The Protective Effect of Bariatric Surgery on the Development of Colorectal Cancer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
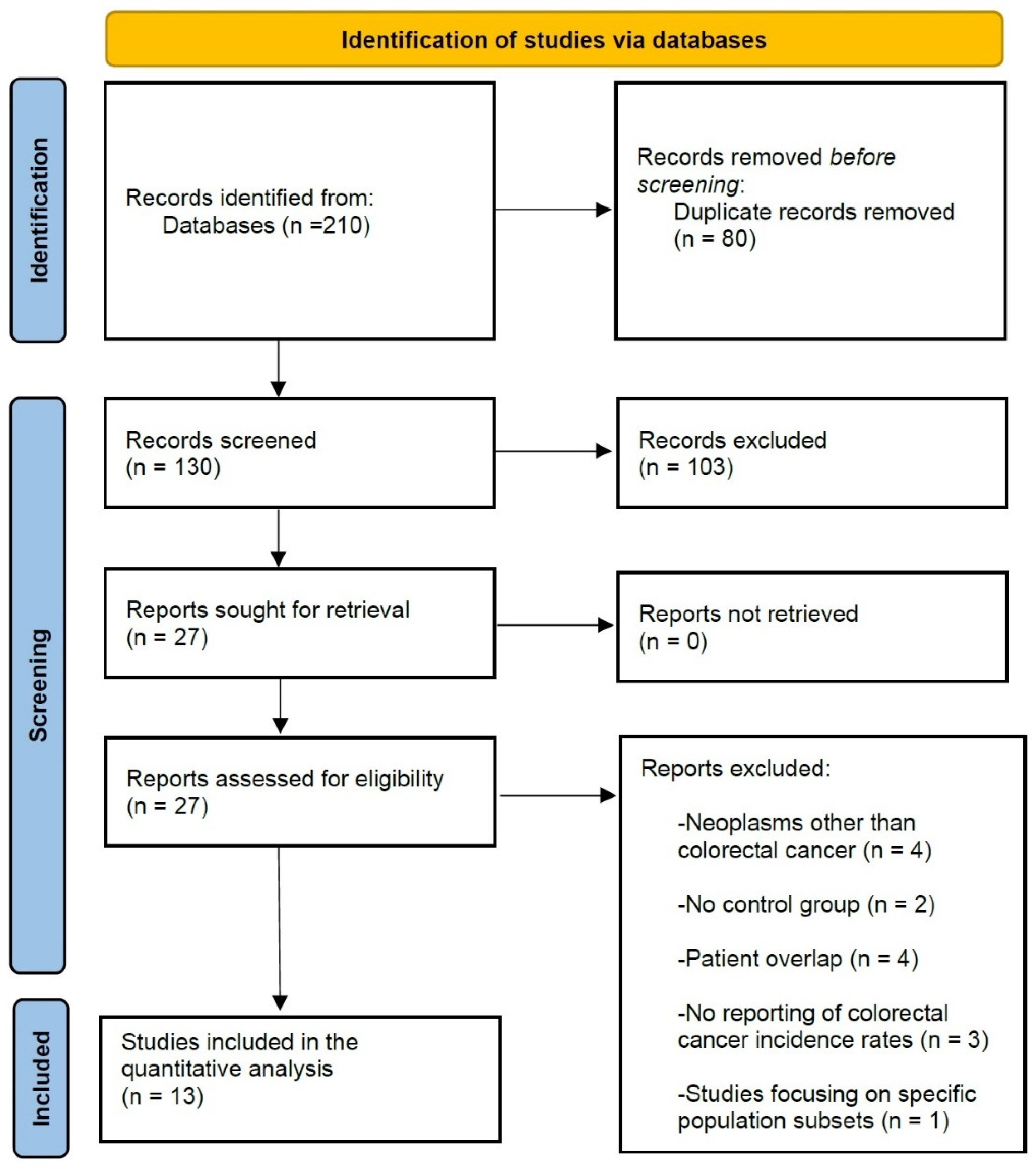
2.1. Literature Search and Study Selection Process
2.2. Data Extraction and Outcomes of Interest
2.3. Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
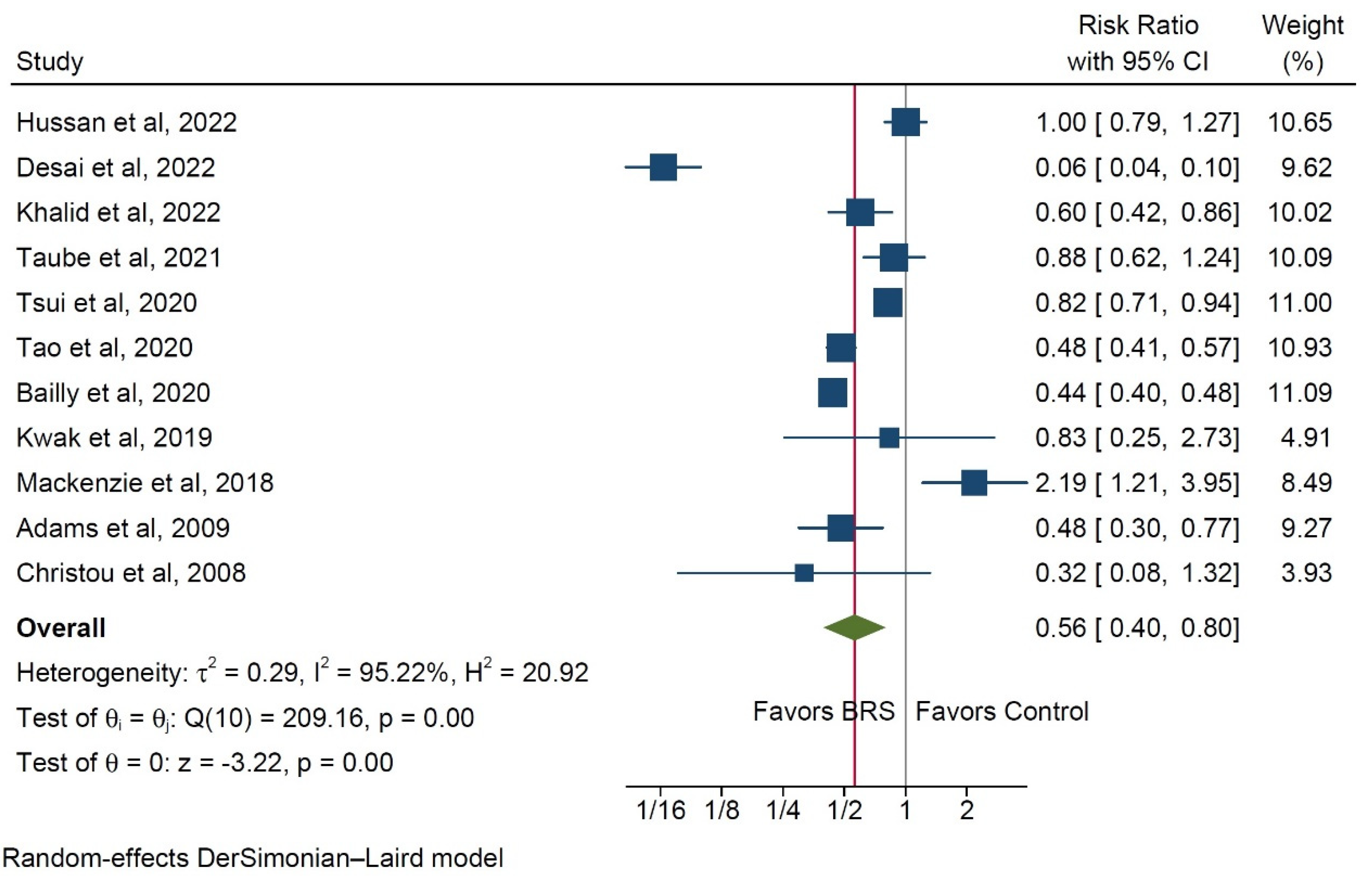
3.1. Risk for Colorectal Cancer
3.2. Risk of Colorectal Adenomas
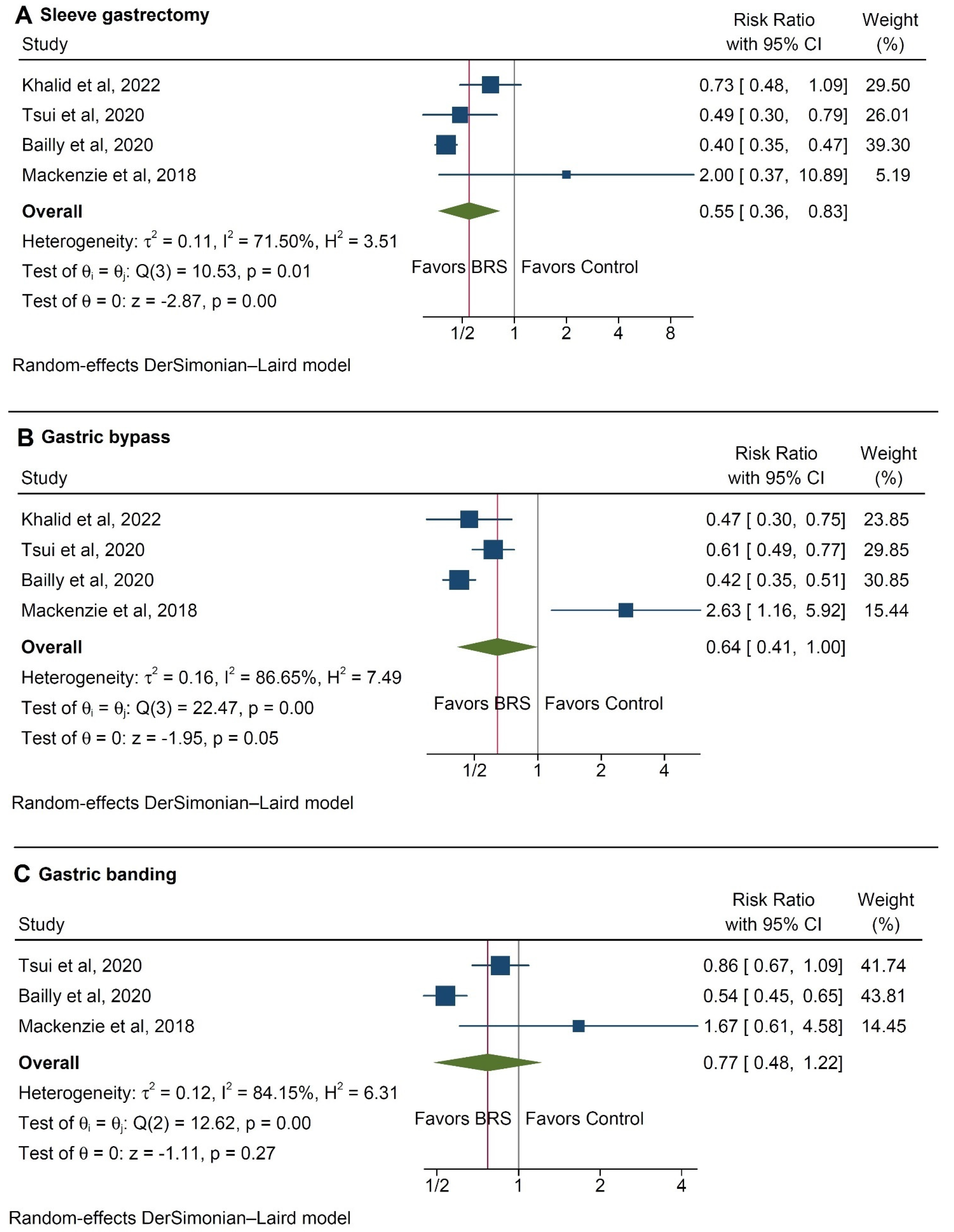
3.3. Subgroup Analysis
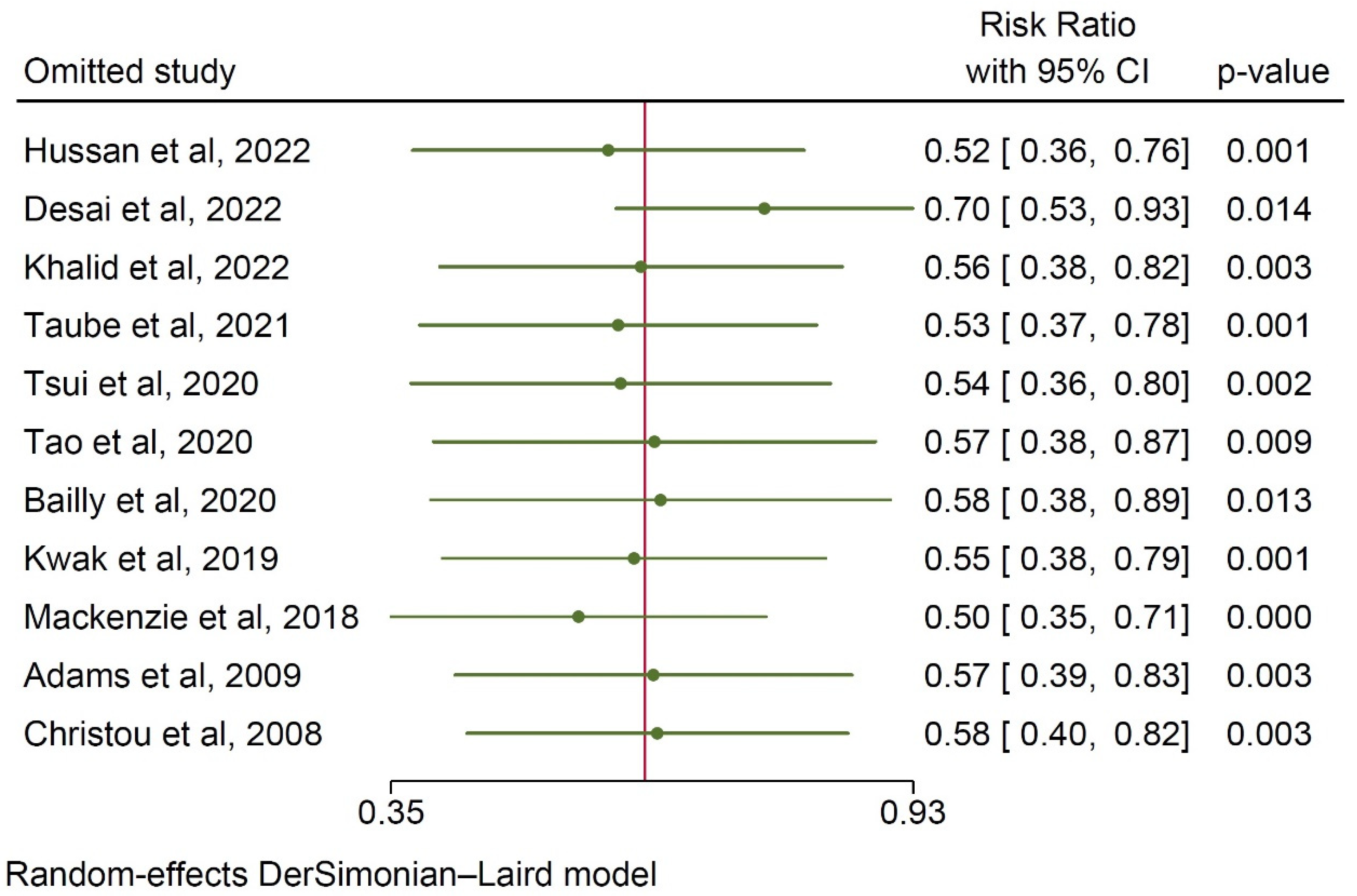
3.4. Publication Bias Assessment
3.5. Risk of Bias and Critical Appraisal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Goding Sauer, A.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA. Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef]
- Decker, K.M.; Lambert, P.; Bravo, J.; Demers, A.; Singh, H. Time Trends in Colorectal Cancer Incidence Rates by Income and Age at Diagnosis in Canada From 1992 to 2016. JAMA Netw. Open 2021, 4, e2117556. [Google Scholar] [CrossRef] [PubMed]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Thun, M.J. Obesity and cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Kaaks, R.; Vainio, H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002, 3, 565–574. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Courcoulas, A.P. Bariatric surgery for obesity and metabolic conditions in adults. BMJ 2014, 349, g3961. [Google Scholar] [CrossRef]
- Taube, M.; Peltonen, M.; Sjöholm, K.; Palmqvist, R.; Andersson-Assarsson, J.C.; Jacobson, P.; Svensson, P.-A.; Carlsson, L.M.S. Long-term incidence of colorectal cancer after bariatric surgery or usual care in the Swedish Obese Subjects study. PLoS ONE 2021, 16, e0248550. [Google Scholar] [CrossRef]
- Mackenzie, H.; Markar, S.R.; Askari, A.; Faiz, O.; Hull, M.; Purkayastha, S.; Møller, H.; Lagergren, J. Obesity surgery and risk of cancer. Br. J. Surg. 2018, 105, 1650–1657. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Artama, M.; von Euler-Chelpin, M.; Hull, M.; Ljung, R.; Lynge, E.; Ólafsdóttir, G.H.; Pukkala, E.; Romundstad, P.; Talbäck, M.; et al. Colon and rectal cancer risk after bariatric surgery in a multicountry Nordic cohort study. Int. J. Cancer 2020, 147, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Singhal, S.; Koka, J. Evaluating the Correlation of Bariatric Surgery and the Prevalence of Cancers in Obese Patients: A Study of the National Inpatient Sample (NIS) Database. Cureus 2022, 14, e23976. [Google Scholar] [CrossRef] [PubMed]
- Tsui, S.T.; Yang, J.; Zhang, X.; Docimo, S.J.; Spaniolas, K.; Talamini, M.A.; Sasson, A.R.; Pryor, A.D. Development of cancer after bariatric surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2020, 16, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Bailly, L.; Fabre, R.; Pradier, C.; Iannelli, A. Colorectal Cancer Risk Following Bariatric Surgery in a Nationwide Study of French Individuals With Obesity. JAMA Surg. 2020, 155, 395–402. [Google Scholar] [CrossRef]
- Adams, T.D.; Stroup, A.M.; Gress, R.E.; Adams, K.F.; Calle, E.E.; Smith, S.C.; Halverson, R.C.; Simper, S.C.; Hopkins, P.N.; Hunt, S.C. Cancer incidence and mortality after gastric bypass surgery. Obesity 2009, 17, 796–802. [Google Scholar] [CrossRef]
- Hussan, H.; Akinyeye, S.; Mihaylova, M.; McLaughlin, E.; Chiang, C.; Clinton, S.K.; Lieberman, D. Colorectal Cancer Risk Is Impacted by Sex and Type of Surgery After Bariatric Surgery. Obes. Surg. 2022, 32, 2880–2890. [Google Scholar] [CrossRef]
- Christou, N.V.; Lieberman, M.; Sampalis, F.; Sampalis, J.S. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2008, 4, 691–695. [Google Scholar] [CrossRef]
- Khalid, S.I.; Maasarani, S.; Wiegmann, J.; Wiegmann, A.L.; Becerra, A.Z.; Omotosho, P.; Torquati, A. Association of Bariatric Surgery and Risk of Cancer in Patients With Morbid Obesity. Ann. Surg. 2022, 275, 1–6. [Google Scholar] [CrossRef]
- Kwak, M.; Mehaffey, J.H.; Hawkins, R.B.; Hedrick, T.L.; Slingluff, C.L.J.; Schirmer, B.; Hallowell, P.T.; Friel, C.M. Bariatric surgery is independently associated with a decrease in the development of colorectal lesions. Surgery 2019, 166, 322–326. [Google Scholar] [CrossRef]
- Droney, A.C.; Sellers, W.; Gupta, A.; Johnson, K.R.; Fluck, M.; Petrick, A.; Bannon, J.; Erchinger, T.; Protyniak, B. Incidence of polyp formation following bariatric surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2021, 17, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Hussan, H.; Drosdak, A.; Le Roux, M.; Patel, K.; Porter, K.; Clinton, S.K.; Focht, B.; Noria, S. The Long-term Impact of Roux-en-Y Gastric Bypass on Colorectal Polyp Formation and Relation to Weight Loss Outcomes. Obes. Surg. 2020, 30, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Almazeedi, S.; El-Abd, R.; Al-Khamis, A.; Albatineh, A.N.; Al-Sabah, S. Role of bariatric surgery in reducing the risk of colorectal cancer: A meta-analysis. Br. J. Surg. 2020, 107, 348–354. [Google Scholar] [CrossRef]
- Afshar, S.; Kelly, S.B.; Seymour, K.; Lara, J.; Woodcock, S.; Mathers, J.C. The effects of bariatric surgery on colorectal cancer risk: Systematic review and meta-analysis. Obes. Surg. 2014, 24, 1793–1799. [Google Scholar] [CrossRef]
- Derogar, M.; Hull, M.A.; Kant, P.; Östlund, M.; Lu, Y.; Lagergren, J. Increased risk of colorectal cancer after obesity surgery. Ann. Surg. 2013, 258, 983–988. [Google Scholar] [CrossRef]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected, U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Stival, C.; Lugo, A.; Odone, A.; van den Brandt, P.A.; Fernandez, E.; Tigova, O.; Soriano, J.B.; José López, M.; Scaglioni, S.; Gallus, S. Prevalence and Correlates of Overweight and Obesity in 12 European Countries in 2017–2018. Obes. Facts 2022, 15, 655–665. [Google Scholar] [CrossRef]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jørgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef]
- Peleg, N.; Sapoznikov, S.; Levi, Z.; Dotan, I.; Shamah, S. Incidence of Colorectal Adenomas After Bariatric Surgery: Pre-operative Super Morbid Obesity Is Independently Associated with Increased Risk. Obes. Surg. 2021, 31, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Spychalski, P.; Kobiela, J.; Wieszczy, P.; Bugajski, M.; Reguła, J.; Kaminski, M.F. Adenoma to Colorectal Cancer Estimated Transition Rates Stratified by BMI Categories-A Cross-Sectional Analysis of Asymptomatic Individuals from Screening Colonoscopy Program. Cancers 2021, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Kedrin, D.; Gandhi, S.-C.C.; Wolf, M.; Roper, J.; Yilmaz, O.; Corey, K.; Khalili, H.; Stanford, F.C.; Gala, M. Bariatric Surgery Prior to Index Screening Colonoscopy Is Associated With a Decreased Rate of Colorectal Adenomas in Obese Individuals. Clin. Transl. Gastroenterol. 2017, 8, e73. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, Y.; Wang, F.; Zhang, P.; Shi, C.; Zou, Y.; Qin, H. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS ONE 2013, 8, e53916. [Google Scholar] [CrossRef] [PubMed]
- Karahalios, A.; Simpson, J.A.; Baglietto, L.; MacInnis, R.J.; Hodge, A.M.; Giles, G.G.; English, D.R. Change in weight and waist circumference and risk of colorectal cancer: Results from the Melbourne Collaborative Cohort Study. BMC Cancer 2016, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ness-Jensen, E.; Martling, A.; Hveem, K. Anthropometry-based Obesity Phenotypes and Risk of Colorectal Adenocarcinoma: A Large Prospective Cohort Study in Norway. Epidemiology 2016, 27, 423–432. [Google Scholar] [CrossRef]


| Author | Study Type | Country of Origin | Interval of Data Collection | Patients, n (%) | Age | Gender (M/F), n (%) | Mean Follow-Up Duration (Months) | ||
|---|---|---|---|---|---|---|---|---|---|
| BRS | Control | BRS | Control | ||||||
| Hussan et al., 2022 [17] | Retrospective, PSM | USA | 2012–2020 | 88,630 | 327,734 | 43.4 ± 10.8 | 43.7 ± 11 | 19,864 (22.4)/68,766 (77.6) | 36.4 ± 24.8 |
| Desai et al., 2022 [13] | Retrospective, PSM | USA | 1999–2014 | 174,765 | 524,355 | 43.9 ± 25.2 | 54.9 ± 36.4 | n/a | n/a |
| Khalid et al., 2022 [19] | Retrospective, PSM | USA | 2010–2018 | 19,272 | 9636 | n/a | n/a | 23,742 (82.1)/5166 (17.9) | 60 |
| Droney et al., 2021 [21] | Retrospective | USA | 2010–2017 | 417 | 1360 | 50.1 ± 10.4 | 49.9 ± 11.8 | 516 (29)/1261 (71) | n/a |
| Taube et al., 2021 [9] | Retrospective | Sweden | 1987–2001 | 2006 | 2038 | 47.2 ± 5.9 | 48.7 ± 6.3 | 1180 (29.2)/2864 (70.8) | 266.4 (172.8) * |
| Tsui et al., 2020 [14] | Retrospective | USA | 2006–2012 | 71,000 | 694,500 | n/a | n/a | n/a | n/a |
| Tao et al., 2020 [12] | Retrospective | Multinational | 1980–2015 | 302,576 | 2,977,526 | n/a | n/a | 172,039 (31.7)/370,319 (68.3) | n/a |
| Hussan et al., 2020 [22] | Retrospective, PSM | USA | 1994–2018 | 84 | 107 | 55.7 ± 7.8 | 56.1 ± 5.9 | 38 (19.9)/153 (80.1) | >60 |
| Bailly et al., 2020 [15] | Retrospective, PSM | France | 2009–2018 | 74,131 | 971,217 | 57.3 ± 5.5 | 63.4 ± 7 | n/a | 63.6 ± 25.2 |
| Kwak et al., 2019 [20] | Retrospective, PSM | USA | 1985–2015 | 2231 | 2231 | 42.6 (10.3) * | 42.8 (13.4) | 734 (16.4)/3728 (83.6) | 93.6 * |
| Mackenzie et al., 2018 [10] | Retrospective, PSM | England | 1997–2012 | 8794 | 8794 | 42 (15) | 42 (15) | 3450 (19.6)/14,138 (80.4) | 55 * |
| Adams et al., 2009 [16] | Retrospective | USA | 1984–2002 | 6709 | 9609 | 38.9 ± 10.3 | 39.1 ± 10.7 | 2512 (15.4)/13,806 (84.6) | 144 ± 67 |
| Christou et al., 2008 [18] | Retrospective | Canada | 1986–2002 | 1035 | 5746 | 45.1 ± 11.6 | 46.7 ± 13.1 | 2424 (35.7)/4357 (64.3) | 60 |
| Study | R1 | R2 | R3 | R4 | R5 | R6 | R7 | Overall |
|---|---|---|---|---|---|---|---|---|
| Taube et al., 2021 [9] | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Mackenzie et al., 2018 [10] | Low | Low | Low | Low | Low | Low | Low | Low |
| Tao et al., 2020 [12] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Desai et al., 2022 [13] | Low | Low | Low | Low | Low | Low | Low | Low |
| Tsui et al., 2020 [14] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Bailly et al., 2020 [15] | Low | Low | Low | Low | Low | Low | Low | Low |
| Adams et al., 2009 [16] | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Hussan et al., 2022 [17] | Low | Serious | Low | Low | Low | Moderate | Low | Serious |
| Christou et al., 2008 [18] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Khalid et al., 2022 [19] | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Kwak et al., 2019 [20] | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Droney et al., 2021 [21] | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Hussan et al., 2020 [22] | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pararas, N.; Pikouli, A.; Dellaportas, D.; Nastos, C.; Charalampopoulos, A.; Muqresh, M.A.; Bagias, G.; Pikoulis, E.; Papaconstantinou, D. The Protective Effect of Bariatric Surgery on the Development of Colorectal Cancer: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 3981. https://doi.org/10.3390/ijerph20053981
Pararas N, Pikouli A, Dellaportas D, Nastos C, Charalampopoulos A, Muqresh MA, Bagias G, Pikoulis E, Papaconstantinou D. The Protective Effect of Bariatric Surgery on the Development of Colorectal Cancer: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(5):3981. https://doi.org/10.3390/ijerph20053981
Chicago/Turabian StylePararas, Nikolaos, Anastasia Pikouli, Dionysios Dellaportas, Constantinos Nastos, Anestis Charalampopoulos, Mohamad Ayham Muqresh, George Bagias, Emmanouil Pikoulis, and Dimitrios Papaconstantinou. 2023. "The Protective Effect of Bariatric Surgery on the Development of Colorectal Cancer: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 5: 3981. https://doi.org/10.3390/ijerph20053981
APA StylePararas, N., Pikouli, A., Dellaportas, D., Nastos, C., Charalampopoulos, A., Muqresh, M. A., Bagias, G., Pikoulis, E., & Papaconstantinou, D. (2023). The Protective Effect of Bariatric Surgery on the Development of Colorectal Cancer: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(5), 3981. https://doi.org/10.3390/ijerph20053981








