Removal of Copper Ions from Wastewater: A Review
Abstract
1. Introduction
2. Restoration Techniques
2.1. Physical–Chemical Technology
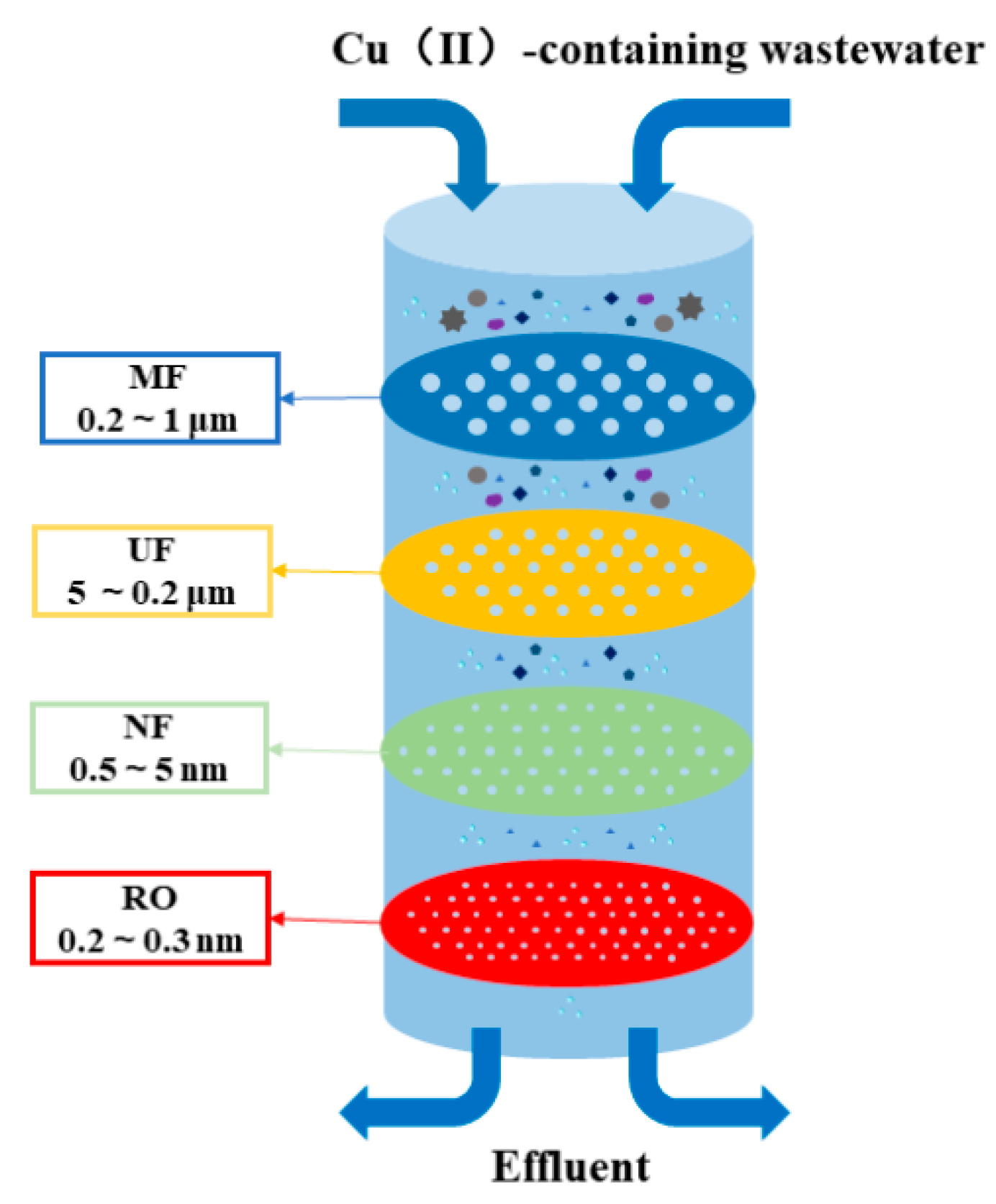
2.1.1. Membrane Separation
Microfiltration, Ultrafiltration, and Nanofiltration
Reverse Osmosis and Electrodialysis
2.1.2. Ion Exchange
2.1.3. Electrochemistry
Electrodeposition
Electroflocculation
2.1.4. Chemical Precipitation
Hydroxide Precipitation
Sulfide Precipitation
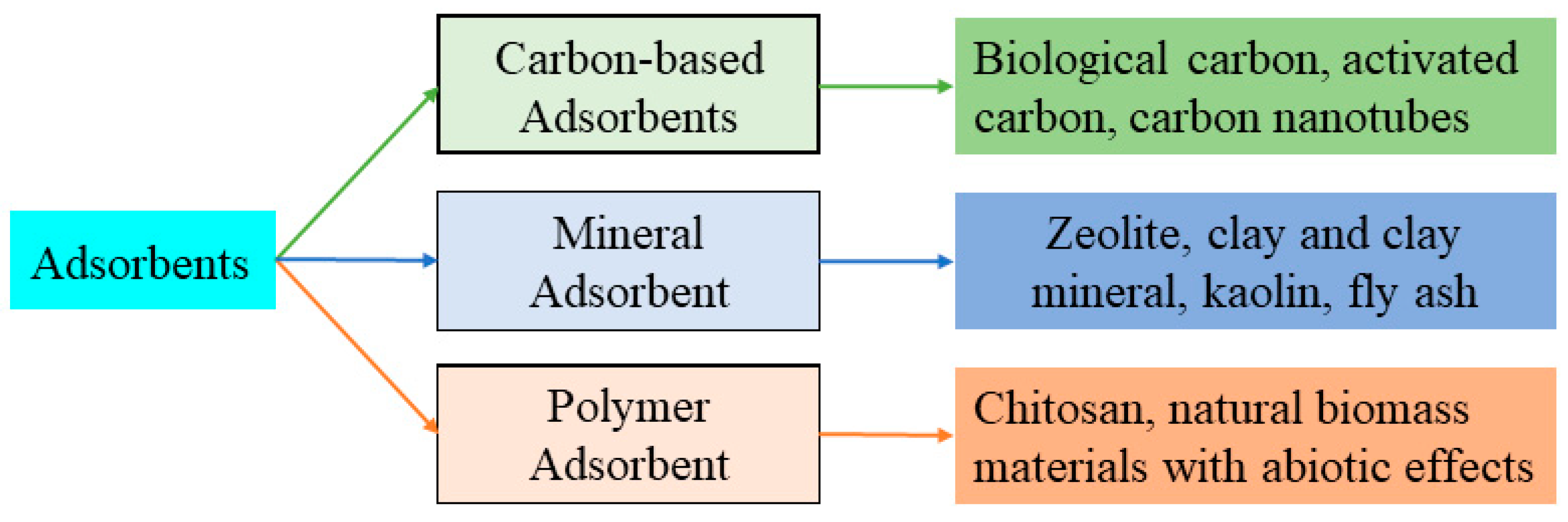
2.1.5. Adsorption
Carbon-Based Adsorbents
Mineral Adsorbents
Polymer Adsorbents
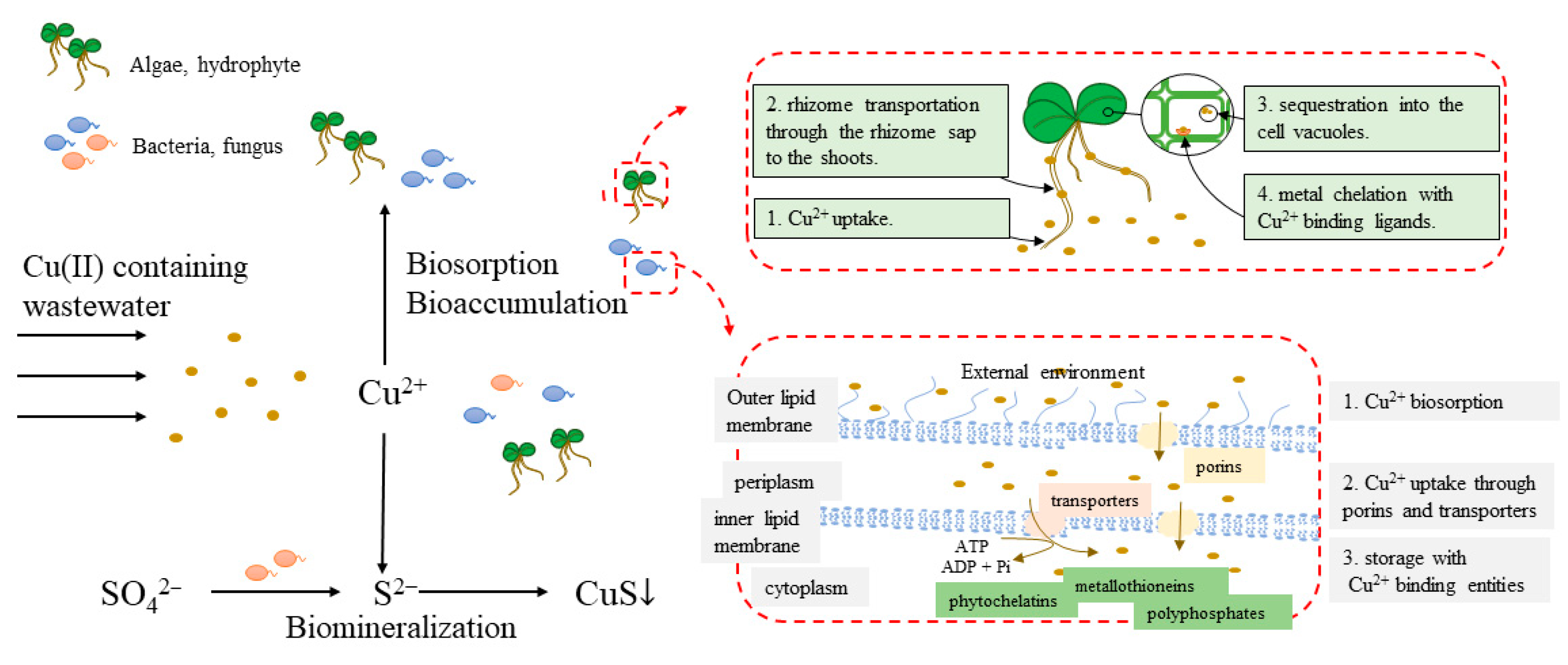
2.2. Biotechnology
3. Copper-Containing AMD Treatment Technology
4. Conclusions and Outlooks for Cu(II) Removal and Recovery
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Nover, D.; Wang, Y. Overview Assessment of Risk Evaluation and Treatment Technologies for Heavy Metal Pollution of Water and Soil. J. Clean. Prod. 2022, 379, 134043. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Majumder, C.B. Novel Biofiltration Methods for the Treatment of Heavy Metals from Industrial Wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Min, X.; Tang, C.-J.; Sillanpää, M.; Zhao, F. Recent Advances in Membrane Filtration for Heavy Metal Removal from Wastewater: A Mini Review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, X.; Xu, J. Heavy Metal Pollution in the East China Sea: A Review. Mar. Pollut. Bull. 2020, 159, 111473. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Wang, W.-X. Trace Metal Contamination in Estuarine and Coastal Environments in China. Sci. Total Environ. 2012, 421–422, 3–16. [Google Scholar] [CrossRef]
- Yan, C.; Qu, Z.; Wang, J.; Cao, L.; Han, Q. Microalgal Bioremediation of Heavy Metal Pollution in Water: Recent Advances, Challenges, and Prospects. Chemosphere 2022, 286, 131870. [Google Scholar] [CrossRef]
- Xia, F.; Qu, L.; Wang, T.; Luo, L.; Chen, H.; Dahlgren, R.A.; Zhang, M.; Mei, K.; Huang, H. Distribution and Source Analysis of Heavy Metal Pollutants in Sediments of a Rapid Developing Urban River System. Chemosphere 2018, 207, 218–228. [Google Scholar] [CrossRef]
- Yari, S.; Abbasizadeh, S.; Mousavi, S.E.; Moghaddam, M.S.; Moghaddam, A.Z. Adsorption of Pb(II) and Cu(II) Ions from Aqueous Solution by an Electrospun CeO2 Nanofiber Adsorbent Functionalized with Mercapto Groups. Process Saf. Environ. Prot. 2015, 94, 159–171. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of Nanomaterials as Adsorbents in Heavy Metal Ion Removal from Waste Water: A Review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Saleh, H.N.; Panahande, M.; Yousefi, M.; Asghari, F.B.; Oliveri Conti, G.; Talaee, E.; Mohammadi, A.A. Carcinogenic and Non-Carcinogenic Risk Assessment of Heavy Metals in Groundwater Wells in Neyshabur Plain, Iran. Biol. Trace Elem. Res. 2019, 190, 251–261. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to Improve the Adsorption Properties of Graphene-Based Adsorbent towards Heavy Metal Ions and Their Compound Pollutants: A Review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.K.; Kabir, S.F.; Bin Abdur Rahman, F.; Sakib, M.N.; Efty, S.S.; Rahman, M.M. Cu(II) Removal from Wastewater Using Chitosan-Based Adsorbents: A Review. J. Environ. Chem. Eng. 2022, 10, 108048. [Google Scholar] [CrossRef]
- Feng, Z.; Feng, C.; Chen, N.; Lu, W.; Wang, S. Preparation of Composite Hydrogel with High Mechanical Strength and Reusability for Removal of Cu(II) and Pb(II) from Water. Sep. Purif. Technol. 2022, 300, 121894. [Google Scholar] [CrossRef]
- Varma, G.; Misra, A. Copper Contaminated Wastewater—An Evaluation of Bioremedial Options. Indoor Built Environ. 2018, 27, 84–95. [Google Scholar] [CrossRef]
- Margalioth, E.J.; Schenker, J.G.; Chevion, M. Copper and Zinc Levels in Normal and Malignant Tissues. Cancer 1983, 52, 868–872. [Google Scholar] [CrossRef]
- Goodman, V.L.; Brewer, G.J.; Merajver, S.D. Copper Deficiency as an Anti-Cancer Strategy. Endocr. Relat. Cancer 2004, 11, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Pensini, E.; Laredo, T.; Earnden, L.; Marangoni, A.G.; Ghazani, S.M. A ‘Three in One’ Complexing Agent Enables Copper Desorption from Polluted Soil, Its Removal from Groundwater and Its Detection. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126840. [Google Scholar] [CrossRef]
- Ortega, P.; Sánchez, E.; Gil, E.; Matamoros, V. Use of Cover Crops in Vineyards to Prevent Groundwater Pollution by Copper and Organic Fungicides. Soil Column Studies. Chemosphere 2022, 303, 134975. [Google Scholar] [CrossRef] [PubMed]
- Donnachie, R.L.; Johnson, A.C.; Moeckel, C.; Pereira, M.G.; Sumpter, J.P. Using Risk-Ranking of Metals to Identify Which Poses the Greatest Threat to Freshwater Organisms in the UK. Environ. Pollut. 2014, 194, 17–23. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W.; Römkens, P.F.A.M.; Bonten, L.T.C. Spatially Explicit Integrated Risk Assessment of Present Soil Concentrations of Cadmium, Lead, Copper and Zinc in The Netherlands. Water Air Soil Pollut. 2008, 191, 199–215. Available online: https://link.springer.com/article/10.1007/s11270-008-9617-z (accessed on 4 October 2022). [CrossRef]
- Chen, C.S. Ecological Risk Assessment for Aquatic Species Exposed to Contaminants in Keelung River, Taiwan. Chemosphere 2005, 61, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yuan, Z.; Xiaona, H.; Wei, M. Distribution and Bioaccumulation of Heavy Metals in Aquatic Organisms of Different Trophic Levels and Potential Health Risk Assessment from Taihu Lake, China. Ecotoxicol. Environ. Saf. 2012, 81, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Wang, G.; Li, X.; Wang, S.; Zhao, Y. Pollution, Sources and Environmental Risk Assessment of Heavy Metals in the Surface AMD Water, Sediments and Surface Soils around Unexploited Rona Cu Deposit, Tibet, China. Chemosphere 2020, 248, 125988. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, P.; Wang, X.; Hu, B.; Liu, S.; Ma, J. Abundant Microbial Communities Act as More Sensitive Bio-Indicators for Ecological Evaluation of Copper Mine Contamination than Rare Taxa in River Sediments. Environ. Pollut. 2022, 305, 119310. [Google Scholar] [CrossRef] [PubMed]
- Rizo, O.D.; Castillo, F.E.; López, J.O.A.; Merlo, M.H. Assessment of Heavy Metal Pollution in Urban Soils of Havana City, Cuba. Bull. Environ. Contam. Toxicol. 2011, 87, 414–419. [Google Scholar] [CrossRef]
- Abraham, M.R.; Susan, T.B. Water Contamination with Heavy Metals and Trace Elements from Kilembe Copper Mine and Tailing Sites in Western Uganda; Implications for Domestic Water Quality. Chemosphere 2017, 169, 281–287. [Google Scholar] [CrossRef]
- Chabukdhara, M.; Nema, A.K. Heavy Metals Assessment in Urban Soil around Industrial Clusters in Ghaziabad, India: Probabilistic Health Risk Approach. Ecotoxicol. Environ. Saf. 2013, 87, 57–64. [Google Scholar] [CrossRef]
- Damous, N.R.; Wagener, A.d.L.R.; Patchineelam, S.R.; Wagene, K. Baseline Studies on Water and Sediments in the Copper Mining Region of Salobo-3A, Carajas: Amazon, Brazil. J. Braz. Chem. Soc. 2002, 13, 140–150. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Wu, F.; Fang, X.; Tan, K. Recovery of Copper-Dominated Resources from Copper Mine Drainage by Chemical Oxidation and Sulfur Biocycling: A Pilot-Scale Study. J. Clean. Prod. 2022, 378, 134525. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, X.; Xu, Q.; Qian, Y. Water Quality Criteria and Ecological Risk Assessment for Copper in Liaodong Bay, China. Mar. Pollut. Bull. 2022, 185, 114164. [Google Scholar] [CrossRef]
- Jong, T.; Parry, D.L. Removal of Sulfate and Heavy Metals by Sulfate Reducing Bacteria in Short-Term Bench Scale Upflow Anaerobic Packed Bed Reactor Runs. Water Res. 2003, 37, 3379–3389. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Charcosset, C. Ultrafiltration, Microfiltration, Nanofiltration and Reverse Osmosis in Integrated Membrane Processes—Integrated Membrane Systems and Processes—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781118739167.ch1 (accessed on 7 October 2022).
- Li, S.; Wang, X.; Guo, Y.; Hu, J.; Lin, S.; Tu, Y.; Chen, L.; Ni, Y.; Huang, L. Recent Advances on Cellulose-Based Nanofiltration Membranes and Their Applications in Drinking Water Purification: A Review. J. Clean. Prod. 2022, 333, 130171. [Google Scholar] [CrossRef]
- Menzel, K.; Barros, L.; García, A.; Ruby-Figueroa, R.; Estay, H. Metal Sulfide Precipitation Coupled with Membrane Filtration Process for Recovering Copper from Acid Mine Drainage. Sep. Purif. Technol. 2021, 270, 118721. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of Heavy Metals from Water Using Polyvinylamine by Polymer-Enhanced Ultrafiltration and Flocculation. Sep. Purif. Technol. 2016, 158, 124–136. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, H.; Wu, Z. Removal of Cu(II) Ions from Contaminated Waters Using a Conducting Microfiltration Membrane. J. Hazard. Mater. 2017, 339, 182–190. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration Membranes Review: Recent Advances and Future Prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, X.; Li, G.; Fei, G.; Jin, P.; Liu, Y.; Wouters, C.; Meir, G.; Li, Y.; Van der Bruggen, B. Selective Removal of Heavy Metals from Saline Water by Nanofiltration. Desalination 2022, 525, 115380. [Google Scholar] [CrossRef]
- Raaijmakers, M.J.T.; Benes, N.E. Current Trends in Interfacial Polymerization Chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- Lim, M.-Y.; Choi, Y.-S.; Kim, J.; Kim, K.; Shin, H.; Kim, J.-J.; Shin, D.M.; Lee, J.-C. Cross-Linked Graphene Oxide Membrane Having High Ion Selectivity and Antibacterial Activity Prepared Using Tannic Acid-Functionalized Graphene Oxide and Polyethyleneimine. J. Membr. Sci. 2017, 521, 1–9. [Google Scholar] [CrossRef]
- Anantharaman, A.; Chun, Y.; Hua, T.; Chew, J.W.; Wang, R. Pre-Deposited Dynamic Membrane Filtration—A Review. Water Res. 2020, 173, 115558. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, L.; Shen, X.; Sotto, A.; Gao, C.; Shen, J. Polythyleneimine-Modified Original Positive Charged Nanofiltration Membrane: Removal of Heavy Metal Ions and Dyes. Sep. Purif. Technol. 2019, 222, 117–124. [Google Scholar] [CrossRef]
- Tian, J.; Chang, H.; Gao, S.; Zhang, R. How to Fabricate a Negatively Charged NF Membrane for Heavy Metal Removal via the Interfacial Polymerization between PIP and TMC? Desalination 2020, 491, 114499. [Google Scholar] [CrossRef]
- Tang, S.; Jiao, Y.; Yan, F.; Qin, Q.; Qin, S.; Ma, X.; Li, J.; Cui, Z. Construction of Hollow Fiber Nanofiltration Separation Layer with Bridging Network Structure by Polymer-Anchored Co-Deposition for High-Concentration Heavy Metal Ion Removal. J. Membr. Sci. 2022, 661, 120864. [Google Scholar] [CrossRef]
- Gao, J.; Sun, S.-P.; Zhu, W.-P.; Chung, T.-S. Chelating Polymer Modified P84 Nanofiltration (NF) Hollow Fiber Membranes for High Efficient Heavy Metal Removal. Water Res. 2014, 63, 252–261. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Bae, J. Membrane Processes and Renewable Energies. Renew. Sustain. Energy Rev. 2015, 43, 1343–1398. [Google Scholar] [CrossRef]
- Anis, S.F.; Lalia, B.S.; Hashaikeh, R.; Hilal, N. Ceramic Nanofiltration Membranes for Efficient Fouling Mitigation through Periodic Electrolysis. Sep. Purif. Technol. 2022, 303, 122228. [Google Scholar] [CrossRef]
- Qasim, M.; Darwish, N.N.; Mhiyo, S.; Darwish, N.A.; Hilal, N. The Use of Ultrasound to Mitigate Membrane Fouling in Desalination and Water Treatment. Desalination 2018, 443, 143–164. [Google Scholar] [CrossRef]
- Zhao, S.; Minier-Matar, J.; Chou, S.; Wang, R.; Fane, A.G.; Adham, S. Gas Field Produced/Process Water Treatment Using forward Osmosis Hollow Fiber Membrane: Membrane Fouling and Chemical Cleaning. Desalination 2017, 402, 143–151. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, J.; Wang, Z.; Østerhus, S.W. Backpulsing Technology Applied in MF and UF Processes for Membrane Fouling Mitigation: A Review. J. Membr. Sci. 2019, 587, 117136. [Google Scholar] [CrossRef]
- Ku, Y.; Chen, S.-W.; Wang, W.-Y. Effect of Solution Composition on the Removal of Copper Ions by Nanofiltration. Sep. Purif. Technol. 2005, 43, 135–142. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse Osmosis Desalination: A State-of-the-Art Review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Coman, V.; Robotin, B.; Ilea, P. Nickel Recovery/Removal from Industrial Wastes: A Review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Porada, S.; Elimelech, M.; Dykstra, J.E. Tutorial Review of Reverse Osmosis and Electrodialysis. J. Membr. Sci. 2022, 647, 120221. [Google Scholar] [CrossRef]
- Peng, W.; Escobar, I.C.; White, D.B. Effects of Water Chemistries and Properties of Membrane on the Performance and Fouling—A Model Development Study. J. Membr. Sci. 2004, 238, 33–46. [Google Scholar] [CrossRef]
- Ozaki, H.; Sharma, K.; Saktaywin, W. Performance of an Ultra-Low-Pressure Reverse Osmosis Membrane (ULPROM) for Separating Heavy Metal: Effects of Interference Parameters. Desalination 2002, 144, 287–294. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Qu, X.; Li, Z.; Ni, J. Mechanism of Combination Membrane and Electro-Winning Process on Treatment and Remediation of Cu2+ Polluted Water Body. J. Environ. Sci. 2009, 21, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, T.; Taha, S.; Taleb Ahmed, M.; Maachi, R.; Dorange, G. Removal of Copper from Industrial Effluent Using a Spiral Wound Module—Film Theory and Hydrodynamic Approach. Desalination 2006, 200, 403–405. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Cséfalvay, E.; Pauer, V.; Mizsey, P. Recovery of Copper from Process Waters by Nanofiltration and Reverse Osmosis. Desalination 2009, 240, 132–142. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Darveau, O.; Meers, E. Fate of Micronutrients and Heavy Metals in Digestate Processing Using Vibrating Reversed Osmosis as Resource Recovery Technology. Sep. Purif. Technol. 2019, 223, 81–87. [Google Scholar] [CrossRef]
- Nemati, M.; Hosseini, S.M.; Shabanian, M. Novel Electrodialysis Cation Exchange Membrane Prepared by 2-Acrylamido-2-Methylpropane Sulfonic Acid; Heavy Metal Ions Removal. J. Hazard. Mater. 2017, 337, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, L.; García, I.; Arriagada, P.; Casas, J.M. The Use of Electrodialysis for Metal Separation and Water Recovery from CuSO4–H2SO4–Fe Solutions. Sep. Purif. Technol. 2009, 68, 105–108. [Google Scholar] [CrossRef]
- Baraka, A.; Hall, P.J.; Heslop, M.J. Melamine–Formaldehyde–NTA Chelating Gel Resin: Synthesis, Characterization and Application for Copper(II) Ion Removal from Synthetic Wastewater. J. Hazard. Mater. 2007, 140, 86–94. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and Equilibrium Studies for the Removal of Nickel and Zinc from Aqueous Solutions by Ion Exchange Resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Hu, H.; Yang, J.; Wang, C.; Cheng, Z. Removal of Trace Copper from Simulated Nickel Electrolytes Using a New Chelating Resin. Hydrometallurgy 2018, 180, 121–131. [Google Scholar] [CrossRef]
- Li, Q.; Ji, M.; Li, X.; Song, H.; Wang, G.; Qi, C.; Li, A. Efficient Co-Removal of Copper and Tetracycline from Aqueous Solution by Using Permanent Magnetic Cation Exchange Resin. Bioresour. Technol. 2019, 293, 122068. [Google Scholar] [CrossRef]
- Ulloa, L.; Bringas, E.; San-Román, M.-F. Simultaneous Separation of Nickel and Copper from Sulfuric Acid Using Chelating Weak Base Resins. Available online: https://onlinelibrary.wiley.com/doi/10.1002/jctb.6364 (accessed on 9 October 2022).
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of Iron, Aluminium, Manganese and Copper from Leach Solutions of Lithium-Ion Battery Waste Using Ion Exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Murray, A.; Örmeci, B. Use of Polymeric Sub-Micron Ion-Exchange Resins for Removal of Lead, Copper, Zinc, and Nickel from Natural Waters. J. Environ. Sci. 2019, 75, 247–254. [Google Scholar] [CrossRef]
- van Deventer, J. Selected Ion Exchange Applications in the Hydrometallurgical Industry: Solvent Extraction and Ion Exchange. Volume 29, No. 5–6. Available online: https://www.tandfonline.com/doi/abs/10.1080/07366299.2011.595626 (accessed on 9 October 2022).
- Ulloa, L.; Martínez-Minchero, M.; Bringas, E.; Cobo, A.; San-Román, M.F. Split Regeneration of Chelating Resins for the Selective Recovery of Nickel and Copper. Sep. Purif. Technol. 2020, 253, 117516. [Google Scholar] [CrossRef]
- Porto, M.B.; Alvim, L.B.; de Almeida Neto, A.F. Nickel Removal from Wastewater by Induced Co-Deposition Using Tungsten to Formation of Metallic Alloys. J. Clean. Prod. 2017, 142, 3293–3299. [Google Scholar] [CrossRef]
- Carpanedo de Morais Nepel, T.; Landers, R.; Gurgel Adeodato Vieira, M.; Florêncio de Almeida Neto, A. Metallic Copper Removal Optimization from Real Wastewater Using Pulsed Electrodeposition. J. Hazard. Mater. 2020, 384, 121416. [Google Scholar] [CrossRef]
- Wu, H.; Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Liang, S.; Hu, Z.; Liu, H. Strategies and Techniques to Enhance Constructed Wetland Performance for Sustainable Wastewater Treatment. Environ. Sci. Pollut. Res. 2015, 22, 14637–14650. [Google Scholar] [CrossRef] [PubMed]
- Fedje, K.K.; Strömvall, A.-M. Enhanced Soil Washing with Copper Recovery Using Chemical Precipitation. J. Environ. Manag. 2019, 236, 68–74. [Google Scholar] [CrossRef]
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy Metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) Removal from Water in Malaysia: Post Treatment by High Quality Limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and Applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of Heavy Metal Removals from Aqueous Solutions by Chemical Precipitation and Characteristics of Precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Mirbagheri, S.A.; Hosseini, S.N. Pilot Plant Investigation on Petrochemical Wastewater Treatmentfor the Removal of Copper and Chromium with the Objective of Reuse. Desalination 2005, 171, 85–93. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Soklun, H.; Qu, G.; Xia, T.; Guo, X.; Jia, H.; Zhu, L. A Green Strategy for Simultaneous Cu(II)-EDTA Decomplexation and Cu Precipitation from Water by Bicarbonate-Activated Hydrogen Peroxide/Chemical Precipitation. Chem. Eng. J. 2019, 370, 1298–1309. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, J.; Chen, X.; Du, D.; Wu, R.; Qu, G.; Guo, X.; Jia, H.; Wang, T. Non-Thermal Plasma Oxidation of Cu(II)-EDTA and Simultaneous Cu(II) Elimination by Chemical Precipitation. J. Environ. Manag. 2019, 248, 109237. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, K.; Chen, Q.; Wang, A.; Chen, W. Application of Magnetic Ferrite Nanoparticles for Removal of Cu(II) from Copper-Ammonia Wastewater. J. Alloy. Compd. 2019, 773, 140–149. [Google Scholar] [CrossRef]
- Monhemius, A.J. Precipitation Diagrams for Metal Hydroxides, Sulphides, Arsenates and Phosphates. J.-GLOBAL 1977, 68, 202–206. [Google Scholar]
- Jiang, S.; Fu, F.; Qu, J.; Xiong, Y. A Simple Method for Removing Chelated Copper from Wastewaters: Ca(OH)2-Based Replacement-Precipitation. Chemosphere 2008, 73, 785–790. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Adsorption of Copper on Chitin-Based Materials: Kinetic and Thermodynamic Studies. J. Taiwan Inst. Chem. Eng. 2016, 65, 140–148. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA Functionalized Magnetic Graphene Oxide for Removal of Pb(II), Hg(II) and Cu(II) in Water Treatment: Adsorption Mechanism and Separation Property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Koppula, S.; Jagasia, P.; Panchangam, M.K.; Manabolu Surya, S.B. Synthesis of Bimetallic Metal-Organic Frameworks Composite for the Removal of Copper(II), Chromium(VI), and Uranium(VI) from the Aqueous Solution Using Fixed-Bed Column Adsorption. J. Solid State Chem. 2022, 312, 123168. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y.; et al. Investigation of the Adsorption-Reduction Mechanisms of Hexavalent Chromium by Ramie Biochars of Different Pyrolytic Temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, Z.; Feng, Q.; Yao, D.; Yu, J.; Wang, D.; Lv, S.; Liu, Y.; Zhou, N.; Zhong, M. Effect of Pyrolysis Condition on the Adsorption Mechanism of Lead, Cadmium and Copper on Tobacco Stem Biochar. J. Clean. Prod. 2018, 187, 996–1005. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of Copper and Zinc by Biochars Produced from Pyrolysis of Hardwood and Corn Straw in Aqueous Solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef]
- Ma, J.; Huang, W.; Zhang, X.; Li, Y.; Wang, N. The Utilization of Lobster Shell to Prepare Low-Cost Biochar for High-Efficient Removal of Copper and Cadmium from Aqueous: Sorption Properties and Mechanisms. J. Environ. Chem. Eng. 2021, 9, 104703. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, X.; Miao, R.; Wang, M.; Zhou, H.; He, L.; Guan, Q. Mn-Embedded Porous Rubber Seed Shell Biochar for Enhanced Removal of Copper Ions and Catalytic Efficacy of the Used Adsorbent for Hydrogenation of Furfural. Chem. Eng. J. 2022, 441, 136065. [Google Scholar] [CrossRef]
- Angın, D.; Altintig, E.; Köse, T.E. Influence of Process Parameters on the Surface and Chemical Properties of Activated Carbon Obtained from Biochar by Chemical Activation. Bioresour. Technol. 2013, 148, 542–549. [Google Scholar] [CrossRef]
- Pu, X.; Yao, L.; Yang, L.; Jiang, W.; Jiang, X. Utilization of Industrial Waste Lithium-Silicon-Powder for the Fabrication of Novel Nap Zeolite for Aqueous Cu(II) Removal. J. Clean. Prod. 2020, 265, 121822. [Google Scholar] [CrossRef]
- Solanki, P.; Gupta, V.; Kulshrestha, R. Synthesis of Zeolite from Fly Ash and Removal of Heavy Metal Ions from Newly Synthesized Zeolite. E-J. Chem. 2010, 7, 1200–1205. [Google Scholar] [CrossRef]
- Sahu, J.N.; Acharya, J.; Meikap, B.C. Optimization of Production Conditions for Activated Carbons from Tamarind Wood by Zinc Chloride Using Response Surface Methodology. Bioresour. Technol. 2010, 101, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.; Chaudhuri, S.; Sigmund, G.; Robertson, I.; Hawkins, N.; Dunlop, T.; Hofmann, T. Wood Ash Amended Biochar for the Removal of Lead, Copper, Zinc and Cadmium from Aqueous Solution. Environ. Technol. Innov. 2021, 24, 101961. [Google Scholar] [CrossRef]
- Sinha, R.; Kumar, R.; Sharma, P.; Kant, N.; Shang, J.; Aminabhavi, T.M. Removal of Hexavalent Chromium via Biochar-Based Adsorbents: State-of-the-Art, Challenges, and Future Perspectives. J. Environ. Manag. 2022, 317, 115356. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A Review of Potentially Low-Cost Sorbents for Heavy Metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Darweesh, M.A.; Elgendy, M.Y.; Ayad, M.I.; Ahmed, A.M.; Elsayed, N.M.K.; Hammad, W.A. Adsorption Isotherm, Kinetic, and Optimization Studies for Copper (II) Removal from Aqueous Solutions by Banana Leaves and Derived Activated Carbon. S. Afr. J. Chem. Eng. 2022, 40, 10–20. [Google Scholar] [CrossRef]
- Verma, B.; Balomajumder, C. Surface Modification of One-Dimensional Carbon Nanotubes: A Review for the Management of Heavy Metals in Wastewater. Environ. Technol. Innov. 2020, 17, 100596. [Google Scholar] [CrossRef]
- Khanday, W.A.; Marrakchi, F.; Asif, M.; Hameed, B.H. Mesoporous Zeolite–Activated Carbon Composite from Oil Palm Ash as an Effective Adsorbent for Methylene Blue. J. Taiwan Inst. Chem. Eng. 2017, 70, 32–41. [Google Scholar] [CrossRef]
- Xie, W.-M.; Zhou, F.-P.; Bi, X.-L.; Chen, D.-D.; Li, J.; Sun, S.-Y.; Liu, J.-Y.; Chen, X.-Q. Accelerated Crystallization of Magnetic 4A-Zeolite Synthesized from Red Mud for Application in Removal of Mixed Heavy Metal Ions. J. Hazard. Mater. 2018, 358, 441–449. [Google Scholar] [CrossRef]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Jiang, M.; Jin, X.; Lu, X.-Q.; Chen, Z. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto Natural Kaolinite Clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Abou-El-Sherbini, K.S.; Hassanien, M.M. Study of Organically-Modified Montmorillonite Clay for the Removal of Copper(II). J. Hazard. Mater. 2010, 184, 654–661. [Google Scholar] [CrossRef]
- Benavente, M.; Moreno, L.; Martinez, J. Sorption of Heavy Metals from Gold Mining Wastewater Using Chitosan. J. Taiwan Inst. Chem. Eng. 2011, 42, 976–988. [Google Scholar] [CrossRef]
- Ahmad, M.; Zhang, B.; Wang, J.; Xu, J.; Manzoor, K.; Ahmad, S.; Ikram, S. New Method for Hydrogel Synthesis from Diphenylcarbazide Chitosan for Selective Copper Removal. Int. J. Biol. Macromol. 2019, 136, 189–198. [Google Scholar] [CrossRef]
- Miretzky, P.; Saralegui, A.; Fernández Cirelli, A. Simultaneous Heavy Metal Removal Mechanism by Dead Macrophytes. Chemosphere 2006, 62, 247–254. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and Plant Derived Biomass for Removal of Heavy Metals from Wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef]
- Areco, M.M.; Hanela, S.; Duran, J.; dos Santos Afonso, M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by Dead Biomasses of Green Alga Ulva Lactuca and the Development of a Sustainable Matrix for Adsorption Implementation. J. Hazard. Mater. 2012, 213–214, 123–132. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Hassan, A.F.; Azab, Y.A. Biosorption of Toxic Heavy Metals from Aqueous Solution by Ulva Lactuca Activated Carbon. Egypt. J. Basic Appl. Sci. 2016, 3, 241–249. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Das, S. Biosorption and Removal of Toxic Heavy Metals by Metal Tolerating Bacteria for Bioremediation of Metal Contamination: A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 104686. [Google Scholar] [CrossRef]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current Perspectives on Concept, Definition and Application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Lau, P.S.; Lee, H.Y.; Tsang, C.C.K.; Tam, N.F.Y.; Wong, Y.S. Effect of Metal Interference, PH and Temperature on Cu and Ni Biosorption by Chlorella Vulgaris and Chlorella Miniata. Available online: https://www.tandfonline.com/doi/abs/10.1080/09593332008616890 (accessed on 25 November 2022).
- Cornu, J.-Y.; Huguenot, D.; Jézéquel, K.; Lollier, M.; Lebeau, T. Bioremediation of Copper-Contaminated Soils by Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 26. [Google Scholar] [CrossRef]
- López, A.; Lázaro, N.; Priego, J.M.; Marqués, A.M. Effect of PH on the Biosorption of Nickel and Other Heavy Metals by Pseudomonas Fluorescens 4F39. J. Ind. Microbiol. Biotechnol. 2000, 24, 146–151. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, Y.; Yang, X.; Zhang, B.; He, X.; Xu, W.; Huang, K. Cadmium Tolerant Characteristic of a Newly Isolated Lactococcus Lactis Subsp. Lactis. Environ. Toxicol. Pharmacol. 2016, 48, 183–190. [Google Scholar] [CrossRef]
- Palanivel, T.M.; Sivakumar, N.; Al-Ansari, A.; Victor, R. Bioremediation of Copper by Active Cells of Pseudomonas Stutzeri LA3 Isolated from an Abandoned Copper Mine Soil. J. Environ. Manag. 2020, 253, 109706. [Google Scholar] [CrossRef]
- Solioz, M.; D Vulpe, C. (PDF) CPx-Type ATPases: A Class of P-Type ATPases That Pump Heavy Metals. Available online: https://www.researchgate.net/publication/14447898_CPx-type_ATPases_A_class_of_P-type_ATPases_that_pump_heavy_metals (accessed on 25 November 2022).
- Albarracin, V.H.; Amoroso, M.J.; Abate, C.M. Isolation and Characterization of Indigenous Copper-Resistant Actinomycete Strains. Chem. Erde-Geochem. 2005, 65, 145–156. [Google Scholar] [CrossRef]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial Resistance to Metals in the Environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207. [Google Scholar] [CrossRef]
- White, C.; Gadd, G.M. Uptake and Cellular Distribution of Copper, Cobalt and Cadmium in Strains of Saccharomyces Cerevisiae Cultured on Elevated Concentrations of These Metals. FEMS Microbiol. Lett. 1986, 38, 277–283. [Google Scholar] [CrossRef]
- Dönmez, G.; Aksu, Z. Bioaccumulation of Copper(Ii) and Nickel(Ii) by the Non-Adapted and Adapted Growing CANDIDA SP. Water Res. 2001, 35, 1425–1434. [Google Scholar] [CrossRef]
- Chen, C.; Song, Y.; Zhuang, K.; Li, L.; Xia, Y.; Shen, Z. Proteomic Analysis of Copper-Binding Proteins in Excess Copper-Stressed Roots of Two Rice (Oryza Sativa L.) Varieties with Different Cu Tolerances. PLoS ONE 2015, 10, e0125367. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0125367 (accessed on 11 October 2022).
- Colica, G.; Li, H.; Rossi, F.; Li, D.; Liu, Y.; De Philippis, R. Microbial Secreted Exopolysaccharides Affect the Hydrological Behavior of Induced Biological Soil Crusts in Desert Sandy Soils. Soil Biol. Biochem. 2014, 68, 62–70. [Google Scholar] [CrossRef]
- Bonilla, J.O.; Callegari, E.A.; Paez, M.D.; Gil, R.A.; Villegas, L.B. Characterization of Copper Stress Response in Fusarium Tricinctum M6: A Metal-Resistant Microorganism Isolated from an Acid Mine Drainage-Affected Environment. J. Hazard. Mater. 2021, 412, 125216. [Google Scholar] [CrossRef]
- Solioz, M.; Abicht, H.K.; Mermod, M.; Mancini, S. Response of Gram-Positive Bacteria to Copper Stress. J. Biol. Inorg. Chem. 2009, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism Remediation Strategies towards Heavy Metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Smith, S.R. A Critical Review of the Bioavailability and Impacts of Heavy Metals in Municipal Solid Waste Composts Compared to Sewage Sludge. Environ. Int. 2009, 35, 142–156. [Google Scholar] [CrossRef]
- Shutcha, M.N.; Faucon, M.P.; Kissi, C.K.; Colinet, G.; Mahy, G.; Luhembwe, M.N.; Visser, M.; Meerts, P. Three Years of Phytostabilisation Experiment of Bare Acidic Soil Extremely Contaminated by Copper Smelting Using Plant Biodiversity of Metal-Rich Soils in Tropical Africa (Katanga, DR Congo). Ecol. Eng. 2015, 82, 81–90. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A Critical Review on Remediation, Reuse, and Resource Recovery from Acid Mine Drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of Acid Mine Drainage-Impacted Water. Curr. Pollut. Rep. 2015, 1, 131–141. [Google Scholar] [CrossRef]
- Santos Jallath, J.E.; Romero, F.M.; Iturbe Argüelles, R.; Cervantes Macedo, A.; Goslinga Arenas, J. Acid Drainage Neutralization and Trace Metals Removal by a Two-Step System with Carbonated Rocks, Estado de Mexico, Mexico. Environ. Earth Sci. 2018, 77, 86. [Google Scholar] [CrossRef]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of Passive Systems for Acid Mine Drainage Treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, M.; Wang, J.; Zhang, Z.; Duan, C.; Wang, X.; Zhao, S.; Bai, X.; Li, Z.; Li, Z.; et al. A Global Meta-Analysis of Heavy Metal(Loid)s Pollution in Soils near Copper Mines: Evaluation of Pollution Level and Probabilistic Health Risks. Sci. Total Environ. 2022, 835, 155441. [Google Scholar] [CrossRef]
- Covre, W.P.; Ramos, S.J.; Pereira, W.V.D.S.; Souza, E.S.; Martins, G.C.; Teixeira, O.M.M.; Amarante, C.B.D.; Dias, Y.N.; Fernandes, A.R. Impact of Copper Mining Wastes in the Amazon: Properties and Risks to Environment and Human Health. J. Hazard. Mater. 2022, 421, 126688. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Mohamed, I.M.A.; Huang, W.; Liu, C. Membrane Fouling Mitigation for Enhanced Water Flux and High Separation of Humic Acid and Copper Ion Using Hydrophilic Polyurethane Modified Cellulose Acetate Ultrafiltration Membranes. React. Funct. Polym. 2020, 150, 104538. [Google Scholar] [CrossRef]
- Rajeswari, A.; Jackcina Stobel Christy, E.; Ida Celine Mary, G.; Jayaraj, K.; Pius, A. Cellulose Acetate Based Biopolymeric Mixed Matrix Membranes with Various Nanoparticles for Environmental Remediation-A Comparative Study. J. Environ. Chem. Eng. 2019, 7, 103278. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Yuan, X.; Ding, W.; Li, Y.; Wang, J. Separation of Oil-Water Emulsion and Adsorption of Cu(II) on a Chitosan-Cellulose Acetate-TiO2 Based Membrane. Chemosphere 2019, 235, 239–247. [Google Scholar] [CrossRef]
- Keane, M.A. The Removal of Copper and Nickel from Aqueous Solution Using Y Zeolite Ion Exchangers. Colloids Surf. A Physicochem. Eng. Asp. 1998, 138, 11–20. [Google Scholar] [CrossRef]
- Li, Q.; Fu, L.; Wang, Z.; Li, A.; Shuang, C.; Gao, C. Synthesis and Characterization of a Novel Magnetic Cation Exchange Resin and Its Application for Efficient Removal of Cu2+ and Ni2+ from Aqueous Solutions. J. Clean. Prod. 2017, 165, 801–810. [Google Scholar] [CrossRef]
- Ahmed Basha, C.; Saravanathamizhan, R.; Nandakumar, V.; Chitra, K.; Lee, C.W. Copper Recovery and Simultaneous COD Removal from Copper Phthalocyanine Dye Effluent Using Bipolar Disc Reactor. Chem. Eng. Res. Des. 2013, 91, 552–559. [Google Scholar] [CrossRef]
- Najafpoor, A.A.; Davoudi, M.; Salmani, E.R. Optimization of Copper Removal from Aqueous Solutions in a Continuous Electrochemical Cell Divided by Cellulosic Separator. Water Sci. Technol. 2017, 75, 1233–1242. [Google Scholar] [CrossRef]
- Sulonen, M.L.K.; Kokko, M.E.; Lakaniemi, A.-M.; Puhakka, J.A. Simultaneous Removal of Tetrathionate and Copper from Simulated Acidic Mining Water in Bioelectrochemical and Electrochemical Systems. Hydrometallurgy 2018, 176, 129–138. [Google Scholar] [CrossRef]
- Navarro, R.R.; Navarro, R.C.; Alfafara, C.G.; Demafelis, R.B.; Tatsumi, K. Simultaneous Treatment of Semiconductor Wastewater and Distillery Slops by Mixing and Precipitation/Coagulation. J. Chem. Technol. Biotechnol. 2005, 80, 1125–1130. [Google Scholar] [CrossRef]
- Shan, Q.; Zhang, Y.; Xue, X. Removal of Copper from Wastewater by Using the Synthetic Nesquehonite. Environ. Prog. Sustain. Energy 2013, 32, 543–546. [Google Scholar] [CrossRef]
- Peng, C.; Chai, L.-Y.; Tang, C.-J.; Min, X.-B.; Ali, M.; Song, Y.-X.; Qi, W.-M. Feasibility and Enhancement of Copper and Ammonia Removal from Wastewater Using Struvite Formation: A Comparative Research. J. Chem. Technol. Biotechnol. 2017, 92, 325–333. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lee, M.G.-H.; Chanaka Udayanga, W.D.; Veksha, A.; Bao, Y.; Giannis, A.; Lim, J.-W.; Lisak, G. Insights into the Single and Binary Adsorption of Copper(II) and Nickel(II) on Hexagonal Boron Nitride: Performance and Mechanistic Studies. J. Environ. Chem. Eng. 2019, 7, 102872. [Google Scholar] [CrossRef]
- Pfeifer, A.; Škerget, M.; Čolnik, M. Removal of Iron, Copper, and Lead from Aqueous Solutions with Zeolite, Bentonite, and Steel Slag. Sep. Sci. Technol. 2021, 56, 2989–3000. [Google Scholar] [CrossRef]
- Meseldzija, S.; Petrovic, J.; Onjia, A.; Volkov-Husovic, T.; Nesic, A.; Vukelic, N. Utilization of Agro-Industrial Waste for Removal of Copper Ions from Aqueous Solutions and Mining-Wastewater. J. Ind. Eng. Chem. 2019, 75, 246–252. [Google Scholar] [CrossRef]
- Tian, Y.; Ye, J.; Yin, H.; Peng, H.; Li, Q.; Bai, J.; Xie, D. Characteristics of Copper Removal and Ion Release during Copper Biosorption by Stenotrophomonas Maltophilia in Presence of Benzo[a]Pyrene. J. Cent. South Univ. 2013, 20, 2796–2805. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Wong, Y.S.; Simpson, C.G. Repeated Removal of Copper by Alginate Beads and the Enhancement by Microalgae. Biotechnol. Tech. 1998, 12, 187–190. [Google Scholar] [CrossRef]
- Contreras-Cortés, A.; Almendariz-Tapia, F.; Gómez-Álvarez, A.; Burgos-Hernández, A.; Luque-Alcaraz, A.; Rodríguez-Félix, F.; Quevedo-López, M.; Plascencia-Jatomea, M. Toxicological Assessment of Cross-Linked Beads of Chitosan-Alginate and Aspergillus Australensis Biomass, with Efficiency as Biosorbent for Copper Removal. Polymers 2019, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, J.S.D.; Lin, J.-Y.; Dalida, M.L.P.; Lu, M.-C. Abatement Technologies for Copper Containing Industrial Wastewater Effluents—A Review. J. Environ. Chem. Eng. 2023, 11, 109336. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste Biomass Adsorbents for Copper Removal from Industrial Wastewater—A Review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef]

| Country/Region | Concentration | Paper |
|---|---|---|
| China/Tibet, Rona | Water: 2114.00 ± 65.89 μg/L Soil: 19.01–1763.1 mg/kg | [23] |
| China/Yunnan Copper Mine WWTP | Sediments: 1200 mg/kg | [24] |
| Cuba/Havana City | Soil: 101 ± 51 mg/kg | [25] |
| Uganda/Kilembe copper mine and tailing sites | Tailings: 10,217 mg/kg Sediments: 4110 mg/kg Water: 1.9–61 μg/L | [26] |
| India/Ghaziabad | Soil: 122 mg/kg | [27] |
| Brazil/Carajas-Amazon | Water: 50–60 nmol/L | [28] |
| China/Dexing copper mine sewage station | 38.24–47.86 mg/L | [29] |
| China/Liaodong Bay | Water: 6.8–11.9 μg/L | [30] |
| Techniques | Materials/Reactors | Removal Efficiency of Cu | References |
|---|---|---|---|
| Membrane separation | Hydrophilic polyurethane modified cellulose acetate ultrafiltration membranes | 92% | [140] |
| Cellulose acetate based biopolymeric mixed matrix membranes | 84–88% | [141] | |
| Chitosan-cellulose acetate-TiO2 based membrane | 97% | [142] | |
| Ion exchange | Y zeolite ion exchangers | 64% | [143] |
| Ion exchange resin | 99.14% | [144] | |
| Electrochemical reaction | Bipolar disc reactor | 90.1% | [145] |
| Continuous electrochemical cell | 91% | [146] | |
| Bioelectrochemical and electrochemical systems | 99.9% | [147] | |
| Chemical precipitation | OM in waste distillery slops—precipitation/coagulation | 92% | [148] |
| Synthetic nesquehonite | 99.97% | [149] | |
| struvite | 99.9% | [150] | |
| Adsorption | Hexagonal boron nitride | 92% | [151] |
| Zeolite, bentonite, and steel slag | 98.47–99.98% | [152] | |
| Agro-industrial waste | 89% | [153] | |
| Biotechnology | Stenotrophomonas maltophilia | 88% | [154] |
| Microalgae | >95% | [155] | |
| Aspergillus australensis Biomass | 79% | [156] |
| Technology | Advantages | Disadvantages | Application Scenarios | Cost |
|---|---|---|---|---|
| Membrane filtration | Excellent performance in scale-up applications, such as excellent heavy metal removal, high efficiency, ease of operation, and low space requirements | Membrane fouling, capital cost, maintenance and operational cost, less efficient in case of lower metal ion concentration | Suitable for both high- and low-concentration copper-polluted water; selection of the right polymer/micellar agent is required to improve the rejection efficiency | Treatment cost of membrane fouling |
| Reverse osmosis | Effective removal of metals from wastewater | Membrane scaling problems, low water permeability, high RO operating pressure due to internal concentration polarization, low water flux, and high energy consumption | Use in drinking water | |
| Ion exchange | Selective removal of heavy metals, high treatment capacity, high metal removal rate | Fouling and maintenance costs, high capital cost of equipment and instruments, high operational as well as resin regeneration cost | Treatment of water bodies polluted by a specific metal element, not suitable for large-scale application | High cost of synthetic resin, pollutant recovery costs |
| Electrochemical reaction | Reduced chemical consumption, recovery of pure metals, effective removal of desired metals, suitable for initial high concentration contamination remediation | Low current effect and selectivity, high power consumption | Electrochemical methods, such as electrodialysis, electrocoagulation, electrodeposition, and capacitive deionization, are capable of removing Cu(II) by different mechanisms and are therefore suitable for a wide range of copper concentrations | Electricity costs |
| Chemical precipitation | Low metal concentration in the effluent achieved. This approach can be adapted to handle large quantities of wastewater. Simple to use | High chemical requirement, pH maintenance at optimum level, handling of colloidal particle sludge disposal problem. A large number of factors, such as temperature, pH, precipitant concentration, etc., have to be monitored when implementing this technique, which is quite difficult | For the treatment of concentrated copper wastewater, the preferred method is precipitation | Sludge disposal cost |
| Adsorption | Highly effective for removing heavy metals within permissible limits; the desorption process can produce a concentrated Cu(II) stream with recovery potential | Chemical regeneration requirement, fouling and corrosion of treatment plant, disposal of exhausted adsorbents, preparation of the adsorbent involve high costs, such as in the case of activated carbon, loss of adsorption capacity by the adsorbent in each cycle, frequent regeneration, which reduce the simplicity of the adsorption process | When treating diluted wastewater, adsorption is preferred due to its simplicity and effectiveness | Cost of desorption and regeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of Copper Ions from Wastewater: A Review. Int. J. Environ. Res. Public Health 2023, 20, 3885. https://doi.org/10.3390/ijerph20053885
Liu Y, Wang H, Cui Y, Chen N. Removal of Copper Ions from Wastewater: A Review. International Journal of Environmental Research and Public Health. 2023; 20(5):3885. https://doi.org/10.3390/ijerph20053885
Chicago/Turabian StyleLiu, Yongming, Haishuang Wang, Yuanyuan Cui, and Nan Chen. 2023. "Removal of Copper Ions from Wastewater: A Review" International Journal of Environmental Research and Public Health 20, no. 5: 3885. https://doi.org/10.3390/ijerph20053885
APA StyleLiu, Y., Wang, H., Cui, Y., & Chen, N. (2023). Removal of Copper Ions from Wastewater: A Review. International Journal of Environmental Research and Public Health, 20(5), 3885. https://doi.org/10.3390/ijerph20053885





