Application of Natural and Calcined Oyster Shell Powders to Improve Latosol and Manage Nitrogen Leaching
Abstract
:1. Introduction
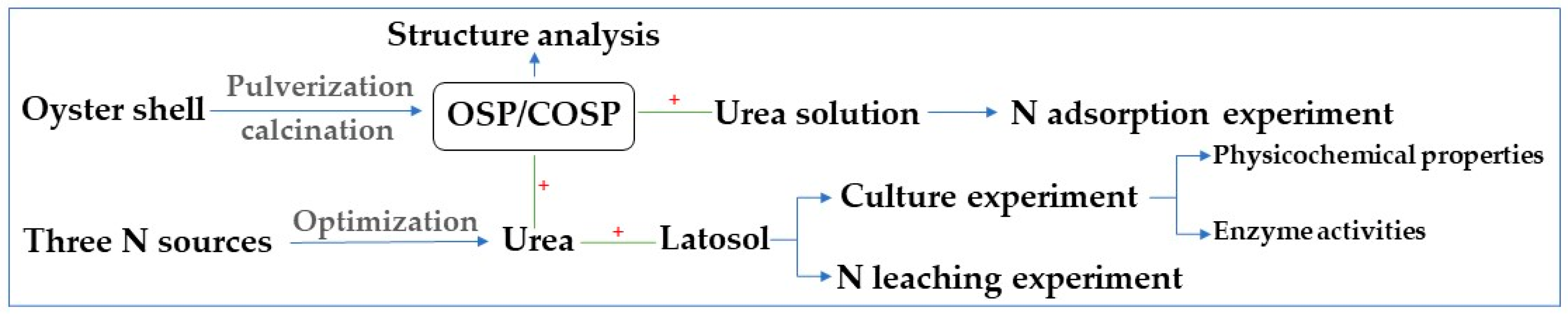
2. Materials and Methods
2.1. Experimental Materials
2.2. Leaching Simulation Device
2.3. Experimental Design
2.3.1. Descriptive Experiments for the OSP and COSPs
Structure Analysis for the OSP and COSPs
N Adsorption Experiment for the OSP and COSPs
2.3.2. Culture and Leaching Experiments
Culture Experiment for the OSP and COSPs
Leaching Experiment for the OSP and COSPs
2.4. Analytical Items and Methods
2.5. Data Processing
3. Results
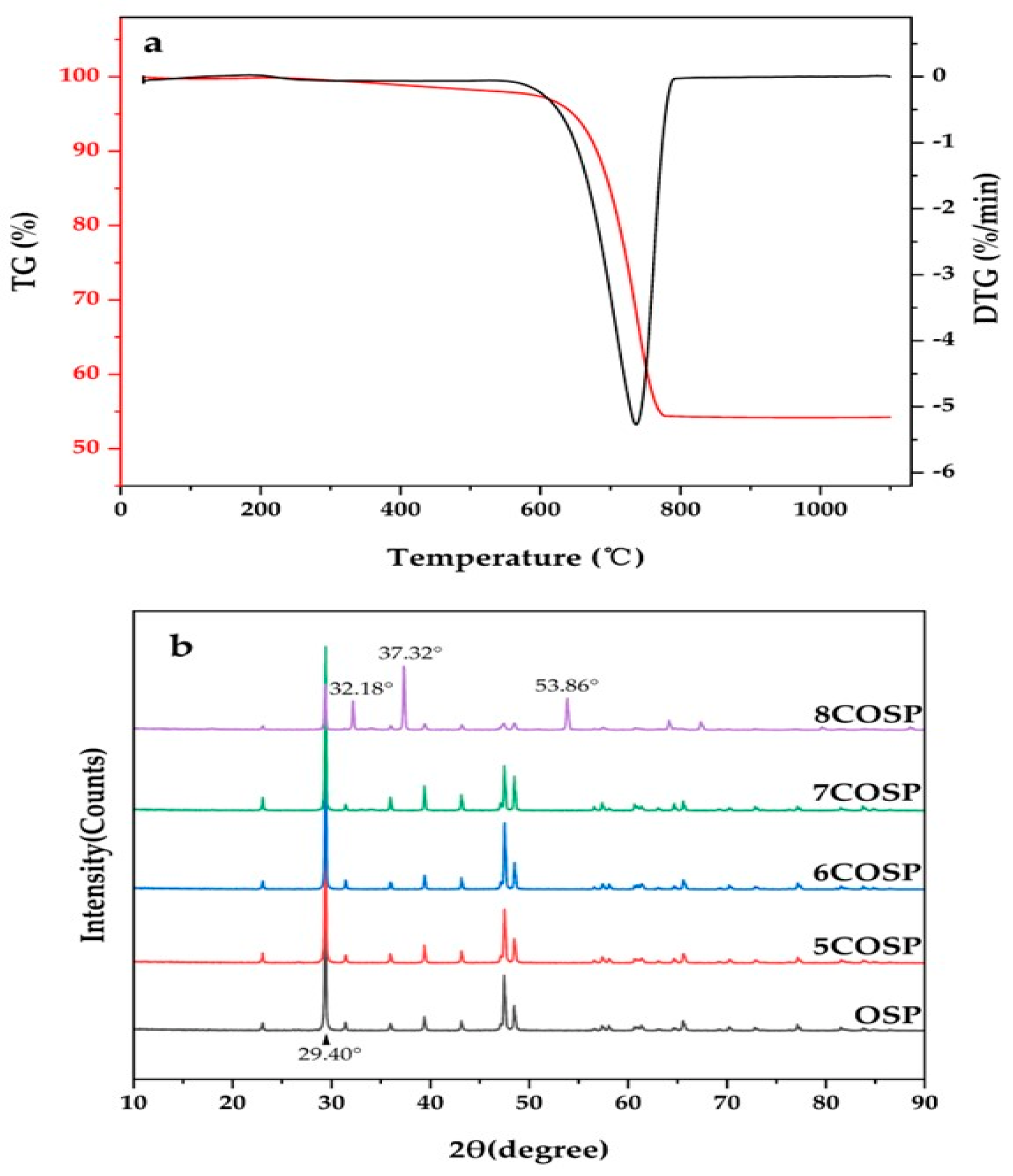
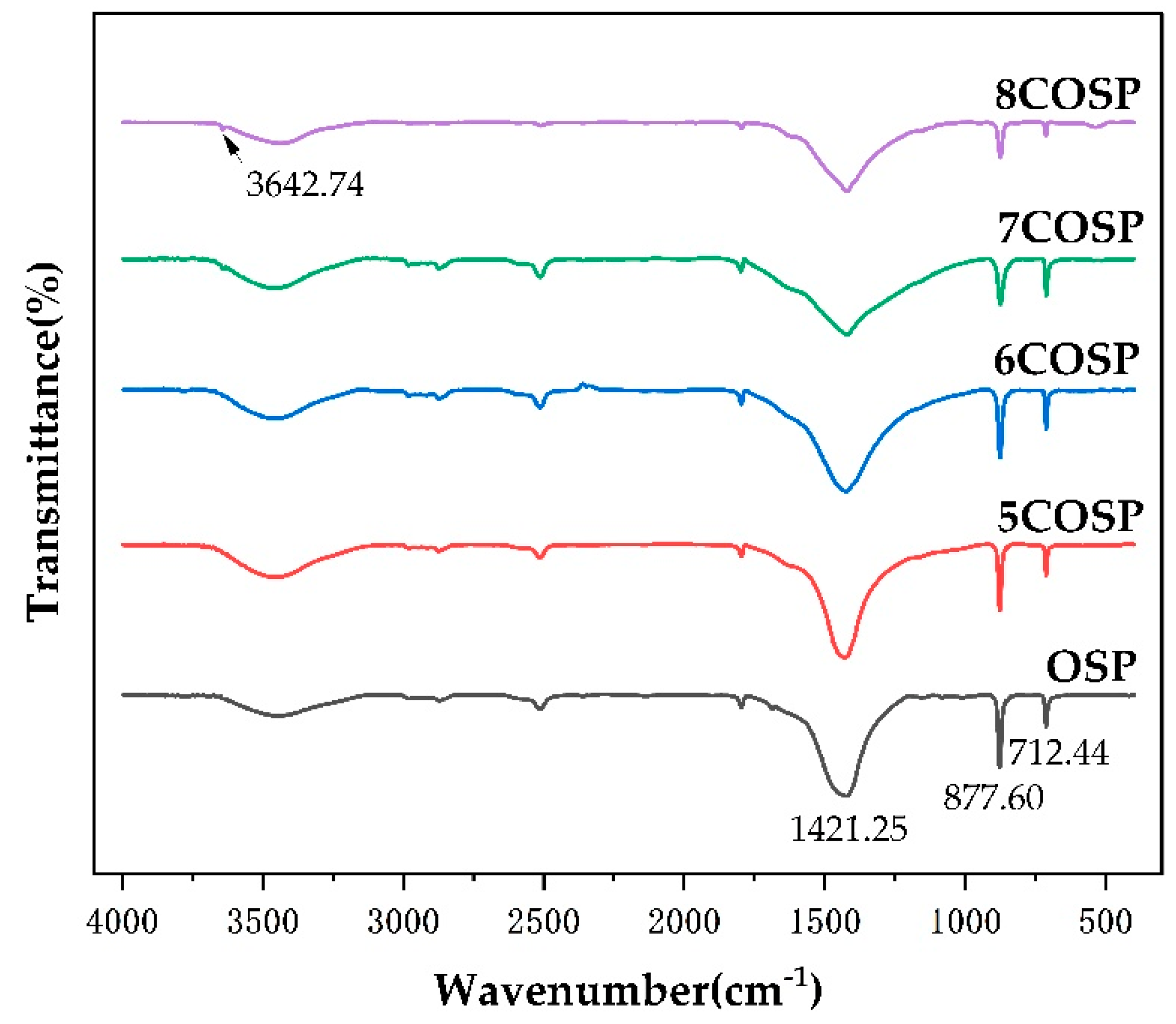
3.1. Structural Characteristics of the OSP and Four COSPs
3.2. Adsorption Characteristics of Urea by the OSP and COSPs
3.3. Impact of OSP and COSPs on the Physicochemical Properties of Latosol
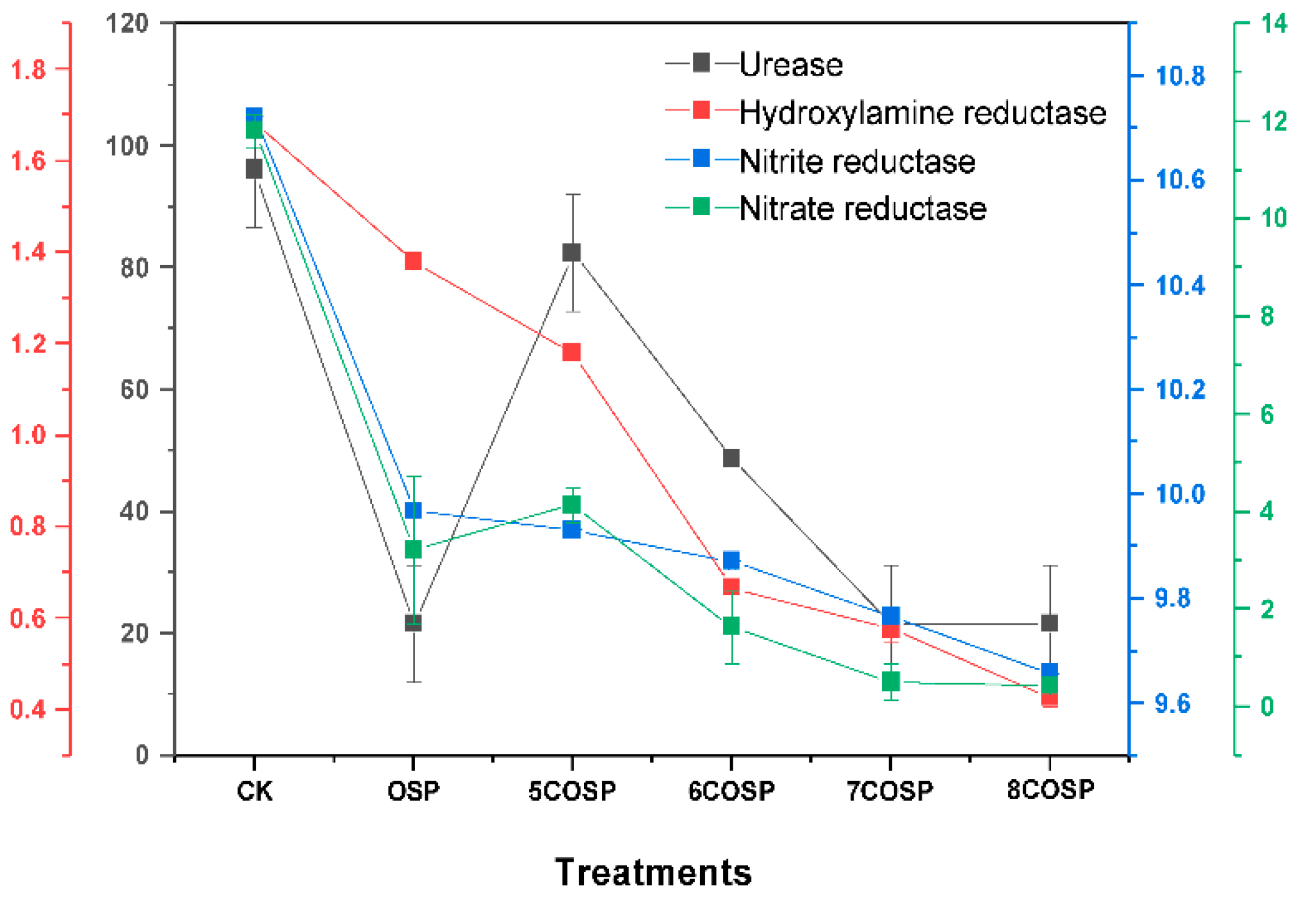
3.4. Impact of OSP and COSPs on Enzyme Activities in Latosol
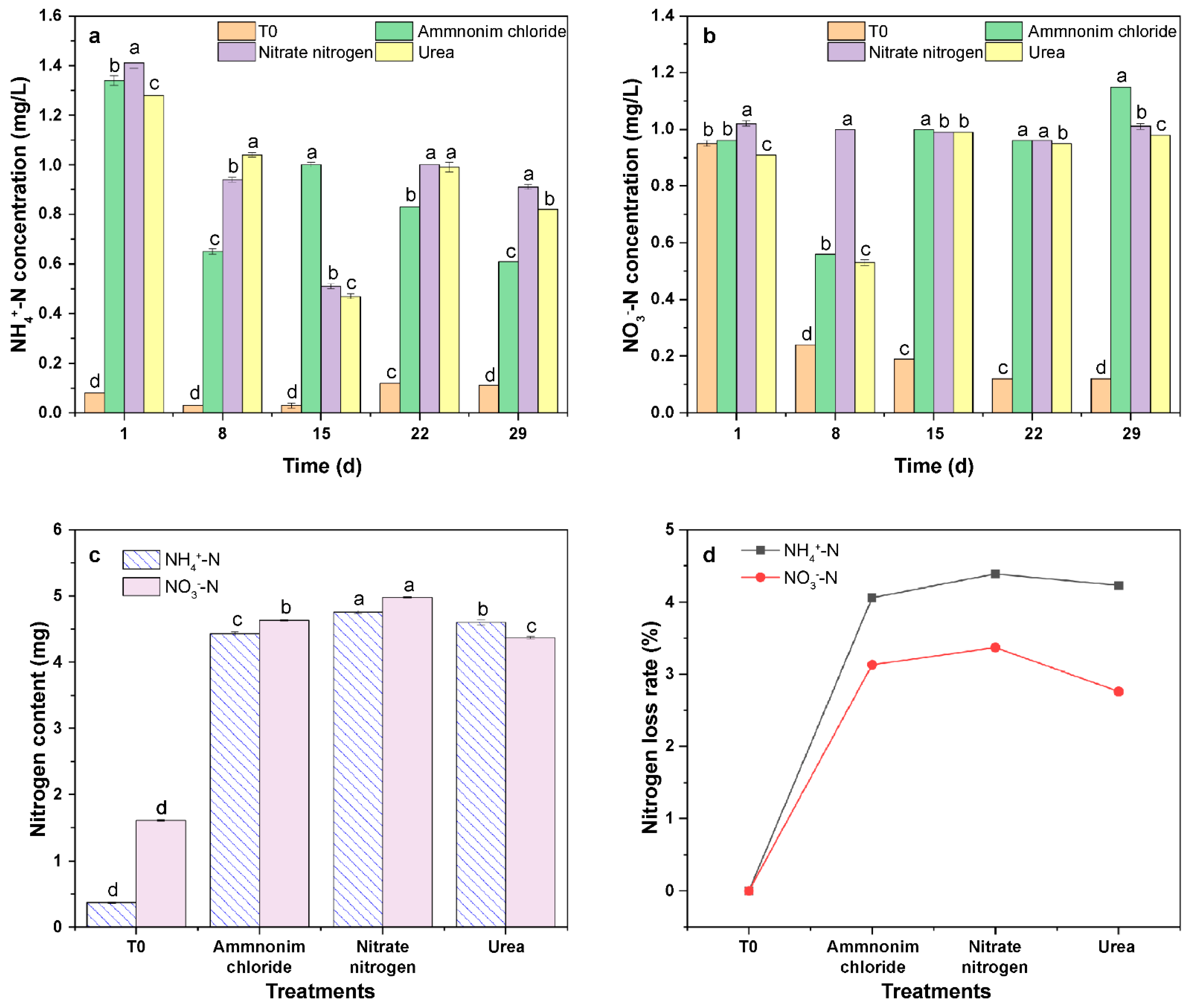
3.5. Impact of N Fertilizer Types on the Characteristics of NH4+-N and NO3−-N Leaching in Latosol
3.6. Impact of the OSP and COSPs on the N Leaching Characteristics of Latosol
3.6.1. Impact of OSP and COSPs on the pH and Ca in Leachate
3.6.2. Impact of the OSP and COSPs on NH4+-N and NO3−-N in the Leachate
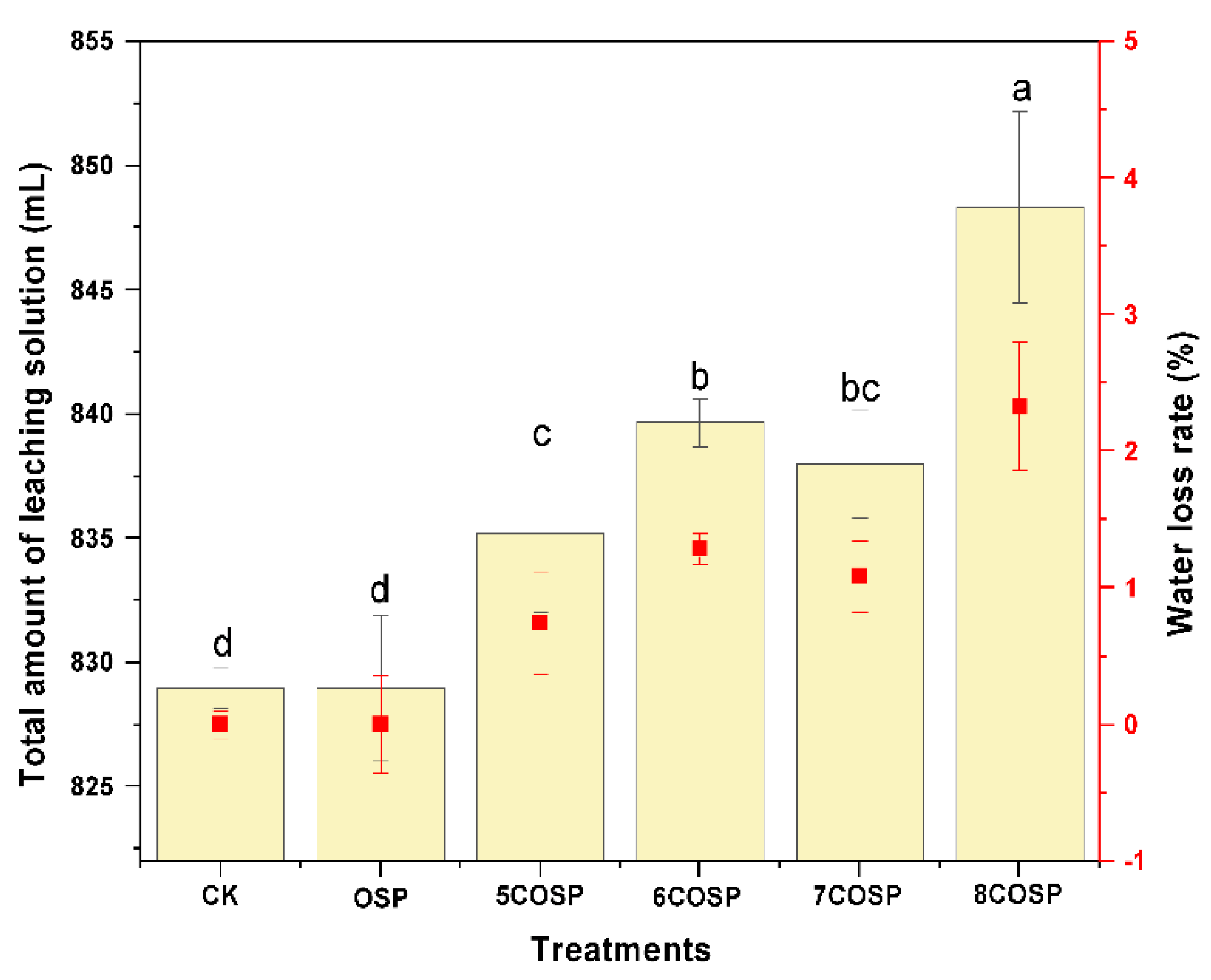
3.6.3. Impact of the OSP and COSPs on the Leachate Volume
3.6.4. Impact of OSP and COSPs on Cumulative N Leaching and Leaching Rate of Leachate
4. Discussion
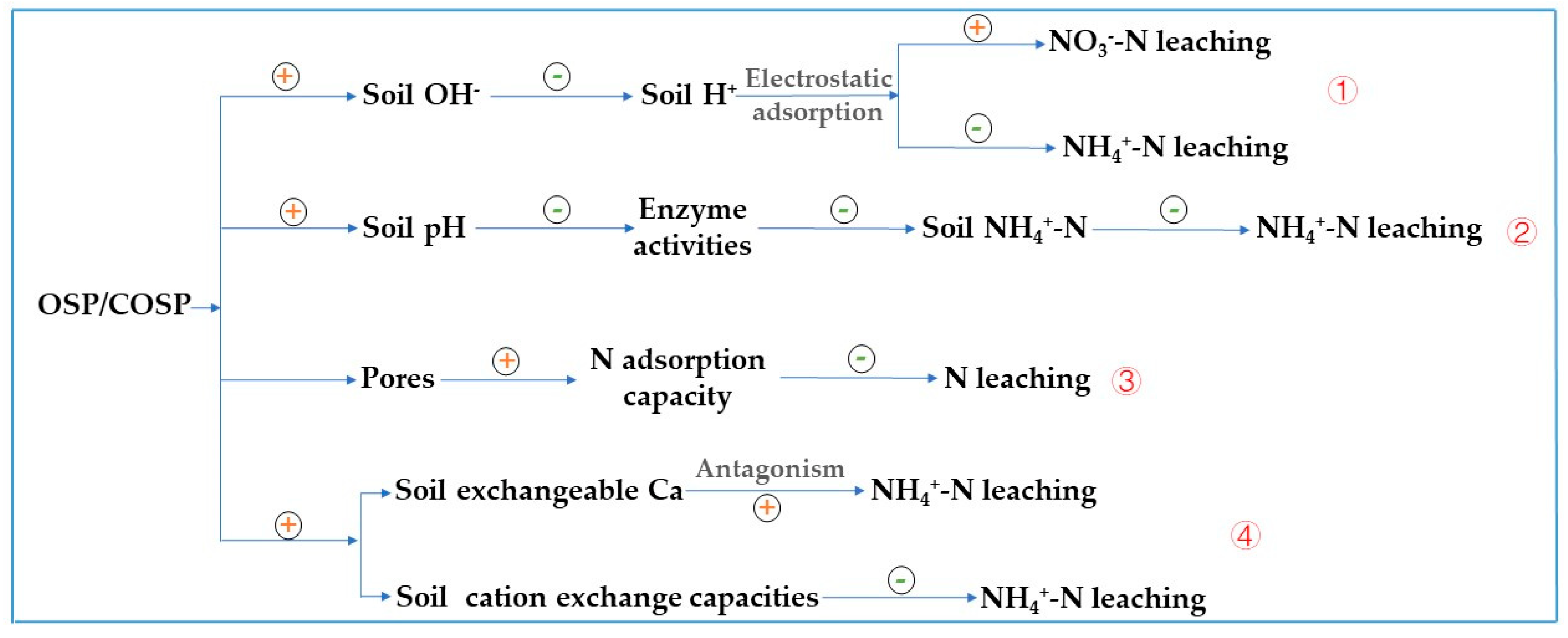
4.1. Impact of the OSP and COSPs on Latosol Acidification
4.2. Impact of OSP and COSPs on N Leaching in Latosol
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Li, X.; Jiang, Y. Response of Nitrogen Losses to Excessive Nitrogen Fertilizer Application in Intensive Greenhouse Vegetable Production. Sustainability 2019, 11, 1513. [Google Scholar] [CrossRef] [Green Version]
- Elhanafi, L.; Houhou, M.; Rais, C.; Mansouri, I.; Elghadraoui, L.; Greche, H. Impact of Excessive Nitrogen Fertilization on the Biochemical Quality, Phenolic Compounds, and Antioxidant Power of Sesamum indicum L. Seeds. J. Food Qual. 2019, 2019, 9428092. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Liu, G.; Chen, Y.; Jiang, Y.; Shi, Y.; Zhao, L.; Liao, P.; Wang, W.; Xu, K.; Dai, Q.; et al. Excessive Nitrogen Application Leads to Lower Rice Yield and Grain Quality by Inhibiting the Grain Filling of Inferior Grains. Agriculture 2022, 12, 962. [Google Scholar] [CrossRef]
- Hruška, J.; Oulehle, F.; Šamonil, P.; Šebesta, J.; Tahovská, K.; Hleb, R.; Houška, J.; Šikl, J. Long-Term Forest Soil Acidification, Nutrient Leaching and Vegetation Development: Linking Modelling and Surveys of a Primeval Spruce Forest in the Ukrainian Transcarpathian Mts. Ecol. Model. 2012, 244, 28–37. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Phan, B.T.; Nguyen, T.X.; Nguyen, V.N.; Van Tran, T.; Bach, Q.-V. Contrastive Nutrient Leaching from Two Differently Textured Paddy Soils as Influenced by Biochar Addition. J. Soils Sediments 2020, 20, 297–307. [Google Scholar] [CrossRef]
- Guddisa, B.; Tana, T.; Legesse, H. Application of Different Lime Rates and Phosphorus on Soil Physico-Chemical Properties of Acid Soils in Western Ethiopia. Sci. Technol. Arts Res. J. 2018, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Meena, H.M.; Prakasha, H.C. The Impact of Biochar, Lime and Fertilizer on Soil Acidity and Microbiological Properties and Their Relationship with Yield of Rice and Cowpea in an Acidic Soil of Southern India. J. Plant Nutr. 2022, 45, 358–368. [Google Scholar] [CrossRef]
- Mosharrof, M.; Uddin, M.K.; Sulaiman, M.F.; Mia, S.; Shamsuzzaman, S.M.; Haque, A.N.A. Combined Application of Biochar and Lime Increases Maize Yield and Accelerates Carbon Loss from an Acidic Soil. Agronomy 2021, 11, 1313. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Zhang, R.; Chen, Y.; Lei, L.; Qiu, C.; Xu, H. Effect of Slow-Released Biomass Alkaline Amendments Oyster Shell on Microecology in Acidic Heavy Metal Contaminated Paddy Soils. J. Environ. Manag. 2022, 319, 115683. [Google Scholar] [CrossRef]
- Ohta, T.; Hiura, T. Root Exudation of Low-Molecular-Mass-Organic Acids by Six Tree Species Alters the Dynamics of Calcium and Magnesium in Soil. Can. J. Soil Sci. 2016, 96, 199–206. [Google Scholar] [CrossRef]
- Yan, J. Oyster Shell Soil Conditioner: Effects on Peanut Yield and Acidified Soil Amendment in Yellow Clayey Field. J. Agric. 2019, 9, 17. [Google Scholar] [CrossRef]
- Moon, D.H.; Chang, Y.-Y.; Ok, Y.S.; Cheong, K.H.; Koutsospyros, A.; Park, J.-H. Amelioration of Acidic Soil Using Various Renewable Waste Resources. Environ. Sci. Pollut. Res. 2014, 21, 774–780. [Google Scholar] [CrossRef]
- Suwannasingha, N.; Kantavong, A.; Tunkijjanukij, S.; Aenglong, C.; Liu, H.-B.; Klaypradit, W. Effect of Calcination Temperature on Structure and Characteristics of Calcium Oxide Powder Derived from Marine Shell Waste. J. Saudi Chem. Soc. 2022, 26, 101441. [Google Scholar] [CrossRef]
- Feng, M.; Li, M.; Zhang, L.; Luo, Y.; Zhao, D.; Yuan, M.; Zhang, K.; Wang, F. Oyster Shell Modified Tobacco Straw Biochar: Efficient Phosphate Adsorption at Wide Range of PH Values. Int. J. Environ. Res. Public Health 2022, 19, 7227. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Q.; Wang, Y.; Weng, L.; Chen, H.; Yang, F.; Zhao, H.; Cao, M. Effect of Calcined Oyster Shell Powder on Soil Acidification and Fruit Quality of Mushroom Melon. J. Jimei Univ. Nat. Sci. 2020, 25, 336–343. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Huang, Y.; Zhuang, X.; Xu, S.; Zhuang, S.; Fang, H.; Cao, M. Improvement Effect on Acidic Soil and the Quality of Guanxi Pomelo by Calcined Oyster Shell Powder. J. Jimei Univ. Nat. Sci. 2020, 25, 256–264. [Google Scholar] [CrossRef]
- Song, H.; Che, Z.; Cao, W.; Huang, T.; Wang, J.; Dong, Z. Changing Roles of Ammonia-Oxidizing Bacteria and Archaea in a Continuously Acidifying Soil Caused by over-Fertilization with Nitrogen. Environ. Sci. Pollut. Res. 2016, 23, 11964–11974. [Google Scholar] [CrossRef]
- Jay, J.G.; Tyler-Plog, M.; Brown, S.L.; Grothkopp, F. Nutrient, Metal, and Organics Removal from Stormwater Using a Range of Bioretention Soil Mixtures. J. Environ. Qual. 2019, 48, 493–501. [Google Scholar] [CrossRef]
- Lee, C.-W.; Jeon, H.-P.; Kwon, H.-B. Physical Properties of Pyrolized Oyster Shell Consisting of Porous CaO/CaCO 3 and Phosphorus Removal Efficiency. J. Korean Ceram. Soc. 2010, 47, 524–528. [Google Scholar] [CrossRef]
- Yun, S.-I.; Seo, D.-H.; Kang, H.S.; Cheng, H.; Lee, G.; Choi, W.-J.; Lee, C.-K.; Jung, M.H. Effects of Dolomite and Oyster Shell on Nitrogen Processes in an Acidic Mine Soil Applied with Livestock Manure Compost. Korean J. Soil Sci. Fertil. 2016, 49, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Awad, Y.M.; Lee, S.S.; Kim, K.-H.; Ok, Y.S.; Kuzyakov, Y. Carbon and Nitrogen Mineralization and Enzyme Activities in Soil Aggregate-Size Classes: Effects of Biochar, Oyster Shells, and Polymers. Chemosphere 2018, 198, 40–48. [Google Scholar] [CrossRef]
- Lian, W.; Li, H.; Yang, J.; Joseph, S.; Bian, R.; Liu, X.; Zheng, J.; Drosos, M.; Zhang, X.; Li, L.; et al. Influence of Pyrolysis Temperature on the Cadmium and Lead Removal Behavior of Biochar Derived from Oyster Shell Waste. Bioresour. Technol. Rep. 2021, 15, 100709. [Google Scholar] [CrossRef]
- FAO/UNESCO Soil Map of the World | FAO SOILS PORTAL | Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/faounesco-soil-map-of-the-world/en/ (accessed on 16 November 2022).
- Yang, X.; Huang, Y.; Liu, K.; Zheng, C. Effects of Oyster Shell Powder on Leaching Characteristics of Nutrients in Low-Fertility Latosol in South China. Environ. Sci. Pollut. Res. 2022, 29, 56200–56214. [Google Scholar] [CrossRef]
- Xu, D.D.; Cheng, Y.T.; Xiao, L.Y.; Jian, B.C.; Yu, K.J.; Shu, B.Y. High-Efficiency Biogenic Calcium Carbonate for Adsorption of Pb(II) and Methyl Orange from Wastewater. J. Inorg. Mater. 2019, 35, 349. [Google Scholar] [CrossRef]
- Ok, Y.S.; Oh, S.-E.; Ahmad, M.; Hyun, S.; Kim, K.-R.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.-T.; Yang, J.E. Effects of Natural and Calcined Oyster Shells on Cd and Pb Immobilization in Contaminated Soils. Environ. Earth Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Jia, X.; Shao, L.; Liu, P.; Zhao, B.; Gu, L.; Dong, S.; Bing, S.H.; Zhang, J.; Zhao, B. Effect of Different Nitrogen and Irrigation Treatments on Yield and Nitrate Leaching of Summer Maize (Zea mays L.) under Lysimeter Conditions. Agric. Water Manag. 2014, 137, 92–103. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zou, J.-D.; Xu, L.-L.; Zhang, X.-Y.; Yang, F.-T.; Dai, X.-Q.; Wang, Z.-Q.; Sun, X.-M. Effects of different fertilizer species on carbon and nitrogen leaching in a reddish paddy soil. Huan Jing Ke Xue 2014, 35, 3083–3090. [Google Scholar]
- Qasim, W.; Xia, L.; Lin, S.; Wan, L.; Zhao, Y.; Butterbach-Bahl, K. Global Greenhouse Vegetable Production Systems Are Hotspots of Soil N2O Emissions and Nitrogen Leaching: A Meta-Analysis. Environ. Pollut. 2021, 272, 116372. [Google Scholar] [CrossRef]
- Wu, L.; Green, R.; Klein, G.; Hartin, J.S.; Burger, D.W. Nitrogen Source and Rate Influence on Tall Fescue Quality and Nitrate Leaching in a Southern California Lawn. Agron. J. 2010, 102, 31–38. [Google Scholar] [CrossRef]
- Wu, B.; Li, J.; Sheng, M.; Peng, H.; Peng, D.; Xu, H. The Application of Biochar and Oyster Shell Reduced Cadmium Uptake by Crops and Modified Soil Fertility and Enzyme Activities in Contaminated Soil. SOIL 2022, 8, 409–419. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Johanson, J.B.; Nelson, C.O. Urease Activity in Sewage Sludge-Amended Soils. Soil Biol. Biochem. 1983, 15, 543–549. [Google Scholar] [CrossRef]
- Huh, J.-H.; Choi, Y.-H.; Lee, H.-J.; Choi, W.J.; Ramakrishna, C.; Lee, H.-W.; Lee, S.-H.; Ahn, J.-W. The Use of Oyster Shell Powders for Water Quality Improvement of Lakes by Algal Blooms Removal. J. Korean Ceram. Soc 2016, 53, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tahir, S.; Marschner, P. Clay Addition to Sandy Soil Reduces Nutrient Leaching—Effect of Clay Concentration and Ped Size. Commun. Soil Sci. Plant Anal. 2017, 48, 1813–1821. [Google Scholar] [CrossRef]
- Kim, T.; Kim, D.; Jeon, J.; Son, Y. Improvement of Desalinization Performance in Reclaimed Land through Recycling of Bottom Ash and Oyster Shells. Paddy Water Environ. 2021, 19, 529–538. [Google Scholar] [CrossRef]
- Zhang, B.W.; Mu, Q.; Liu, D.W.; Li, L.; Wan, S.B.; Wang, J.G.; Guo, F. Effects of calcium application on physical and chemical properties of peanut in barren upland red soil. Chin. J. Oil Crop Sci. 2020, 42, 896–902. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, H.; Chang, X.; Wu, G. Higher Species Diversity Improves Soil Water Infiltration Capacity by Increasing Soil Organic Matter Content in Semiarid Grasslands. Land Degrad. Dev. 2019, 30, 1599–1606. [Google Scholar] [CrossRef]




| Tested Material | Data | pH | Organic Matter (g/kg) | Total N (g/kg) | NO3−-N (mg/kg) | NH4+-N (mg/kg) | Exchangeable Ca (g/kg) |
|---|---|---|---|---|---|---|---|
| Latosol | Mean | 4.85 | 4.94 | 0.26 | 6.97 | 0.96 | 12.88 |
| SE | 0.03 | 0.49 | 0.02 | 0.04 | 0.00 | 0.10 |
| Absorption Peak Position (cm−1) | Assignment | ||||
|---|---|---|---|---|---|
| OSP | 5COSP | 6COSP | 7COSP | 8COSP | |
| High-frequency region | |||||
| 3440.48 | 3433.51 | 3434.77 | 3434.98 | 3431.03 | O-H symmetric stretching vibration |
| 2872.59 | 2873.18 | 2873.16 | 2872.74 | 2872.65 | -CH2- and -CH3 stretching vibrations |
| 2509.87 | 2511.7 | 2511.7 | 2510.98 | 2511.91 | -S-H symmetric stretching vibration |
| Medium-frequency region | |||||
| 1796.49 | 1796.82 | 1797.22 | 1798.58 | 1796.5 | C-O symmetric stretching vibration |
| 1421.23 | 1428.36 | 1423.15 | 1420.95 | 1421.25 | C-O asymmetric stretching vibration |
| 874.6 | 877.12 | 875.85 | 874.85 | 877.6 | CO32− out-of-plane bending vibration |
| 712.9 | 712.43 | 712.26 | 711.81 | 712.44 | CO32− in-plane bending vibration |
| Treatment | pH | Organic Matter (g/kg) | Total N (g/kg) | NO3−-N (mg/kg) | NH4+-N (mg/kg) | Ca (g/kg) | Cation Exchange Capacity (cmol/kg) |
|---|---|---|---|---|---|---|---|
| CK | 5.47 ± 0.02 d | 13.13 ± 0.06 c | 0.68 ± 0.00 c | 15.15 ± 0.08 e | 1.97 ± 0.03 a | 17.69 ± 0.14 e | 9.92 ± 0.51 f |
| OSP | 7.47 ± 0.03 c | 14.12 ± 0.17 b | 0.77 ± 0.01 b | 16.81 ± 0.06 a | 1.98 ± 0.02 a | 26.48 ± 0.54 d | 21.53 ± 0.09 a |
| 5COSP | 7.57 ± 0.02 b | 13.94 ± 0.15 b | 0.78 ± 0.03 b | 16.00 ± 0.02 b | 1.99 ± 0.01 a | 26.67 ± 0.23 d | 18.25 ± 0.47 b |
| 6COSP | 7.58 ± 0.01 b | 13.96 ± 0.32 ab | 0.79 ± 0.01 ab | 15.48 ± 0.07 c | 1.99 ± 0.00 a | 28.59 ± 0.50 c | 11.88 ± 0.54 e |
| 7COSP | 7.63 ± 0.02 a | 14.53 ± 0.24 a | 0.83 ± 0.03 a | 15.33 ± 0.07 d | 1.99 ± 0.00 a | 30.92 ± 0.26 b | 15.89 ± 0.41 d |
| 8COSP | 7.64 ± 0.01 a | 14.85 ± 0.10 a | 0.86 ± 0.03 a | 15.03 ± 0.10 e | 2.00 ± 0.01 a | 32.60 ± 0.36 a | 17.45 ± 0.16 c |
| Treatment | Cumulative Leaching (mg) | NH4+-N/NH4+-N + NO3−-N (%) | NO3−-N/NH4+-N + NO3−-N (%) | Leaching Rate (%) | ||||
|---|---|---|---|---|---|---|---|---|
| NH4+-N | NO3−-N | NH4+-N + NO3−-N | NH4+-N | NO3−-N | NH4+-N + NO3−-N | |||
| CK | 4.60 ± 0.04 b | 4.37 ± 0.02 b | 8.97 ± 0.06 a | 51.31 ± 0.11 b | 48.69 ± 0.11 d | 4.16 ± 0.04 b | 2.76 ± 0.02 b | 6.99 ± 0.06 a |
| OSP | 3.19 ± 0.02 f | 4.15 ± 0.03 e | 7.34 ± 0.02 d | 43.47 ± 0.29 e | 56.53 ± 0.29 a | 2.82 ± 0.02 f | 2.54 ± 0.03 e | 5.36 ± 0.02 d |
| 5COSP | 4.77 ± 0.05 a | 4.13 ± 0.00 e | 8.90 ± 0.05 a | 53.61 ± 0.23 a | 46.39 ± 0.23 e | 4.40 ± 0.05 a | 2.52 ± 0.00 e | 6.92 ± 0.05 a |
| 6COSP | 4.53 ± 0.02 c | 4.23 ± 0.01 d | 8.78 ± 0.01 b | 51.61 ± 0.21 b | 48.39 ± 0.21 d | 4.16 ± 0.02 c | 2.64 ± 0.01 d | 6.80 ± 0.01 b |
| 7COSP | 4.14 ± 0.03 d | 4.33 ± 0.01 c | 8.47 ± 0.03 c | 48.88 ± 0.21 c | 51.12 ± 0.21 c | 3.77 ± 0.03 d | 2.72 ± 0.01 c | 6.49 ± 0.03 c |
| 8COSP | 3.93 ± 0.03 e | 4.47 ± 0.03 a | 8.40 ± 0.06 c | 46.75 ± 0.09 d | 53.25 ± 0.09 b | 3.56 ± 0.03 e | 2.86 ± 0.03 a | 6.42 ± 0.06 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Liu, K.; Wen, Y.; Huang, Y.; Zheng, C. Application of Natural and Calcined Oyster Shell Powders to Improve Latosol and Manage Nitrogen Leaching. Int. J. Environ. Res. Public Health 2023, 20, 3919. https://doi.org/10.3390/ijerph20053919
Yang X, Liu K, Wen Y, Huang Y, Zheng C. Application of Natural and Calcined Oyster Shell Powders to Improve Latosol and Manage Nitrogen Leaching. International Journal of Environmental Research and Public Health. 2023; 20(5):3919. https://doi.org/10.3390/ijerph20053919
Chicago/Turabian StyleYang, Xiaofei, Kexing Liu, Yanmei Wen, Yongxiang Huang, and Chao Zheng. 2023. "Application of Natural and Calcined Oyster Shell Powders to Improve Latosol and Manage Nitrogen Leaching" International Journal of Environmental Research and Public Health 20, no. 5: 3919. https://doi.org/10.3390/ijerph20053919





