Cost-Impact Analysis of a Novel Diagnostic Test to Assess Community-Acquired Pneumonia Etiology in the Emergency Department Setting: A Multi-Country European Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Overview
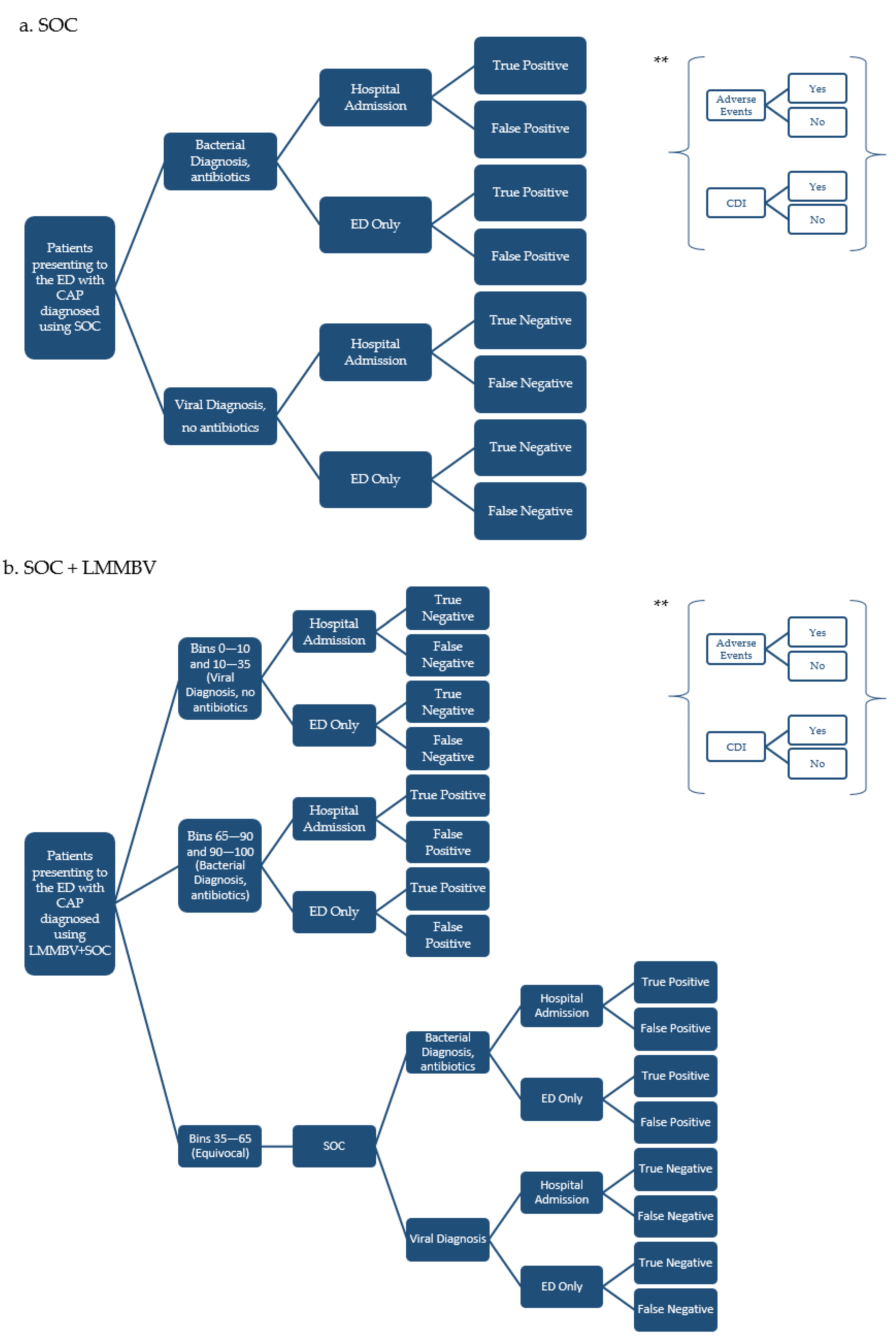
2.2. Model Structure
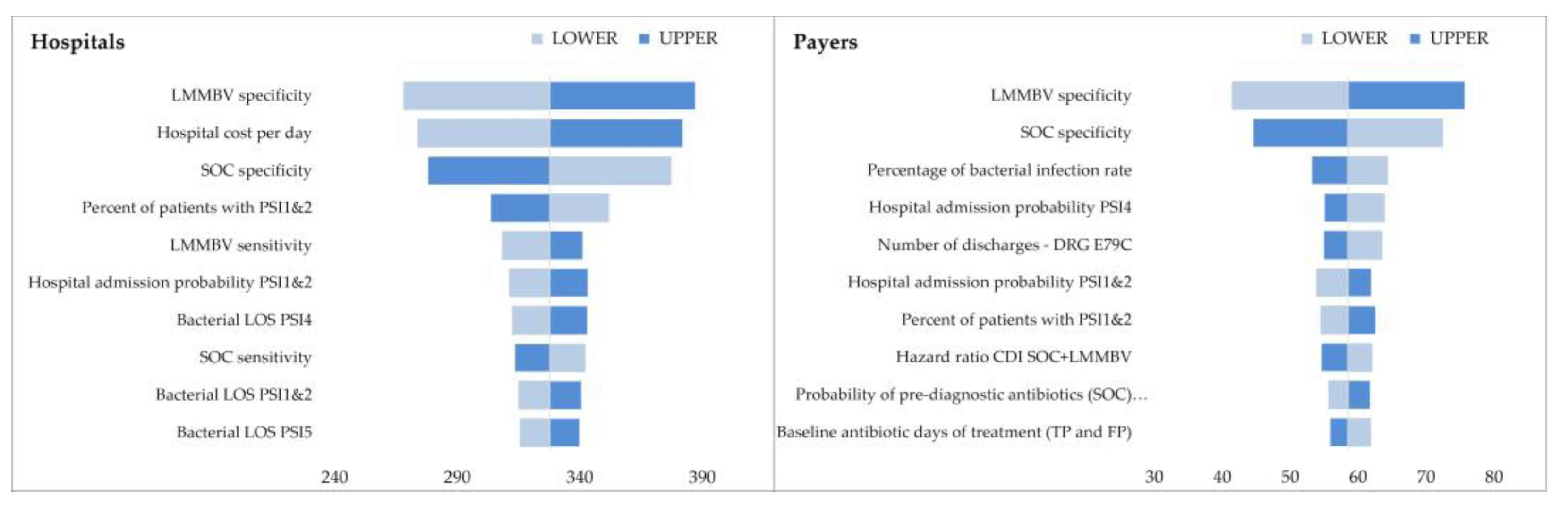
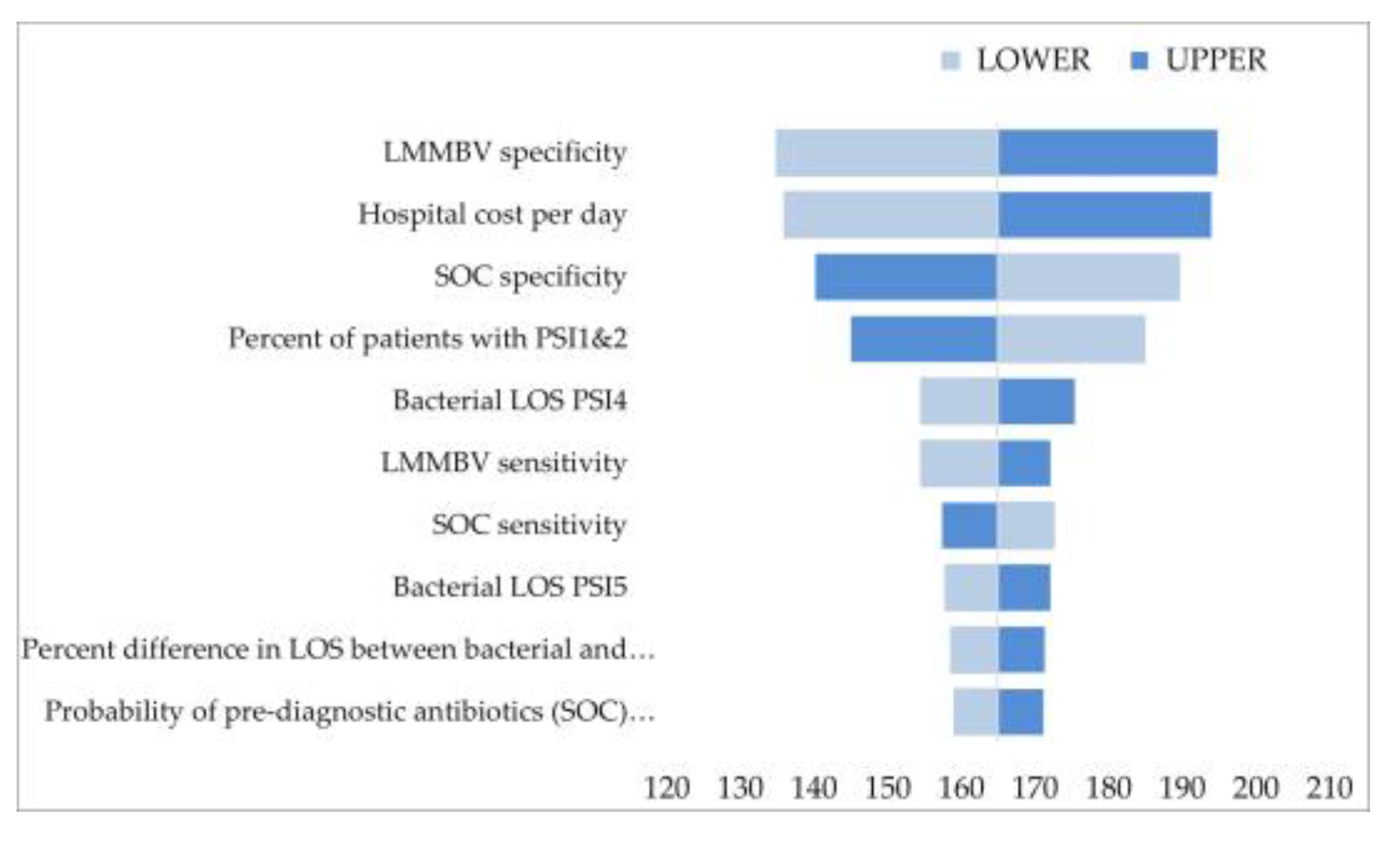
2.3. Sensitivity Analysis
2.4. Inputs
2.4.1. Clinical Inputs
| Value | Ref | |||||
|---|---|---|---|---|---|---|
| Italy | Germany | Spain | IT | GE | SP | |
| Baseline antibiotic treatment days (TP and FP patients) | 5 | [12] | ||||
| Antibiotic treatment days for FN patients | 7.5 | Assumption | ||||
| Baseline hospital LOS for hospitalized patients (days) | ||||||
| PSI score 1 and 2 | 4.74 | 7.04 | 2.56 | [30] | Assumption [29] | [31] |
| PSI score 3 | 8.71 | 7.80 | 5.39 | |||
| PSI score 4 | 8.09 | 9.01 | 8.82 | |||
| PSI score 5 | 11.76 | 12.15 | 10.22 | |||
2.4.2. Costs
2.4.3. Scenarios
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aliberti, S.; Dela Cruz, C.S.; Amati, F.; Sotgiu, G.; I Restrepo, M. Community-acquired pneumonia. Lancet 2021, 398, 906–919. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death—Factsheet. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 17 October 2022).
- Gibson, G.J.; Loddenkemper, R.; Lundbäck, B.; Sibille, Y. Respiratory health and disease in Europe: The new European Lung White Book. Eur. Respir. J. 2013, 42, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira-Coimbra, J.; Sarda, C.; Rello, J. Burden of Community-Acquired Pneumonia and Unmet Clinical Needs. Adv. Ther. 2020, 37, 1302–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolditz, M.; Tesch, F.; Mocke, L.; Höffken, G.; Ewig, S.; Schmitt, J. Burden and risk factors of ambulatory or hospitalized CAP: A population based cohort study. Respir. Med. 2016, 121, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welte, T.; Köhnlein, T. Global and Local Epidemiology of Community-Acquired Pneumonia: The Experience of the CAPNETZ Network. Semin. Respir. Crit. Care Med. 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Rivero-Calle, I.; Pardo-Seco, J.; Aldaz, P.; Vargas, D.A.; Mascarós, E.; Redondo, E.; Díaz-Maroto, J.L.; Linares-Rufo, M.; Fierro-Alacio, M.J.; Gil, A.; et al. Incidence and risk factor prevalence of community-acquired pneumonia in adults in primary care in Spain (NEUMO-ES-RISK project). BMC Infect. Dis. 2016, 16, 645. [Google Scholar] [CrossRef] [Green Version]
- Eurostat—Ageing Europe—Statistics on Population Developments. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Ageing_Europe_-_statistics_on_population_developments (accessed on 17 October 2022).
- Torres, A.; Peetermans, W.E.; Viegi, G.; Blasi, F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax 2013, 68, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.E.; Cooper, J.T. Cost impact analysis of novel host-response diagnostic for patients with community-acquired pneumonia in the emergency department. J. Med. Econ. 2022, 25, 138–151. [Google Scholar] [CrossRef]
- Welte, T.; Torres, A.; Nathwani, D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012, 67, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-Acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Klompas, M.; Ochoa, A.; Ji, W.; McKenna, C.; Clark, R.; Shenoy, E.S.; Hooper, D.; Rhee, C.; CDC Prevention Epicenters Program. Prevalence of Clinical Signs within Reference Ranges among Hospitalized Patients Prescribed Antibiotics for Pneumonia. JAMA Netw. Open 2020, 3, e2010700. [Google Scholar] [CrossRef]
- DiaSorin LIAISON® MeMed BV®. Available online: https://www.diasorin.com/it/immunodiagnostica/aree-cliniche/malattie-infettive/liaisonr-memed-bvr (accessed on 18 October 2022).
- Ashkenazi-Hoffnung, L.; Oved, K.; Navon, R.; Friedman, T.; Boico, O.; Paz, M.; Kronenfeld, G.; Etshtein, L.; Cohen, A.; Gottlieb, T.M.; et al. A host-protein signature is superior to other biomarkers for differentiating between bacterial and viral disease in patients with respiratory infection and fever without source: A prospective observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Stein, M.; Lipman-Arens, S.; Oved, K.; Cohen, A.; Bamberger, E.; Navon, R.; Boico, O.; Friedman, T.; Etshtein, L.; Paz, M.; et al. A novel host-protein assay outperforms routine parameters for distinguishing between bacterial and viral lower respiratory tract infections. Diagn. Microbiol. Infect. Dis. 2018, 90, 206–213. [Google Scholar] [CrossRef]
- Oved, K.; Cohen, A.; Boico, O.; Navon, R.; Friedman, T.; Etshtein, L.; Kriger, O.; Bamberger, E.; Fonar, Y.; Yacobov, R.; et al. A Novel Host-Proteome Signature for Distinguishing between Acute Bacterial and Viral Infections. PLoS ONE 2015, 10, e0120012. [Google Scholar] [CrossRef] [Green Version]
- Srugo, I.; Klein, A.; Stein, M.; Golan-Shany, O.; Kerem, N.; Chistyakov, I.; Genizi, J.; Glazer, O.; Yaniv, L.; German, A.; et al. Validation of a Novel Assay to Distinguish Bacterial and Viral Infections. Pediatrics 2017, 140, e20163453. [Google Scholar] [CrossRef] [Green Version]
- van Houten, C.B.; de Groot, J.; Klein, A.; Srugo, I.; Chistyakov, I.; de Waal, W.; Meijssen, C.B.; Avis, W.; Wolfs, T.F.W.; Shachor-Meyouhas, Y.; et al. A host-protein based assay to differentiate between bacterial and viral infections in preschool children (Opportunity): A double-blind, multicentre, validation study. Lancet Infect. Dis. 2017, 17, 431–440. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Chappell, J.D. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Havers, F.P.; Hicks, L.A.; Chung, J.R.; Gaglani, M.; Murthy, K.; Zimmerman, R.K.; Jackson, L.A.; Petrie, J.G.; McLean, H.Q.; Nowalk, M.P.; et al. Outpatient Antibiotic Prescribing for Acute Respiratory Infections during Influenza Seasons. JAMA Netw. Open 2018, 1, e180243. [Google Scholar] [CrossRef]
- Ruiz-González, A.; Falguera, M.; Vives, M.; Nogués, A.; Porcel, J.; Rubio-Caballero, M. Community-acquired pneumonia: Development of a bedside predictive model and scoring system to identify the aetiology. Respir. Med. 2000, 94, 505–510. [Google Scholar] [CrossRef] [Green Version]
- A Novel, Rapid Host-Response Test Permits Accurate Differentiation of Bacterial and Viral Infection in Febrile Ambulatory Patients: Apollo Study. In Progress. MeMed Data on File. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04690569 (accessed on 12 September 2022).
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A Prediction Rule to Identify Low-Risk Patients with Community-Acquired Pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef]
- Lin, R.Y.; Nuruzzaman, F.; Shah, S.N. Incidence and impact of adverse effects to antibiotics in hospitalized adults with pneumonia. J. Hosp. Med. 2009, 4, E7–E15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Palazuelos-Munoz, S.; Balsells, E.M.; Nair, H.; Chit, A.; Kyaw, M.H. Cost of hospital management of Clostridium difficile infection in United States—A meta-analysis and modelling study. BMC Infect. Dis. 2016, 16, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events with Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, J.D.; Akram, A.R.; Singanayagam, A.; Wilcox, M.H.; Hill, A.T. Risk factors for Clostridium difficile infection in hospitalized patients with community-acquired pneumonia. J. Infect. 2016, 73, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, H.; Garau, J.; Medina, J.; Pascual, E.; McBride, K.; Blasi, F. Resource use by patients hospitalized with community-acquired pneumonia in Europe: Analysis of the REACH study. BMC Pulm. Med. 2014, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Migliorati, P.; Boccoli, E.; Bracci, L.; Sestini, P.; Melani, A. A survey on hospitalised community acquired pneumonia in Italy. Monaldi Arch. Chest Dis. 2006, 65, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Julián-Jiménez, A.; de los Reyes, M.J.P.; Miguez, R.P.; Laín-Terés, N.; Cuena-Boy, R.; Lozano-Ancín, A.A. Improved management of community-acquired pneumonia in the emergency department. Arch. Bronconeumol. 2013, 49, 230–240. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale Ministero Della Salute Decreto 18 Ottobre 2012. Available online: https://www.gazzettaufficiale.it/eli/id/2013/01/28/13A00528/sg (accessed on 17 October 2022).
- OBI Regional Tariffs (Campania, E.R., FVG, Lazio, Liguria, Bolzano, Veneto) Weighted for Resident Population (ISTAT 2020). 2020. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3142_allegato.pdf (accessed on 17 October 2022).
- EBM—Uniform Standard Assessment 2022. Available online: https://www.kbv.de/media/sp/EBM_Gesamt_-_Stand_4._Quartal_2022.pdf (accessed on 9 February 2023).
- Reyes, S.; Martinez, R.; Vallés, J.M.; Cases, E.; Menéndez, R. Determinants of hospital costs in community-acquired pneumonia. Eur. Respir. J. 2008, 31, 1061–1067. [Google Scholar] [CrossRef]
- López, F.; Català, M.; Prats, C.; Estrada, O.; Oliva, I.; Prat, N.; Isnard, M.; Vallès, R.; Vilar, M.; Clotet, B.; et al. A Cost-Benefit Analysis of COVID-19 Vaccination in Catalonia. Vaccines 2021, 10, 59. [Google Scholar] [CrossRef]
- DiaSorin SpA Market Research. Data on File 2021. Available online: https://diasoringroup.com/sites/diasorincorp/files/docs/diasorin_en_0.pdf (accessed on 17 October 2022).
- Martin, M.; Quilici, S.; File, T.; Garau, J.; Kureishi, A.; Kubin, M. Cost-effectiveness of empirical prescribing of antimicrobials in community-acquired pneumonia in three countries in the presence of resistance. J. Antimicrob. Chemother. 2007, 59, 977–989. [Google Scholar] [CrossRef] [Green Version]
- Orden de 17/11/2014, de la Consejería de Sanidad Y Asuntos Sociales, Por la Que se Establecen Los Precios Públicos de la Asistencia Sanitaria Y de los Servicios Prestados en la Red de Centros Sanitarios Dependientes Del Servicio de Salud de Castilla-La Mancha. Available online: https://noticias.juridicas.com/base_datos/CCAA/539877-orden-sanidad-y-asuntos-sociales-17-nov-2014-ca-castilla-la-mancha-precios.html (accessed on 19 October 2022).
- Codifa L’informatore Farmaceutico. Available online: https://www.codifa.it/ (accessed on 17 October 2022).
- Rote Liste Arzneimittelinformationen für Deutschland. Available online: https://www.rote-liste.de/ (accessed on 17 October 2022).
- Ministerio de Sanidad—Profesionales de la Salud—Nomenclator. Available online: https://www.sanidad.gob.es/profesionales/nomenclator.do (accessed on 17 October 2022).
- Giusti, M.; Banfi, F.; Perrone, F.; Pitrelli, A.; Pippo, L.; Giuliani, L. [Community-acquired pneumonia: A budget impact model]. Infez. Med. 2010, 18, 143–153. [Google Scholar]
- OECD. Lorenzoni—International Comparisons of Health Prices and Volumes: New Findings. Available online: https://www.oecd.org/health/health-systems/International-Comparisons-of-Health-Prices-and-Volumes-New-Findings.pdf (accessed on 20 October 2022).
- AGENAS. Agenzia Nazionale per i Servizi Sanitari. Available online: https://www.agenas.gov.it/ (accessed on 21 October 2022).
- Mayordomo, J.I.; López, A.; Viñolas, N.; Castellanos, J.; Pernas, S.; Alonso, J.D.; Frau, A.; Layola, M.; Gasquet, J.A.; Sánchez, J.; et al. Retrospective cost analysis of management of febrile neutropenia in cancer patients in Spain. Curr. Med. Res. Opin. 2009, 25, 2533–2542. [Google Scholar] [CrossRef]
- Ministero della Salute. Rapporto Annuale Sull’attività di Ricovero Ospedaliero (Dati SDO 2018). Giugno 2019. Available online: http://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=22 (accessed on 20 October 2022).
- InEK. Available online: https://www.g-drg.de/ (accessed on 21 October 2022).
- Reimbursement Institute. Available online: https://reimbursement.institute/ (accessed on 21 October 2022).
- Shorr, A.F.; Zilberberg, M.D.; Micek, S.T.; Kollef, M.H. Viruses are prevalent in non-ventilated hospital-acquired pneumonia. Respir. Med. 2017, 122, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Bonine, N.G.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Lodise, T. Impact of Delayed Appropriate Antibiotic Therapy on Patient Outcomes by Antibiotic Resistance Status from Serious Gram-Negative Bacterial Infections. Am. J. Med. Sci. 2019, 357, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Menéndez, R.; Torres, A.; Reyes, S.; Zalacain, R.; Capelastegui, A.; Aspa, J.; Borderias, L.; Martin-Villasclaras, J.J.; Bello, S.; Alfageme, I.; et al. Initial management of pneumonia and sepsis: Factors associated with improved outcome. Eur. Respir. J. 2012, 39, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Peyrani, P.; Mandell, L.; Torres, A.; Tillotson, G.S. The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expert Rev. Respir. Med. 2019, 13, 139–152. [Google Scholar] [CrossRef]
- AIFA Antibiotico-Resistenza: Un Rischio Globale Che Richiede Strategie Condivise 2022. Available online: https://www.aifa.gov.it/-/antibiotico-resistenza-un-rischio-globale-che-richiede-strategie-condivise (accessed on 21 October 2022).

| Scenario | Impact of Antibiotic Prescription | Impact of Hospital Admission | Impact of Hospital LOS |
|---|---|---|---|
| Main analysis—Impact of antibiotic prescription only | x | ||
| Scenario 1—Hospital admission rate impact | x | x | |
| Scenario 2—Length of stay impact/DRG reallocation | x | x | |
| Scenario 3—Reduced hospitalization and length of stay impact/DRG reallocation | x | x | x |
| Value | Ref | |||
|---|---|---|---|---|
| SOC | SOC + LMMBV | SOC | SOC + LMMBV | |
| Bacterial infection rate | 37.8% | [20] | ||
| Viral infection rate | 62.2% | [20] | ||
| Sensitivity | 66.6% | 96.7% | [21,22] | [15,16,17,18,19,23] |
| Specificity | 78.3% | 89.8% | ||
| Test equivocal rate | NA | 8.3% | NA | |
| Probability of prediagnostic antibiotics | ||||
| PSI score 1 and 2 | 75.0% | NA | Assumption | NA |
| PSI score 3 | 90.0% | NA | ||
| PSI score 4 | 100.0% | NA | ||
| PSI score 5 | 100.0% | NA | ||
| Prediagnostic antibiotic treatment days | 1.5 | NA | Assumption | NA |
| Baseline cohort PSI severity distributions | ||||
| PSI score 1 and 2 | 55.0% | [24] | ||
| PSI score 3 | 14.0% | |||
| PSI score 4 | 21.0% | |||
| PSI score 5 | 10.0% | |||
| Hospital admission probability from the ED | ||||
| PSI score 1 and 2 | 33.5% | [24] | ||
| PSI score 3 | 78.0% | |||
| PSI score 4 | 92.0% | |||
| PSI score 5 | 100.0% | |||
| Probability of antibiotic adverse events | ||||
| PSI score 1 and 2 | 0.65% | 0.42% | (c) | |
| PSI score 3 | 0.72% | 0.52% | ||
| PSI score 4 | 0.80% | 0.66% | ||
| PSI score 5 | 0.91% | 0.91% | ||
| Antibiotic adverse event-attributable LOS | 1.3 | [25] | ||
| Probability of CDI | ||||
| PSI score 1 and 2 | 2.43% | 1.71% | (c) | |
| PSI score 3 | 2.92% | 2.28% | ||
| PSI score 4 | 3.24% | 2.93% | ||
| PSI score 5 | 3.24% | 3.24% | ||
| Hospital-onset CDI attributable LOS (days) | 7.8 | [26] | ||
| Community-onset CDI attributable LOS (days) | 5.7 | [26] | ||
| Cost Drivers | Hospitals | Payers | Hospitals/Payers | ||
|---|---|---|---|---|---|
| Italy | Germany | Italy | Germany | Spain | |
| Diagnostic testing | x | x | x | x | x |
| ED visit | x | x | x | x | x |
| Inpatient days of antibiotic (AB) treatment | x | x | x | ||
| Outpatient days of AB treatment | x | x | x | ||
| Adverse Events | x | x | x | ||
| Outpatient CDI | x | x | x | x | x |
| Baseline hospital stay and inpatient CDI | x | x | x | x | x |
| Economic Inputs | Italy | Ref | Germany | Ref | Spain | Ref |
|---|---|---|---|---|---|---|
| Diagnostic test and ED stay cost | ||||||
| X-ray | 15.5 | Tariff 87.44.1 [32] | 9.2 | Tariff 34240 [34] | 9.2 | [35] |
| Complete blood count (CBC) | 3.2 | Tariff 90.62.2 [32] | 1.1 | Tariff 32122 [34] | 6.7 | [35] |
| Viral PCR | 118.5 | [37] | 85.0 | Tariff 32851 [34] | 75.0 | [36] |
| ED visit | 248.2 | [33] | 588.1 | [38] | 236.1 | [39] |
| Antibiotic treatment | ||||||
| Cost of inpatient AB treatment per day | 25.0 | Elaboration [12,40] | 52.7 | Elaboration [12,41] | 18.4 | Elaboration [12,42] |
| Including cost per IV administration | 6.4 | * [43] | 7.6 | Tariff 02100 [34] | 5.6 | [43,44] |
| Cost of outpatient AB treatment per day | 1.3 | Elaboration [12,40] | 5.5 | Elaboration [12,41] | 1.9 | Elaboration [12,42] |
| Hospital stays—providers | ||||||
| Hospital cost per day | 962.6 | [45] | 697.2 | [29] | 488.7 | [46] |
| Hospital stays—payers | ||||||
| Hospital cost per episode—CAP | 3198.8 | DRG 89/90 ** [32,47] | 2657.7 | DRG E79A, E65A, E79C ** [48,49] | - | |
| Hospital cost per episode—inpatient CDI | 3558.0 | DRG 89 [32] | 4059.0 | DRG E79A [48] | - | |
| Hospital cost per episode—outpatient CDI | 3484.0 | DRG 572 [32] | 3798.0 | DRG G77B [48] | - |
| Main Analysis | Scenario 1 | Scenario 2 | Scenario 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Per Patient | Per 1000 | Per Patient | Per 1000 | Per Patient | Per 1000 | Per Patient | Per 1000 | |
| Antibiotic patients avoided | 0.43 | 429 | 0.43 | 429 | 0.43 | 429 | 0.43 | 429 |
| Antibiotic days saved | 1.02 | 1020 | 1.02 | 1020 | 1.02 | 1020 | 1.02 | 1020 |
| Hospital admissions avoided | - | - | 0.01 | 8 | - | - | 0.01 | 8 |
| Hospital days saved | ||||||||
| Italy | 0.04 | 38 | 0.08 | 82 | 0.30 | 303 | 0.35 | 351 |
| Germany | 0.04 | 38 | 0.10 | 100 | 0.32 | 323 | 0.39 | 389 |
| Spain | 0.04 | 38 | 0.06 | 62 | 0.27 | 271 | 0.30 | 297 |
| (A) Italy | Main Analysis | Scenario 1 | Scenario 2 | Scenario 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Hospital | Payer | Hospital | Payer | Hospital | Payer | Hospital | Payer | |
| Diagnostic testing | - | - | - | - | - | - | - | - |
| ED visit | - | - | - | - | - | - | - | - |
| Inpatient days of AB treatment | 24.6 | - | 26.9 | - | 24.6 | - | 26.9 | - |
| Outpatient days of AB treatment | - | 0.0 | - | (0.1) | - | 0.0 | - | (0.1) |
| Adverse events | 1.1 | - | 1.2 | - | 1.1 | - | 1.2 | - |
| Outpatient CDI | 15.9 | 10.1 | 20.7 | 9.2 | 15.9 | 10.1 | 20.7 | 9.2 |
| Baseline Hospital Stay and Inpatient CDI | 19.7 | 0.9 | 57.0 | 26.7 | 274.8 | 55.1 | 315.6 | 82.1 |
| Total | 61.4 | 11.1 | 105.8 | 35.8 | 316.5 | 65.3 | 364.4 | 91.2 |
| (B) Germany | Main Analysis | Scenario 1 | Scenario 2 | Scenario 3 | ||||
| Hospital | Payer | Hospital | Payer | Hospital | Payer | Hospital | Payer | |
| Diagnostic testing | - | - | - | - | - | - | - | - |
| ED visit | - | - | - | - | - | - | - | - |
| Inpatient days of AB treatment | 51.8 | - | 56.7 | - | 51.8 | - | 56.7 | - |
| Outpatient days of AB treatment | - | 0.2 | - | (0.3) | - | 0.2 | - | (0.3) |
| Adverse events | 0.8 | - | 0.9 | - | 0.8 | - | 0.9 | - |
| Outpatient CDI | 11.5 | 11.0 | 15.0 | 10.0 | 11.5 | 11.0 | 15.0 | 10.0 |
| Baseline Hospital Stay and Inpatient CDI | 14.3 | 3.7 | 54.2 | 25.4 | 212.5 | 26.6 | 255.3 | 48.9 |
| Total | 78.4 | 14.9 | 126.7 | 35.1 | 276.7 | 37.8 | 327.8 | 58.5 |
| (C) Spain (Payers/Hospitals) | Main Analysis | Scenario 1 | Scenario 2 | Scenario 3 | ||||
| Diagnostic testing | - | - | - | - | ||||
| ED visit | - | - | - | - | ||||
| Inpatient days of AB treatment | 18.1 | 19.7 | 18.1 | 19.7 | ||||
| Outpatient days of AB treatment | 0.1 | (0.1) | 0.1 | (0.1) | ||||
| Adverse events | 0.6 | 0.6 | 0.6 | 0.6 | ||||
| Outpatient CDI | 8.1 | 10.5 | 8.1 | 10.5 | ||||
| Baseline Hospital Stay and Inpatient CDI | 10.0 | 19.9 | 123.8 | 134.2 | ||||
| Total | 36.8 | 49.8 | 150.5 | 164.9 | ||||
| Scenarios | Italy | Germany | Spain | ||
|---|---|---|---|---|---|
| Hospital | Payer | Hospital | Payer | Hospital/Payer | |
| Main analysis | 61,385 | 11,077 | 78,437 | 14,883 | 36,787 |
| Scenario 1 | 105,830 | 35,817 | 126,731 | 35,079 | 49,770 |
| Scenario 2 | 316,459 | 65,266 | 276,685 | 37,846 | 150,535 |
| Scenario 3 | 364,403 | 91,158 | 327,806 | 58,530 | 164,931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houshmand, H.; Porta, C.; Pradelli, L.; Pinciroli, M.; Sotgiu, G. Cost-Impact Analysis of a Novel Diagnostic Test to Assess Community-Acquired Pneumonia Etiology in the Emergency Department Setting: A Multi-Country European Study. Int. J. Environ. Res. Public Health 2023, 20, 3853. https://doi.org/10.3390/ijerph20053853
Houshmand H, Porta C, Pradelli L, Pinciroli M, Sotgiu G. Cost-Impact Analysis of a Novel Diagnostic Test to Assess Community-Acquired Pneumonia Etiology in the Emergency Department Setting: A Multi-Country European Study. International Journal of Environmental Research and Public Health. 2023; 20(5):3853. https://doi.org/10.3390/ijerph20053853
Chicago/Turabian StyleHoushmand, Hirad, Camilla Porta, Lorenzo Pradelli, Matteo Pinciroli, and Giovanni Sotgiu. 2023. "Cost-Impact Analysis of a Novel Diagnostic Test to Assess Community-Acquired Pneumonia Etiology in the Emergency Department Setting: A Multi-Country European Study" International Journal of Environmental Research and Public Health 20, no. 5: 3853. https://doi.org/10.3390/ijerph20053853





