Endovascular Intervention for Aortic Dissection Is “Ascending”
Abstract
1. Introduction
2. Aim of the Review
3. Guidelines Recommendation and Open Surgery Limitations
4. The Role of Ascending Thoracic Endovascular Aortic Repair
- Technical issues. Standard thoracic aorta endoprosthesis are too long for ascending aorta stenting (Figure 3) and their nose cones may protrude into left ventricle; their delivery systems do not allow devices manipulation into ascending aorta and across aortic valve and most devices do not account for ascending aorta and aortic arch curves. On the other hand, abdominal aortic devices are too small, and their delivery systems are short for endovascular treatment of ascending aorta (Figure 4). Thus, a trans-subclavian or trans-apical approach was used, even if these procedures are not always feasible.
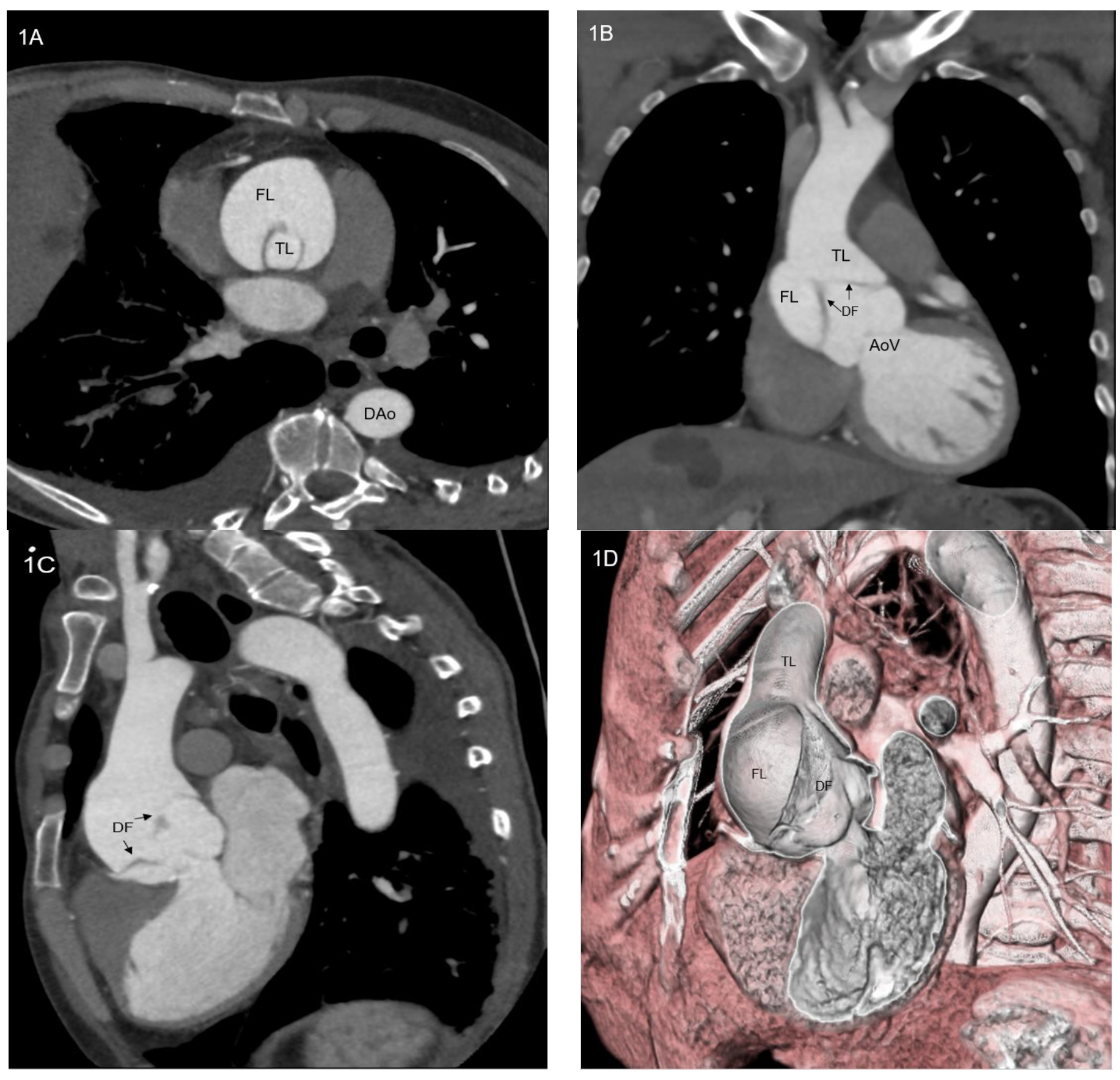
- Physiological issues. Ascending aorta presents a complex motion during the cardiac cycle, characterized by a composite of craniocaudal movement and a rotation of 6–14° [21]. Thus, the risk of endoprosthesis dislodgment is not negligible. Although this circumstance could be avoided by device oversizing, this situation can lead to aortic rupture.
- Anatomical issues. The coverable zone in the ascending aorta is restricted proximally by the aortic sinus with the coronary arteries and distally by the offspring of the brachiocephalic trunk; the most relevant limitation is represented by too proximal entry tears, involving aortic root or aortic valve (<2 cm from the most distal coronary artery); the feasibility of endovascular stenting is not well established and ranges from 2% to 50% of patients with TAAD [22,23,24]; it mainly depends on improper proximal tear sealing, due to the absence of adequate proximal landing zone. Moreover, ascending aorta presents two different curves: a longer outer curve and a shorter inner curve (Figure 5A,B); conventional endoprostheses do not fit this difference in length, so that a proximal shift at inner curve could occur during the endoprosthesis deployment; the proximal shift could provoke impairment of left coronary ostium, leading to serious consequences [25].
5. Towards a Total Endovascular Approach to Ascending Aorta: The Endo-Bentall Procedure
6. Clinical Trials
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| aTEVAR | ascending thoracic endovascular aortic repair |
| MMP | matrix metalloproteinase |
| TAA | thoracic aortic aneurysm |
| TAAD | type A aortic dissection |
| TAD | thoracic aortic dissection |
| TIA | transient ischemic attack |
| TIMP | tissue inhibitor metalloproteinase |
References
- Heather, B.P.; Poskitt, K.R.; Earnshaw, J.J.; Whyman, M.; Shaw, E. Population screening reduces mortality rate from aortic aneurysm in men. Br. J. Surg. 2000, 87, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A.P.; Wilson, N.M.; A Ashton, H.; Kay, D.N. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br. J. Surg. 1995, 82, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Thelin, S.; Ståhle, E.; Ekbom, A.; Granath, F. Thoracic Aortic Aneurysm and Dissection. Circulation 2006, 114, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.P.; Banerjee, A.; Fairhead, J.F.; Perkins, J.; Silver, L.E.; Rothwell, P.M. Population-Based Study of Incidence and Outcome of Acute Aortic Dissection and Premorbid Risk Factor Control. Circulation 2013, 127, 2031–2037. [Google Scholar] [CrossRef]
- Cheung, C.; Bernardo, A.S.; Trotter, M.W.B.; A Pedersen, R.; Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin–dependent disease susceptibility. Nat. Biotechnol. 2012, 30, 165–173. [Google Scholar] [CrossRef]
- Weiss, N.; Rodionov, R.N.; Mahlmann, A. Medical management of abdominal aortic aneurysms. Vasa 2014, 43, 415–421. [Google Scholar] [CrossRef]
- Pape, L.A.; Tsai, T.T.; Isselbacher, E.M.; Oh, J.K.; O’Gara, P.T.; Evangelista, A.; Fattori, R.; Meinhardt, G.; Trimarchi, S.; Bossone, E.; et al. Aortic Diameter ≥ 5.5 cm Is Not a Good Predictor of Type A Aortic Dissection. Circulation 2007, 116, 1120–1127. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, I.J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileaul, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: Document Covering Acute and Chronic Aortic Diseases of the Thoracic and Abdominal Aorta of the Adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar]
- Yamaguchi, T.; Nakai, M.; Sumita, Y.; Miyamoto, Y.; Matsuda, H.; Inoue, Y.; Yoshino, H.; Okita, Y.; Minatoya, K.; Ueda, Y.; et al. Current status of the management and outcomes of acute aortic dissection in Japan: Analyses of nationwide Japanese Registry of All Cardiac and Vascular Diseases-Diagnostic Procedure Combination data. Eur. Heart J. Acute Cardiovasc. Care 2019, 9, S21–S31. [Google Scholar] [CrossRef]
- Pan, E.; Kytö, V.; Savunen, T.; Gunn, J. Early and late outcomes after open ascending aortic surgery: 47-year experience in a single centre. Heart Vessel. 2017, 33, 427–433. [Google Scholar] [CrossRef]
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; DI Eusanio, M.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. Insights from the International Registry of Acute Aortic Dissection. Circulation 2018, 137, 1846–1860. [Google Scholar] [CrossRef]
- Roselli, E.E.; Hasan, S.M.; Idrees, J.J.; Aftab, M.; Eagleton, M.J.; Menon, V.; Svensson, L.G. Inoperable patients with acute type A dissection: Are they candidates for endovascular repair? Interact. Cardiovasc. Thorac. Surg. 2017, 25, 582–588. [Google Scholar] [CrossRef]
- Helder, M.R.; Schaff, H.V.; Day, C.N.; Pochettino, A.; Bagameri, G.; Greason, K.L.; Lansman, S.L.; Girardi, L.N.; Storlie, C.B.; Habermann, E.B. Regional and Temporal Trends in the Outcomes of Repairs for Acute Type A Aortic Dissections. Ann. Thorac. Surg. 2019, 109, 26–33. [Google Scholar] [CrossRef]
- Dorros, G.; Dorros, A.M.; Planton, S.; O’Hair, D.; Zayed, M. Transseptal guidewire stabilization facilitates stent-graft deployment for persistent proximal ascending aortic dissection. J. Endovasc. Ther. 2000, 7, 506–512. [Google Scholar] [CrossRef]
- Roselli, E.E.; Idrees, J.; Greenberg, R.K.; Johnston, D.R.; Lytle, B.W. Endovascular stent grafting for ascending aorta repair in high-risk patients. J. Thorac. Cardiovasc. Surg. 2015, 149, 144–154. [Google Scholar] [CrossRef]
- Preventza, O.; Henry, M.J.; Cheong, B.Y.; Coselli, J.S. Endovascular Repair of the Ascending Aorta: When and How to Implement the Current Technology. Ann. Thorac. Surg. 2014, 97, 1555–1560. [Google Scholar] [CrossRef]
- Roselli, E.E.; Brozzi, N.; Albacker, T.; Lytle, B.W. Transapical Endovascular Ascending Repair for Inoperable Acute Type A Dissection. JACC Cardiovasc. Interv. 2013, 6, 425–426. [Google Scholar] [CrossRef]
- Ahmed, Y.; Houben, I.B.; Figueroa, C.A.; Burris, N.S.; Williams, D.M.; Moll, F.L.; Patel, H.J.; van Herwaarden, J.A. Endovascular ascending aortic repair in type A dissection: A systematic review. J. Card. Surg. 2020, 36, 268–279. [Google Scholar] [CrossRef]
- Harky, A.; Chan, J.; MacCarthy-Ofosu, B. The future of stenting in patients with type A aortic dissection: A systematic review. J. Int. Med. Res. 2019, 48, 300060519871372. [Google Scholar] [CrossRef]
- Beller, C.J.; Labrosse, M.R.; Thubrikar, M.J.; Robicsek, F. Role of Aortic Root Motion in the Pathogenesis of Aortic Dissection. Circulation 2004, 109, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Sobocinski, J.; O’Brien, N.; Maurel, B.; Bartoli, M.; Goueffic, Y.; Sassard, T.; Midulla, M.; Koussa, M.; Vincentelli, A.; Haulon, S. Endovascular Approaches to Acute Aortic Type A Dissection: A CT-Based Feasibility Study. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.C.; Greenberg, R.K.; Morales, J.P.; Martin, Z.; Lu, Q.; Dowdall, J.F.; Hernandez, A.V. Computed tomography-based anatomic characterization of proximal aortic dissection with consideration for endovascular candidacy. J. Vasc. Surg. 2011, 53, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Nissen, A.P.; Ocasio, L.; Tjaden, B.L.; Sandhu, H.K.; Riascos, R.F.; Safi, H.J.; Estrera, A.L.; Charlton-Ouw, K.M. Imaging characteristics of acute type A aortic dissection and candidacy for repair with ascending aortic endografts. J. Vasc. Surg. 2019, 70, 1766–1775.e1. [Google Scholar] [CrossRef]
- Hauck, S.R.; Kupferthaler, A.; Stelzmüller, M.; Eilenberg, W.; Ehrlich, M.; Neumayer, C.; Wolf, F.; Loewe, C.; Funovics, M.A. Endovascular Stent-Graft Repair of the Ascending Aorta: Assessment of a Specific Novel Stent-Graft Design in Phantom, Cadaveric, and Clinical Application. Cardiovasc. Interv. Radiol. 2021, 44, 1448–1455. [Google Scholar] [CrossRef]
- Patel, P.A.; Bavaria, J.E.; Ghadimi, K.; Gutsche, J.T.; Vallabhajosyula, P.; Ko, H.A.; Desai, N.D.; Mackay, E.; Weiss, S.J.; Augoustides, J.G. Aortic Regurgitation in Acute Type-A Aortic Dissection: A Clinical Classification for the Perioperative Echocardiographer in the Era of the Functional Aortic Annulus. J. Cardiothorac. Vasc. Anesth. 2018, 32, 586–597. [Google Scholar] [CrossRef]
- Trimarchi, S.; Grassi, V.; Lomazzi, C.; Domanin, M.; Bissacco, D.; Bellosta, R.; Piffaretti, G. Endovascular type A aortic repair—When? J. Card. Surg. 2020, 36, 1742–1744. [Google Scholar] [CrossRef]
- Rylski, B.; Szeto, W.Y.; Bavaria, J.E.; Branchetti, E.; Moser, W.; Milewski, R.K. Development of a Single Endovascular Device for Aortic Valve Replacement and Ascending Aortic Repair. J. Card. Surg. 2014, 29, 371–376. [Google Scholar] [CrossRef]
- Gaia, D.F.; Bernal, O.; Castilho, E.; Ferreira, C.B.N.D.; Dvir, D.; Simonato, M.; Palma, J.H. First-in-Human Endo-Bentall Procedure for Simultaneous Treatment of the Ascending Aorta and Aortic Valve. JACC Case Rep. 2020, 2, 480–485. [Google Scholar] [CrossRef]
- Gandet, T.; Westermann, D.; Conradi, L.; Panuccio, G.; Heidemann, F.; Rohlffs, F.; Kölbel, T. Modular Endo-Bentall Procedure Using a “Rendez-Vous Access”. J. Endovasc. Ther. 2021, 29, 711–716. [Google Scholar] [CrossRef]
- Roselli, E.E.; Atkins, M.D.; Brinkman, W.; Coselli, J.; Desai, N.; Estrera, A.; Johnston, D.R.; Patel, H.; Preventza, O.; Vargo, P.R.; et al. ARISE: First-In-Human Evaluation of a Novel Stent Graft to Treat Ascending Aortic Dissection. J. Endovasc. Ther. 2022, 15266028221095018. [Google Scholar] [CrossRef]






| Author, Publication Year | Patient’s Age | Gender | Surgical Risk | Aortic Disease | Vascular Access | Diseased Aortic Valve | Endoprosthesis and Valves | Coronary Arteries Perfusion |
|---|---|---|---|---|---|---|---|---|
| Rylski, 2014 [28] | - | - | - | Ascending aorta aneurysm | Transapical | Native aortic valve stenosis | Endovascular valvuled conduit: proximal transcatheter aortic valve connected to an uncovered portion of a covered stent graft | Stented endoprosthesis |
| Gaia, 2020 [29] | 64 | female | EuroSCORE 25.8% | ascending aorta pseudoaneurysm | Transapical | Biological prosthetic valve failure | Custom-made balloon expandable aortic valve and self-expandable aortic stent graft | Coronary branch from the endoprosthesis |
| Gandet, 2021 [30] | 82 | female | - | Ascending aortica aneurysm | Transapical and transfemoral (“Rendez-vous-access”) | Native aortic valve stenosis | Fenestrated physician-modified endograft | Endoprosthesis fnestration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizza, A.; Negro, F.; Mandigers, T.J.; Palmieri, C.; Berti, S.; Trimarchi, S. Endovascular Intervention for Aortic Dissection Is “Ascending”. Int. J. Environ. Res. Public Health 2023, 20, 4094. https://doi.org/10.3390/ijerph20054094
Rizza A, Negro F, Mandigers TJ, Palmieri C, Berti S, Trimarchi S. Endovascular Intervention for Aortic Dissection Is “Ascending”. International Journal of Environmental Research and Public Health. 2023; 20(5):4094. https://doi.org/10.3390/ijerph20054094
Chicago/Turabian StyleRizza, Antonio, Francesco Negro, Tim J. Mandigers, Cataldo Palmieri, Sergio Berti, and Santi Trimarchi. 2023. "Endovascular Intervention for Aortic Dissection Is “Ascending”" International Journal of Environmental Research and Public Health 20, no. 5: 4094. https://doi.org/10.3390/ijerph20054094
APA StyleRizza, A., Negro, F., Mandigers, T. J., Palmieri, C., Berti, S., & Trimarchi, S. (2023). Endovascular Intervention for Aortic Dissection Is “Ascending”. International Journal of Environmental Research and Public Health, 20(5), 4094. https://doi.org/10.3390/ijerph20054094








