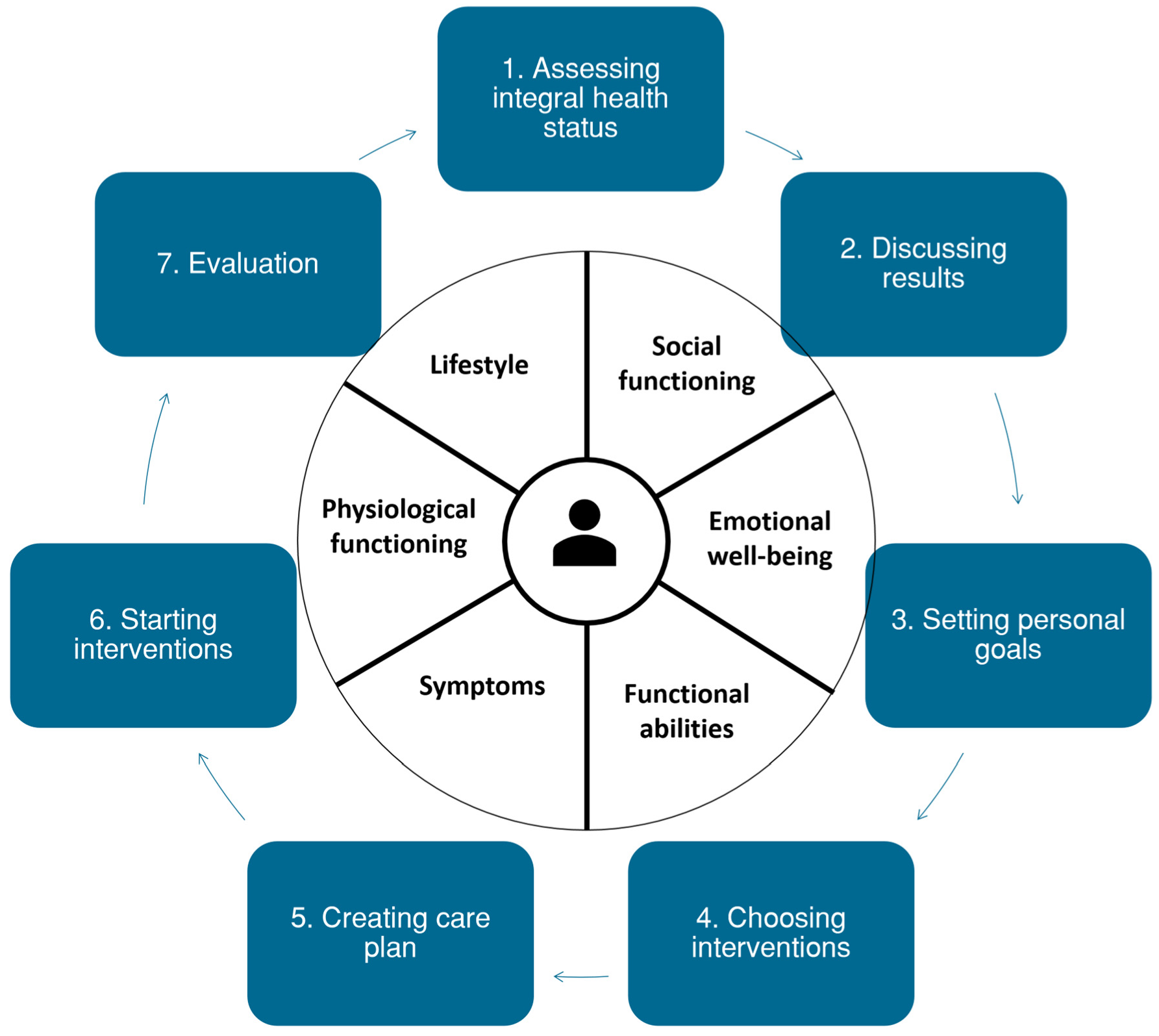
| Phase | Population | Time Period | Result |
|---|---|---|---|
| 1. Literature review & document analysis | N/A | March–September 2019 | Conceptual PC-IC approach |
| 2. Online qualitative surveys | Primary care professionals * purposively selected by the three regional primary care cooperatives involved, supplemented with GPs with a special interest in CVD, DM2, or COPD involved in a national guideline or health policy committees | October–May 2020 | Adaptations to conceptual PC-IC approach |
| 3. Interviews | Patients with DM and/or COPD and/or CVD who received chronic disease management from their GP, recruited through the primary care cooperatives involved, by asking involved practices to recruit 1–2 patients from their chronic care population | May–July 2020 | Adaptations to conceptual PC-IC approach |
| 4. Finalization | Stakeholders involved in chronic disease care:
| June–August 2020 | Final version of the PC-IC approach |
| Analysis of the data obtained from the document analysis, online surveys, one-on-one interviews, and focus group interviews was performed using inductive thematical coding, a commonly used method to identify themes in qualitative data. In all phases, at least two authors independently coded the data using ATLAS.ti version 8.4.15. The authors discussed the selected quotations and respective codes after coding one to five documents or transcripts until they reached a consensus. The quotes in the code book were periodically reviewed to check if they needed adaptation, for example, if two codes could be merged together, or if another code should be added. After coding all the documents or transcripts once, the documents were reviewed in light of the latest version of the codebook. After this second review, we categorized the codes using an affinity diagram method. These categories were discussed within the project team. Preliminary results of the analyses were offered for member checking, where possible. |
| Number | |
|---|---|
| Medical professionals | |
| GP specialized in COPD, CVD, and/or DM2 | 8 |
| Regular GP | 8 |
| Specialist for elderly care | 1 |
| Specialist for internal diseases | 1 |
| Nursing professionals | |
| PN COPD, CVD, and/or DM2 | 13 |
| PN mental healthcare | 2 |
| Home care nurse | 2 |
| Allied healthcare professionals | |
| Dietician | 4 |
| Podotherapist | 2 |
| Medical pedicure | 1 |
| Physiotherapist | 1 |
| Pharmacist | 1 |
| Other | |
| Social worker | 4 |
| Policy officer of primary care cooperative | 3 |
| Lifestyle coach | 1 |
| Total | 52 |
| Quote | Quote | Participant |
|---|---|---|
| Healthcare providers | ||
| Q1 | ‘The holistic view and thinking does not put curing as the highest priority but rather well-being and functioning as desired. A pleasant way for both the caregiver and the patient.’ | 212 |
| Q2 | ‘The integral approach can be more time consuming, but will eventually lead to more time, less frustration and more satisfaction.’ | 201 |
| Q3 | ‘… giving people back the feeling of control.’ | 317 |
| Q4 | ‘It takes a lot of time. Not every healthcare professional is able to do this; not every patient wants to do this.’ | 324 |
| Q5 | ‘It will be difficult for patients with limited health skills, while the conversation about integral health status is so important, especially for them.’ | 206 |
| Q6 | ‘[Assessment of integral health status] will possibly reveal particular subjects or triggers, which have a negative impact on a patient’s health and would otherwise not have been revealed. For example, financial problems or loneliness.’ | 319 |
| Q7 | ‘Some patients might think … some domains are too personal and this might cause resistance in the patient.’ | 410 |
| Q8 | ‘Possibly the goals can improve therapy compliance, because the motivation is better.’ | 207 |
| Q9 | ‘[Personal goals] can be far away from treatment goals. With high blood pressure, high HbA1c and many cigarettes, it is perfectly possible to take care of grandchildren.’ | 204 |
| Q10 | ‘I think it is too detailed, and therefore maybe not usable in practice.’ | 311 |
| Q11 | ‘… In addition, it is clear for other involved healthcare professionals what the goals of the patient are and how they want to achieve them. Care can be co-ordinated better.’ | 306 |
| Q12 | ‘It could lead to medicalisation of problems which do not originate in the somatic corner. It is quite a lot of work to write it.’ | 315 |
| Q13 | ‘Evaluate what worked, also especially what made it work, what didn’t work and what could help to make it work … with a non-judgmental attitude.’ | 416 |
| Patients with DM2, COPD and/or CVD | ||
| Q14 | ‘Let’s be honest, you can’t do anything in ten minutes. […] This plan here, […] the practice nurse having half an hour with you, that’s a luxury.’ | 7 |
| Q15 | “You could say to yourself that you are doing alright, but I think that when you have to fill in a questionnaire like that it would make you wonder. How am I really doing?” | 8 |
| Q16 | “I think that people like myself […] benefit from filling out the questionnaire again after a while and seeing what changes there are, both positive and negative.” | 8 |
| Q17 | “Some people don’t or don’t want to understand what they are told, because [optimal treatment] means changing their lifestyle, and taking medication may be easier.” | 12 |
| Q18 | “The more involved your partner is, the better, […] not just for your own support, but also for theirs. They struggle too, sometimes more than you. That is an aspect that is often overlooked.” | 7 |
| Q19 | “[…]practice nurse, you have to be understanding towards patients that don’t require those strict guidelines.” | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raaijmakers, L.H.A.; Schermer, T.R.; Wijnen, M.; van Bommel, H.E.; Michielsen, L.; Boone, F.; Vercoulen, J.H.; Bischoff, E.W.M.A. Development of a Person-Centred Integrated Care Approach for Chronic Disease Management in Dutch Primary Care: A Mixed-Method Study. Int. J. Environ. Res. Public Health 2023, 20, 3824. https://doi.org/10.3390/ijerph20053824
Raaijmakers LHA, Schermer TR, Wijnen M, van Bommel HE, Michielsen L, Boone F, Vercoulen JH, Bischoff EWMA. Development of a Person-Centred Integrated Care Approach for Chronic Disease Management in Dutch Primary Care: A Mixed-Method Study. International Journal of Environmental Research and Public Health. 2023; 20(5):3824. https://doi.org/10.3390/ijerph20053824
Chicago/Turabian StyleRaaijmakers, Lena H. A., Tjard R. Schermer, Mandy Wijnen, Hester E. van Bommel, Leslie Michielsen, Floris Boone, Jan H. Vercoulen, and Erik W. M. A. Bischoff. 2023. "Development of a Person-Centred Integrated Care Approach for Chronic Disease Management in Dutch Primary Care: A Mixed-Method Study" International Journal of Environmental Research and Public Health 20, no. 5: 3824. https://doi.org/10.3390/ijerph20053824
APA StyleRaaijmakers, L. H. A., Schermer, T. R., Wijnen, M., van Bommel, H. E., Michielsen, L., Boone, F., Vercoulen, J. H., & Bischoff, E. W. M. A. (2023). Development of a Person-Centred Integrated Care Approach for Chronic Disease Management in Dutch Primary Care: A Mixed-Method Study. International Journal of Environmental Research and Public Health, 20(5), 3824. https://doi.org/10.3390/ijerph20053824






