Colles’ Fracture: An Epidemiological Nationwide Study in Italy from 2001 to 2016
Abstract
:1. Introduction
2. Materials and Methods
3. Results
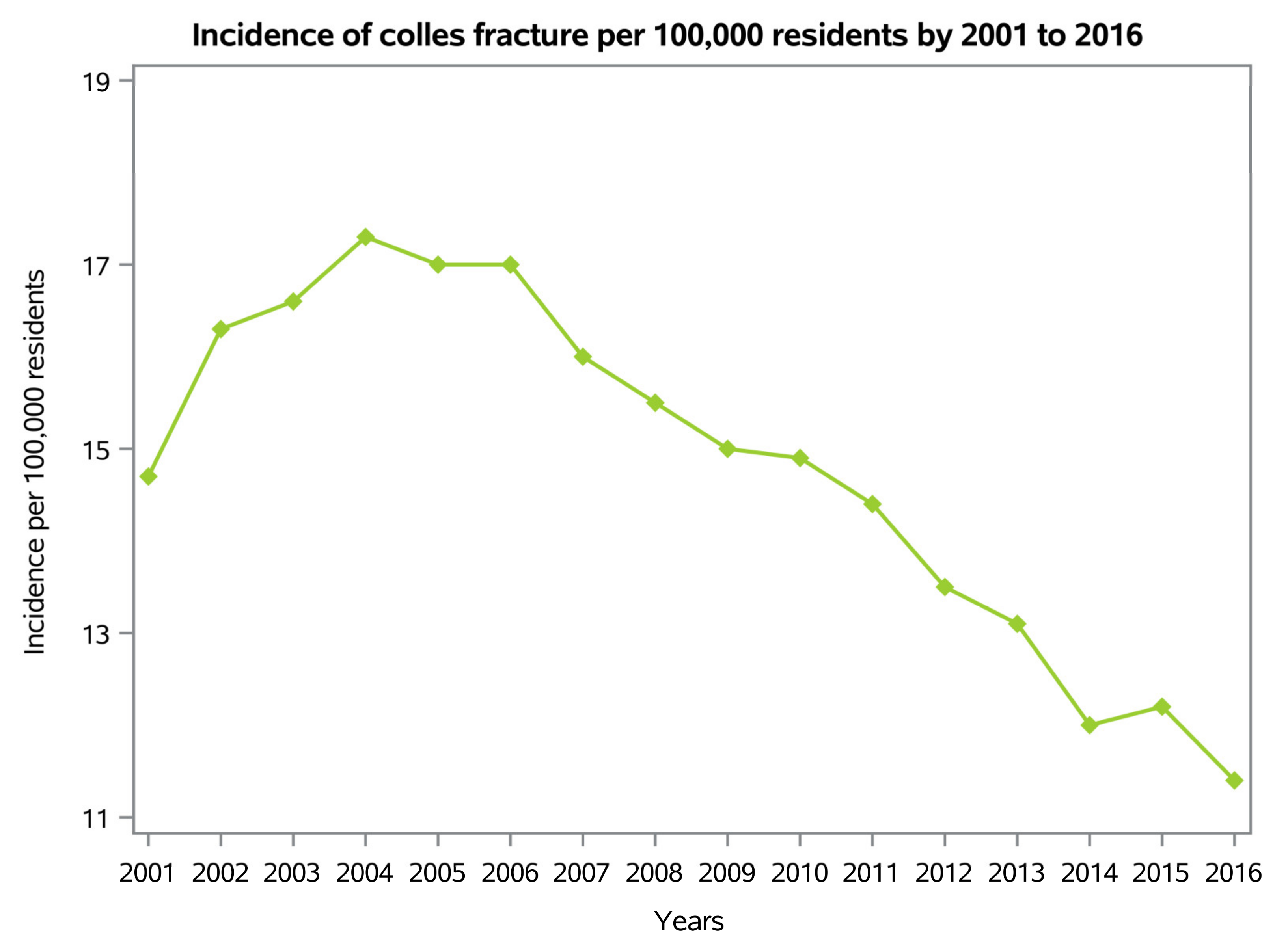
3.1. Demographics
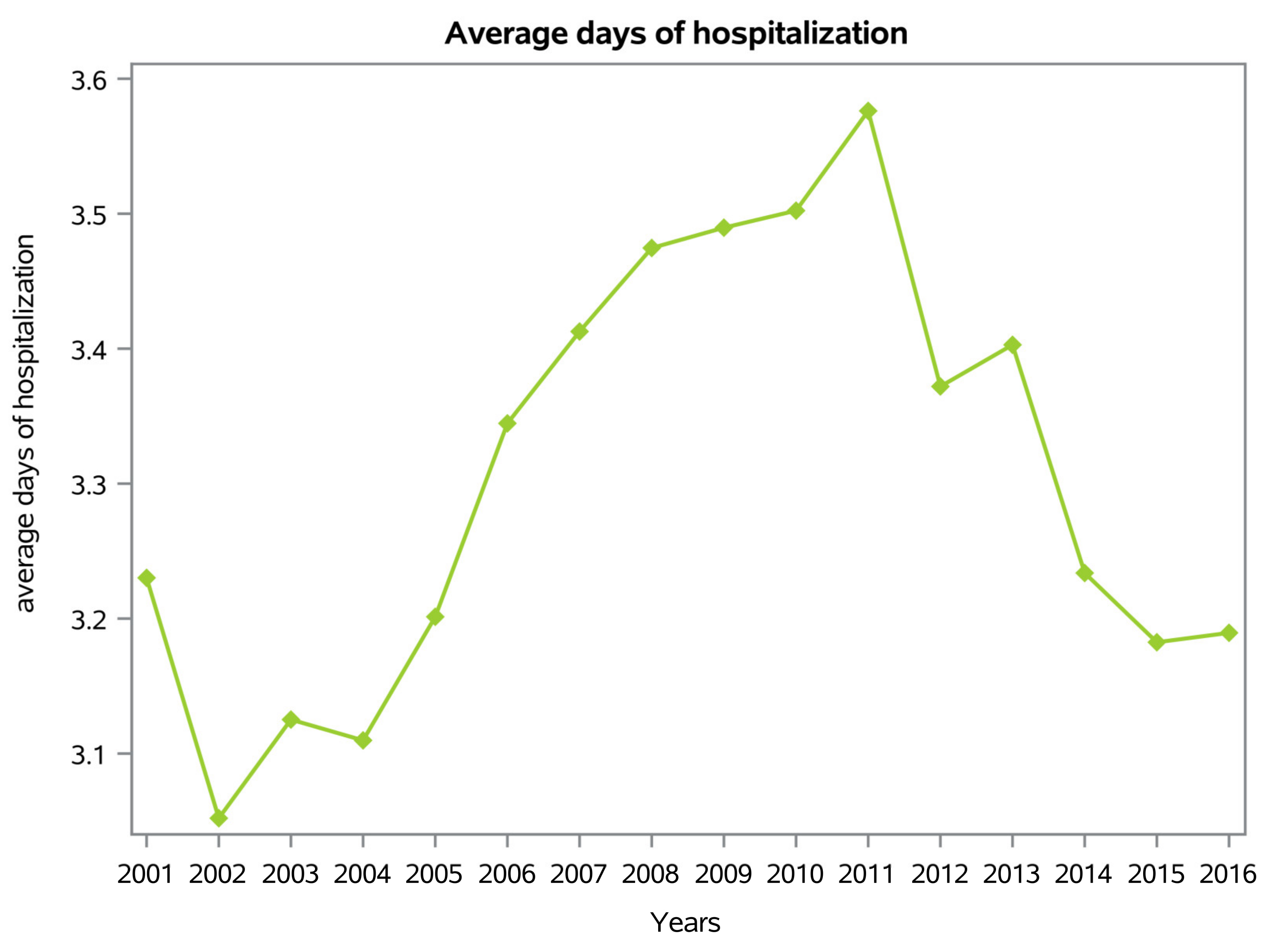
3.2. Length of Hospitalization
3.3. Main Primary Procedures
3.4. Economic Impact
4. Discussion
4.1. Economic Analysis
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porrino, J.A.; Maloney, E.; Scherer, K.; Mulcahy, H.; Ha, A.S.; Allan, C. Fracture of the Distal Radius: Epidemiology and Premanagement Radiographic Characterization. Am. J. Roentgenol. 2014, 203, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Ruzzini, L.; De Salvatore, S.; Lamberti, D.; Maglione, P.; Piergentili, I.; Crea, F.; Ossella, C.; Costici, P. COVID-19 Changed the Incidence and the Pattern of Pediatric Traumas: A Single-Centre Study in a Pediatric Emergency Department. Int. J. Environ. Res. Public Health 2021, 18, 6573. [Google Scholar] [CrossRef] [PubMed]
- Rundgren, J.; Bojan, A.; Navarro, C.M.; Enocson, A. Epidemiology, classification, treatment and mortality of distal radius fractures in adults: An observational study of 23,394 fractures from the national Swedish fracture register. BMC Musculoskelet. Disord. 2020, 21, 88. [Google Scholar] [CrossRef]
- Lindau, T.R.; Aspenberg, P.; Arner, M.; Redlundh-Johnell, I.; Hagberg, L. Fractures of the distal forearm in young adults: An epidemiologic description of 341 patients. Acta Orthop. 1999, 70, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallmin, H.; Ljunghall, S. Distal radius fracture is an early sign of general osteoporosis: Bone mass measurements in a population-based study. Osteoporos. Int. 1994, 4, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Jupiter, J. Future Treatment and Research Directions in Distal Radius Fracture. Hand Clin. 2012, 28, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Nellans, K.W.; Kowalski, E.; Chung, K.C. The Epidemiology of Distal Radius Fractures. Hand Clin. 2012, 28, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Koo, K.O.T.; Tan, D.M.K.; Chong, A.K.S. Distal Radius Fractures: An Epidemiological Review. Orthop. Surg. 2013, 5, 209–213. [Google Scholar] [CrossRef]
- Altizer, L.L. Colles’ fracture. Orthop. Nurs. 2008, 27, 140–145. [Google Scholar] [CrossRef]
- Grafstein, E.; Stenstrom, R.; Christenson, J.; Innes, G.; MacCormack, R.; Jackson, C.; Stothers, K.; Goetz, T. A prospective randomized controlled trial comparing circumferential casting and splinting in displaced Colles fractures. Can. J. Emerg. Med. 2010, 12, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Stirling, E.R.B.; Johnson, N.A.; Dias, J.J. Epidemiology of distal radius fractures in a geographically defined adult population. J. Hand Surg. 2018, 43, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Stirling, E.; Divall, P.; Thompson, J.; Ullah, A.; Dias, J. Risk of hip fracture following a wrist fracture—A meta-analysis. Injury 2016, 48, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D. Distal radius fracture: The rationale of a classification. Chir. De La Main 2001, 20, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Colles, A. On the Fracture of the Carpal Extremity of the Radius. Edinb. Med. Surg. J. 1814, 10, 182–186. [Google Scholar] [CrossRef]
- Jayakumar, P.; Teunis, T.; Giménez, B.B.; Verstreken, F.; Di Mascio, L.; Jupiter, J.B. AO Distal Radius Fracture Classification: Global Perspective on Observer Agreement. J. Wrist Surg. 2017, 6, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Kreder, H.J.; Hanel, D.P.; McKee, M.; Jupiter, J.; McGillivary, G.; Swiontkowski, M.F. Consistency of AO fracture classification for the distal radius. J. Bone Jt. Surg. 1996, 78, 726–731. [Google Scholar] [CrossRef]
- Andersen, D.J.; Blair, W.F.; Stevers, C.M.; Adams, B.D.; El-Khouri, G.Y.; Brandser, E.A. Classification of distal radius fractures: An analysis of interobserver reliability and intraobserver reproducibility. J. Hand Surg. 1996, 21, 574–582. [Google Scholar] [CrossRef]
- Arnold, C.M.; Bello-Haas, V.P.D.; Farthing, J.P.; Crockett, K.L.; Haver, C.R.; Johnston, G.; Basran, J. Falls and Wrist Fracture: Relationship to Women’s Functional Status after Age. Can. J. Aging La Rev. Can. Du Vieil. 2016, 35, 361–371. [Google Scholar] [CrossRef]
- Mallmin, H.; Ljunghall, S.; Naessén, T. Colles’ fracture associated with reduced bone mineral content: Photon densitometry in 74 patients with matched controls. Acta Orthop. 1992, 63, 552–554. [Google Scholar] [CrossRef] [Green Version]
- MacIntyre, N.J.; Dewan, N. Epidemiology of distal radius fractures and factors predicting risk and prognosis. J. Hand Ther. 2016, 29, 136–145. [Google Scholar] [CrossRef]
- Cortet, B.; Blotman, F.; Debiais, F.; Huas, D.; Mercier, F.; Rousseaux, C.; Berger, V.; Gaudin, A.-F.; Cotté, F.-E. Management of osteoporosis and associated quality of life in post menopausal women. BMC Musculoskelet. Disord. 2011, 12, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, M.L.; Orsini, M.R.; Saraifoger, S.; Ortolani, S.; Radaelli, G.; Betti, S. Quality of life in post-menopausal osteoporosis. Health Qual. Life Outcomes 2005, 3, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, A.; Kang, H.P.; Alluri, R.K.; Vakhshori, V.; Kay, H.F.; Ghiassi, A. Epidemiological and Treatment Trends of Distal Radius Fractures across Multiple Age Groups. J. Wrist Surg. 2019, 8, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Mellstrand-Navarro, C.; Pettersson, H.J.; Tornqvist, H.; Ponzer, S. The Operative Treatment of Fractures of the Distal Radius Is Increasing: Results from a nationwide Swedish study. Bone Jt. J. 2014, 96-B, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Hevonkorpi, T.P.; Launonen, A.P.; Huttunen, T.T.; Kannus, P.; Niemi, S.; Mattila, V.M. Incidence of Distal Radius Fracture Surgery in Finns Aged 50 Years or More between 1998 and 2016—Too Many Patients Are yet Operated On? BMC Musculoskelet. Disord. 2018, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Mattila, V.M.; Huttunen, T.T.; Sillanpää, P.; Niemi, S.; Pihlajamäki, H.; Kannus, P. Significant Change in the Surgical Treatment of Distal Radius Fractures: A Nationwide Study between 1998 and 2008 in Finland. J. Trauma Inj. Infect. Crit. Care 2011, 71, 939–942. [Google Scholar] [CrossRef]
- Moore, C.M.; Leonardi-Bee, J. The prevalence of pain and disability one year post fracture of the distal radius in a UK population: A cross sectional survey. BMC Musculoskelet. Disord. 2008, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Loder, R.T.; Skopelja, E.N. The Epidemiology and Demographics of Slipped Capital Femoral Epiphysis. ISRN Orthop. 2011, 2011, 486512. [Google Scholar] [CrossRef] [Green Version]
- Longo, U.G.; Papalia, R.; De Salvatore, S.; Ruzzini, L.; Piergentili, I.; Oggiano, L.; Costici, P.F.; Denaro, V. Developmental Hip Dysplasia: An Epidemiological Nationwide Study in Italy from 2001 to 2016. Int. J. Environ. Res. Public Health 2021, 18, 6589. [Google Scholar] [CrossRef]
- Denaro, L.; Longo, U.G.; Papalia, R.; De Salvatore, S.; Ruzzini, L.; Piergentili, I.; Denaro, V. The burden of percutaneous vertebroplasty: An epidemiological nationwide study in Italy from 2009 to 2015. Eur. Spine J. 2021, 30, 3099–3106. [Google Scholar] [CrossRef]
- Salvatore, G.; Berton, A.; Giambini, H.; Ciuffreda, M.; Florio, P.; Longo, U.G.; Denaro, V.; Thoreson, A.; An, K.-N. Biomechanical effects of metastasis in the osteoporotic lumbar spine: A Finite Element Analysis. BMC Musculoskelet. Disord. 2018, 19, 38. [Google Scholar] [CrossRef]
- Longo, U.G.; Loppini, M.; Denaro, L.; Maffulli, N.; Denaro, V. Osteoporotic vertebral fractures: Current concepts of conservative care. Br. Med. Bull. 2012, 102, 171–189. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-Y.; Hung, M.-C.; Chang, S.-F.; Tsuang, F.-Y.; Chang, J.; Sun, J.-S. Efficacy and Safety of Postmenopausal Osteoporosis Treatments: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 3043. [Google Scholar] [CrossRef]
- Ostergaard, P.J.; Hall, M.J.; Rozental, T.D. Considerations in the Treatment of Osteoporotic Distal Radius Fractures in Elderly Patients. Curr. Rev. Musculoskelet. Med. 2019, 12, 50–56. [Google Scholar] [CrossRef]
- Secretariat, M.A. Utilization of DXA Bone Mineral Densitometry in Ontario: An Evidence-Based Analysis. Ont. Health Technol. Assess. Ser. 2006, 6, 1–180. [Google Scholar]
- Force, U.P.S.T.; Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; A Doubeni, C.; Epling, J.W.; et al. Screening for Vitamin D Deficiency in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1436–1442. [Google Scholar]
- Mäkitie, R.E.; Costantini, A.; Kämpe, A.; Alm, J.J.; Mäkitie, O. New Insights Into Monogenic Causes of Osteoporosis. Front. Endocrinol. 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkitie, O. Causes, mechanisms and management of paediatric osteoporosis. Nat. Rev. Rheumatol. 2013, 9, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, O.; Zillikens, M.C. Early-Onset Osteoporosis. Calcif. Tissue Int. 2022, 110, 546–561. [Google Scholar] [CrossRef]
- Van Oijen, G.W.; Van Lieshout, E.M.M.; Reijnders, M.R.L.; Appalsamy, A.; Hagenaars, T.; Verhofstad, M.H.J. Treatment options in extra-articular distal radius fractures: A systematic review and meta-analysis. Eur. J. Trauma Emerg. Surg. 2021, 48, 4333–4348. [Google Scholar] [CrossRef] [PubMed]
- Mauck, B.M.; Swigler, C.W. Evidence-Based Review of Distal Radius Fractures. Orthop. Clin. N. Am. 2018, 49, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.C.; Kim, H.M.; Malay, S.; Shauver, M.J.; Group, W. Comparison of 24-Month Outcomes After Treatment for Distal Radius Fracture: The WRIST Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2112710. [Google Scholar] [CrossRef] [PubMed]
- Esworthy, G.P.; Johnson, N.A.; Divall, P.; Dias, J.J. Origins of the threshold for surgical intervention in intra-articular distal radius fractures. Bone Jt. J. 2021, 103-B, 1457. [Google Scholar] [CrossRef] [PubMed]
- Farner, S.; Malkani, A.; Lau, E.; Day, J.; Ochoa, J.; Ong, K. Outcomes and Cost of Care for Patients With Distal Radius Fractures. Orthopedics 2014, 37, e866–e878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, S.R.; Kelsey, J.L.; Nevitt, M.C.; O’Dowd, K.J. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol. Rev. 1985, 7, 178–208. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, U.G.; De Salvatore, S.; Mazzola, A.; Salvatore, G.; Mera, B.J.; Piergentili, I.; Denaro, V. Colles’ Fracture: An Epidemiological Nationwide Study in Italy from 2001 to 2016. Int. J. Environ. Res. Public Health 2023, 20, 3956. https://doi.org/10.3390/ijerph20053956
Longo UG, De Salvatore S, Mazzola A, Salvatore G, Mera BJ, Piergentili I, Denaro V. Colles’ Fracture: An Epidemiological Nationwide Study in Italy from 2001 to 2016. International Journal of Environmental Research and Public Health. 2023; 20(5):3956. https://doi.org/10.3390/ijerph20053956
Chicago/Turabian StyleLongo, Umile Giuseppe, Sergio De Salvatore, Alessandro Mazzola, Giuseppe Salvatore, Barbara Juliette Mera, Ilaria Piergentili, and Vincenzo Denaro. 2023. "Colles’ Fracture: An Epidemiological Nationwide Study in Italy from 2001 to 2016" International Journal of Environmental Research and Public Health 20, no. 5: 3956. https://doi.org/10.3390/ijerph20053956





