Human Dietary Exposure to Heavy Metals via Rice in Nepal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Sample Preparation and Analysis
2.4. Quality Assurance and Quality Control (QA/QC)
2.5. Human Health Risk Assessment
2.5.1. Estimated Daily Intakes (EDIs)
2.5.2. Non-Carcinogenic Risk Assessment
2.5.3. Carcinogenic Risk Assessment
2.6. Statistical Analysis
3. Results and Discussion
3.1. Concentrations of Cd, As, Pb, and Cu
3.1.1. Cadmium
3.1.2. Arsenic
3.1.3. Lead
3.1.4. Copper
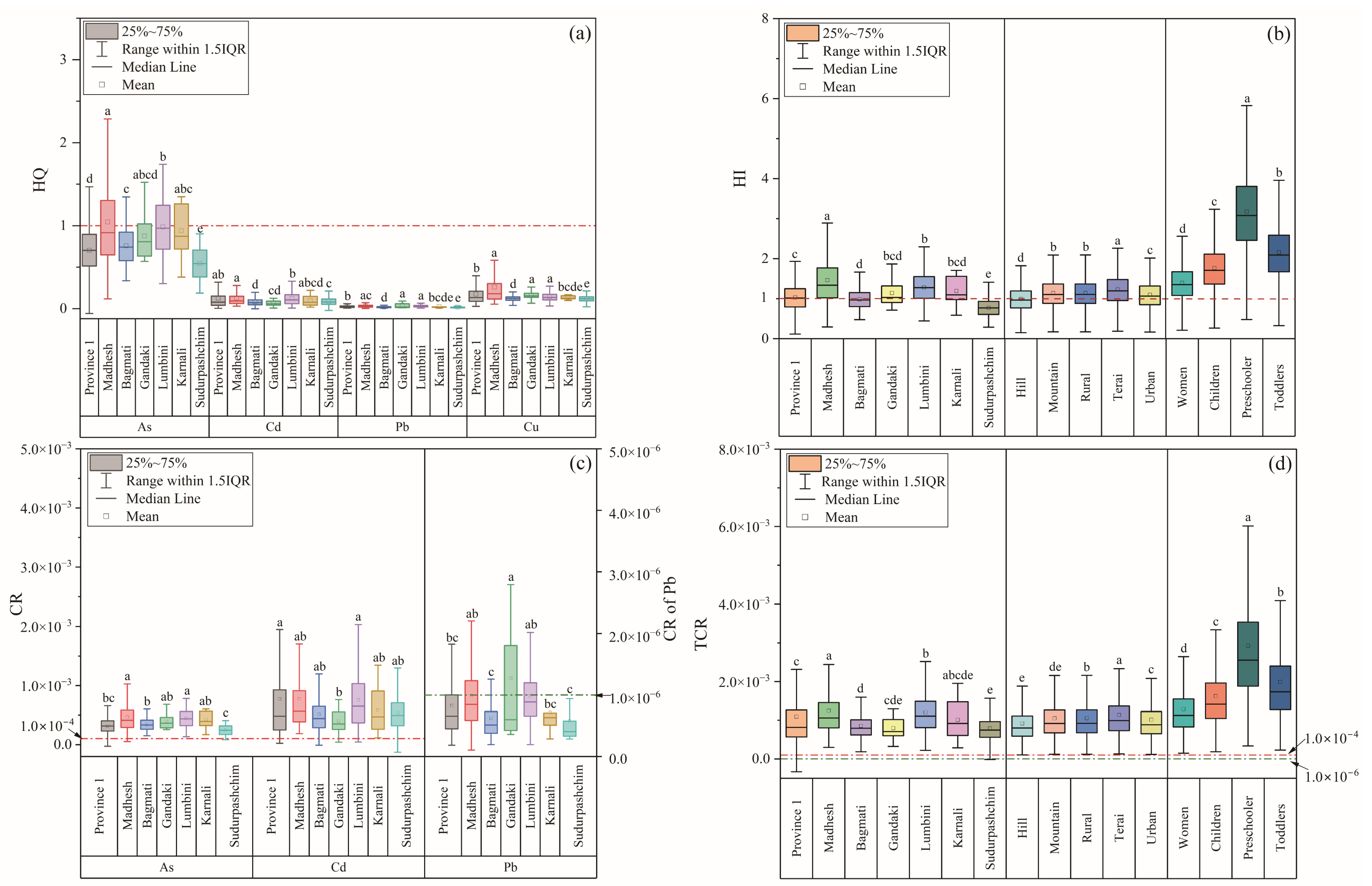
3.2. Human Exposure to HMs
3.3. Risk Assessment
3.3.1. Non-Carcinogenic Risk (NCR)
3.3.2. Carcinogenic Risk (CR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Naseri, M.; Vazirzadeh, A.; Kazemi, R.; Zaheri, F. Concentration of some heavy metals in rice types available in Shiraz market and human health risk assessment. Food Chem. 2015, 175, 243–248. [Google Scholar] [CrossRef]
- Wang, L.; Han, J.; Katuwal, H.B.; Xia, P.; Xu, X.; Feng, X.; Qiu, G. Occurrence of total mercury and methylmercury in rice: Exposure and health implications in Nepal. Ecotoxicol. Environ. Saf. 2021, 228, 113019. [Google Scholar] [CrossRef]
- Abtahi, M.; Fakhri, Y.; Oliveri Conti, G.; Keramati, H.; Zandsalimi, Y.; Bahmani, Z.; Hosseini Pouya, R.; Sarkhosh, M.; Moradi, B.; Amanidaz, N.; et al. Heavy metals (As, Cr, Pb, Cd and Ni) concentrations in rice (Oryza sativa) from Iran and associated risk assessment: A systematic review. Toxin Rev. 2017, 36, 331–341. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Abduljabbar, M. Heavy metals (lead, cadmium, methylmercury, arsenic) in commonly imported rice grains (Oryza sativa) sold in Saudi Arabia and their potential health risk. Int. J. Hyg. Environ. Health 2017, 220, 1168–1178. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Patlolla, A.K.; Centeno, J.A. Carcinogenic and systemic health effects associated with arsenic exposure--A critical review. Toxicol Pathol. 2003, 31, 575–588. [Google Scholar] [CrossRef]
- Nejabat, M.; Kahe, H.; Shirani, K.; Ghorbannejad, P.; Hadizadeh, F.; Karimi, G. Health risk assessment of heavy metals via dietary intake of wheat in Golestan Province, Iran. Hum. Ecol. Risk Assess. 2017, 23, 1193–1201. [Google Scholar] [CrossRef]
- World Health Organization, WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015.
- Zheng, S.; Wang, Q.; Yuan, Y.; Sun, W. Human health risk assessment of heavy metals in soil and food crops in the Pearl River Delta urban agglomeration of China. Food Chem. 2020, 316, 126213. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 4 May 2021).
- Perera, P.A.C.T.; Sundarabarathy, T.V.; Sivananthawerl, T.; Kodithuwakku, S.P.; Edirisinghe, U. Arsenic and Cadmium Contamination in Water, Sediments and Fish is a Consequence of Paddy Cultivation: Evidence of River Pollution in Sri Lanka. Achiev Life Sci. 2016, 10, 144–160. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Feng, H.; Wei, J. Toxic element (As and Hg) content and health risk assessment of commercially available rice for residents in Beijing based on their dietary consumption. Environ. Sci. Pollut. Res. 2020, 27, 13205–13214. [Google Scholar] [CrossRef]
- Shimbo, S.; Zhang, Z.-W.; Watanabe, T.; Nakatsuka, H.; Matsuda-Inoguchi, N.; Higashikawa, K.; Ikeda, M. Cadmium and lead contents in rice and other cereal products in Japan in 1998–2000. Sci. Total Environ. 2001, 281, 165–175. [Google Scholar] [CrossRef]
- Thomas, K.V.; Bijlsma, L.; Castiglioni, S.; Covaci, A.; Emke, E.; Grabic, R.; Hernández, F.; Karolak, S.; Kasprzyk-Hordern, B.; Lindberg, R.H.; et al. Comparing illicit drug use in 19 European cities through sewage analysis. Sci. Total Environ. 2012, 432, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Gao, J.; Cen, K. Levels of eight heavy metals and health risk assessment considering food consumption by China’s residents based on the 5th China total diet study. Sci. Total Environ. 2019, 689, 1141–1148. [Google Scholar] [CrossRef]
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy Metal Contamination in Rice-Producing Soils of Hunan Province, China and Potential Health Risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef]
- Aziz, R.A.; Rahim, S.A.; Sahid, I.; Idris, W.M.R. Speciation and Availability of Heavy Metals On Serpentinized Paddy Soil and Paddy Tissue. Procedia Soc Behav Sci. 2015, 195, 1658–1665. [Google Scholar] [CrossRef] [Green Version]
- Kukusamude, C.; Sricharoen, P.; Limchoowong, N.; Kongsri, S. Heavy metals and probabilistic risk assessment via rice consumption in Thailand. Food Chem. 2021, 334, 127402. [Google Scholar] [CrossRef]
- Proshad, R.; Kormoker, T.; Islam, M.S.; Chandra, K. Potential health risk of heavy metals via consumption of rice and vegetables grown in the industrial areas of Bangladesh. Hum. Ecol. Risk Assess. 2019, 26, 921–943. [Google Scholar] [CrossRef]
- Roychowdhury, T.; Tokunaga, H.; Fau-Ando, M.; Ando, M. Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Sci. Total Environ. 2003, 308, 15–35. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, X.; Yang, W.; Ma, N.; Xin, Z.; Fu, J.; Liu, X.; Liu, M.; Mariga, A.M.; Zhu, X.; et al. Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food Chem. 2014, 147, 147–151. [Google Scholar] [CrossRef]
- Djahed, B.; Kermani, M.; Farzadkia, M.; Taghavi, M.; Norzaee, S. Exposure to heavy metal contamination and probabilistic health risk assessment using Monte Carlo simulation: A study in the Southeast Iran. J. Environ. Health Sci. Eng. 2020, 18, 1217–1226. [Google Scholar] [CrossRef]
- USEPA. Exposure Factors Handbook; US Environmental Protection Agency: Washington, DC, USA, 2011. [Google Scholar]
- Abbasi, A.M.; Iqbal, J.; Khan, M.A.; Shah, M.H. Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Ecotoxicol. Environ. Saf. 2013, 92, 237–244. [Google Scholar] [CrossRef]
- Brtnicky, M.; Pecina, V.; Hladky, J.; Radziemska, M.; Koudelkova, Z.; Klimanek, M.; Richtera, L.; Adamcova, D.; Elbl, J.; Galiova, M.V.; et al. Assessment of phytotoxicity, environmental and health risks of historical urban park soils. Chemosphere 2019, 220, 678–686. [Google Scholar] [CrossRef]
- USEPA. Risk Assessment Guidance for Superfund, Process for Conducting Probabilistic Risk Assessment; USEPA: Washington, DC, USA, 2001; Part A; Volume III, p. 20460. [Google Scholar]
- Hu, X.F.; Lowe, M.; Chan, H.M. Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environ. Res. 2021, 193, 110538. [Google Scholar] [CrossRef]
- Kayastha, S.P. Heavy metal pollution of agricultural soils and vegetables of bhaktapur district, Nepal. Sci. World 2014, 12, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Dahal, B.M.; Fuerhacker, M.; Mentler, A.; Karki, K.B.; Shrestha, R.R.; Blum, W.E. Arsenic contamination of soils and agricultural plants through irrigation water in Nepal. Environ. Pollut. 2008, 155, 157–163. [Google Scholar] [CrossRef]
- Meharg, A.A.; Norton, G.; Deacon, C.; Williams, P.; Adomako, E.E.; Price, A.; Zhu, Y.; Li, G.; Zhao, F.J.; McGrath, S.; et al. Variation in rice cadmium related to human exposure. Environ. Sci. Technol. 2013, 47, 5613–5618. [Google Scholar] [CrossRef]
- Fillman, T.; Shimizu-Furusawa, H.; Ng, C.F.S.; Parajuli, R.P.; Watanabe, C. Association of cadmium and arsenic exposure with salivary telomere length in adolescents in Terai, Nepal. Environ. Res. 2016, 149, 8–14. [Google Scholar] [CrossRef]
- Maharjan, M.; Watanabe, C.; Ahmad, S.A.; Ohtsuka, R. Arsenic contamination in drinking water and skin manifestations in lowland Nepal: The first community-based survey. Am. J. Trop. Med. Hyg. 2005, 73, 477–479. [Google Scholar] [CrossRef] [Green Version]
- Gumma, M.K.; Gauchan, D.; Nelson, A.; Pandey, S.; Rala, A. Temporal changes in rice-growing area and their impact on livelihood over a decade: A case study of Nepal. Agric. Ecosyst. Environ. 2011, 142, 382–392. [Google Scholar] [CrossRef]
- Central Bureau of Statistics (CBS). Statistical Year Book; National PlanningCommission Secretariat: Kathmandu, Nepal, 2016. [Google Scholar]
- Ministry of Finance (MoF). Economic Survey; Government of Nepal: Kathmandu, Nepal, 2014. [Google Scholar]
- Li, D.; Zhang, Q.; Sun, D.; Yang, C.; Luo, G. Accumulation and risk assessment of heavy metals in rice: A case study for five areas of Guizhou Province, China. Environ. Sci. Pollut. Res. 2022, 29, 84113–84124. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Chen, C.; Zhang, Q.; Li, Y.; Chen, Z.; Li, M. Concentrations of cadmium, lead, mercury and arsenic in Chinese market milled rice and associated population health risk. Food Control. 2010, 21, 1757–1763. [Google Scholar] [CrossRef]
- Panter-Brick, C. Women’s work and child nutrition: The food intake of 0–4 year old children in rural Nepal. Ecol. Food Nutr. 1992, 29, 207–230. [Google Scholar] [CrossRef]
- Saville, N.M.; Maharjan, M.; Manandhar, D.S.; Harris-Fry, H.A. Equity implications of rice fortification: A modelling study from Nepal. Public Health Nutr. 2020, 23, 2824–2839. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, M.; Liu, Q.; Zhang, Z.; Fei, X.; Xiao, R.; Lv, X. Contamination assessment, health risk evaluation, and source identification of heavy metals in the soil-rice system of typical agricultural regions on the southeast coast of China. Environ. Sci. Pollut. Res. 2021, 28, 12870–12880. [Google Scholar] [CrossRef]
- USEPA. Risk-Based Concentration Table; United States Environmental Protection Agency: Washington, DC, USA; Philadelphia, PA, USA, 2000. [Google Scholar]
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao City, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar] [CrossRef]
- USEPA. Integrated Risk Information System (IRIS)-Cd Chemical Assessment Summary, National Center for Environmental Assessment. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=141 (accessed on 22 June 2020).
- USEPA. Guidelines for Human Exposure Assessment; EPA/100/B-19/001; Risk Assessment Forum; USEPA: Washington, DC, USA, 2019. [Google Scholar]
- Ferreira-Baptista, L.; De Miguel, E. Geochemistry and risk assessment of street dust in Luanda, Angola: A tropical urban environment. Atmos. Environ. 2005, 39, 4501–4512. [Google Scholar] [CrossRef] [Green Version]
- USEPA (United States Environmental Protection Agency). Risk Assessment Guidance for Superfund (RAGS), Volume I, Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment). Available online: https://www.epa.gov/risk/risk-assessment-guidancesuperfund-rags-part-e (accessed on 25 March 2022).
- Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods; WHO: Geneva, Switzerland, 2011; pp. 21–25.
- Praveena, S.M.; Omar, N.A. Heavy metal exposure from cooked rice grain ingestion and its potential health risks to humans from total and bioavailable forms analysis. Food Chem. 2017, 235, 203–211. [Google Scholar] [CrossRef]
- Liu, L.; Han, J.; Xu, X.; Xu, Z.; Abeysinghe, K.S.; Atapattu, A.J.; De Silva, P.; Lu, Q.; Qiu, G. Dietary exposure assessment of cadmium, arsenic, and lead in market rice from Sri Lanka. Environ. Sci. Pollut. Res. 2020, 27, 42704–42712. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.M.; Reichman, S.M.; Lim, R.P.; Naidu, R. Heavy metals in Australian grown and imported rice and vegetables on sale in Australia: Health hazard. Ecotoxicol. Environ. Saf. 2014, 100, 53–60. [Google Scholar] [CrossRef]
- Zakaria, Z.; Zulkafflee, N.S.; Mohd Redzuan, N.A.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Tóth, G.; Abdull Razis, A.F. Understanding Potential Heavy Metal Contamination, Absorption, Translocation and Accumulation in Rice and Human Health Risks. Plants 2021, 10, 1070. [Google Scholar] [CrossRef]
- Diyabalanage, S.; Fonseka, S.; Dasanayake, D.; Chandrajith, R. Environmental exposures of trace elements assessed using keratinized matrices from patients with chronic kidney diseases of uncertain etiology (CKDu) in Sri Lanka. J. Trace Elem. Med Biol. 2017, 39, 62–70. [Google Scholar] [CrossRef]
- Morekian, R.; Mirlohi, M.; Azadbakht, L.; Maracy, M.R. Heavy metal distribution frequency in Iranian and imported rice varieties marketed in central Iran, Yazd, 2012. Int. J. Environ. Health Eng. 2013, 2, 36. [Google Scholar]
- Jallad, K.N. Heavy metal exposure from ingesting rice and its related potential hazardous health risks to humans. Environ. Sci. Pollut. Res. 2015, 22, 15449–15458. [Google Scholar] [CrossRef]
- Norton, G.J.; Williams, P.N.; Adomako, E.E.; Price, A.H.; Zhu, Y.; Zhao, F.J.; McGrath, S.; Deacon, C.M.; Villada, A.; Sommella, A.; et al. Lead in rice: Analysis of baseline lead levels in market and field collected rice grains. Sci. Total Environ. 2014, 485–486, 428–434. [Google Scholar] [CrossRef]


| Samples Area | Mean ± SD (μg/kg) (Minimum–Maximum) | |||
|---|---|---|---|---|
| Cd | As | Pb | Cu | |
| Province 1 | 15.4 ± 21.9 | 36.6 ± 18.8 | 15.7 ± 17.3 | 1078 ± 1920 |
| (1.76–95.4) | (6.43–92.3) | (5.49–121.4) | (264–10,059) | |
| Madhesh | 17.5 ± 13.5 | 47.9 ± 28.5 | 17.3 ± 10.3 | 1364 ± 1601 |
| (6.80–46.4) | (17.0–121) | (5.72–36.9) | (453–6559) | |
| Bagmati | 14.9 ± 11.5 | 46.4 ± 14.8 | 13.5 ± 9.21 | 1000 ± 251 |
| (2.68–55.9) | (25.3–81.7) | (5.91–43.0) | (519–1556) | |
| Gandaki | 9.93 ± 6.71 | 47.2 ± 15.8 | 19.7 ± 25.5 | 1100 ± 398 |
| (1.31–23.5) | (32.1–85.6) | (7.94–87.4) | (491–1959) | |
| Lumbini | 17.2 ± 14.8 | 49.2 ± 18.1 | 18.4 ± 10.2 | 949 ± 358 |
| (1.73–68.6) | (23.1–85.8) | (6.02–51.3) | (271–2016) | |
| Karnali | 15.7 ± 12.5 | 54.4 ± 20.3 | 15.6 ± 8.97 | 1072 ± 194 |
| (3.86–45.4) | (23.5–83.3) | (6.94–35.1) | (823–1383) | |
| Sudurpashchim | 17.0 ± 12.5 | 35.1 ± 13.9 | 12.9 ± 5.94 | 1051 ± 316 |
| (4.26–51.9) | (17.3–60.2) | (7.98–26.8) | (550–1624) | |
| Total Nepal | 15.5 ± 16.0 | 43.4 ± 19.6 | 16.0 ± 14.0 | 1066 ± 1210 |
| (1.31–95.4) | (6.43–121) | (5.49–121.4) | (264–10,059) | |
| FAO/WHO * | 100 | 200 | 200 | 10,000 |
| Element | Parameter | Average | P50 | P75 | P90 | P95 | P97.5 | P99 | P99.9 |
|---|---|---|---|---|---|---|---|---|---|
| Cd | EDI | 0.108 | 0.0846 | 0.138 | 0.212 | 0.274 | 0.345 | 0.440 | 0.719 |
| HQ | 0.108 | 0.0846 | 0.138 | 0.212 | 0.274 | 0.345 | 0.440 | 0.719 | |
| As | EDI | 0.25 | 0.239 | 0.313 | 0.388 | 0.436 | 0.484 | 0.543 | 0.671 |
| HQ | 0.834 | 0.795 | 1.04 | 1.29 | 1.45 | 1.61 | 1.81 | 2.24 | |
| Pb | EDI | 0.100 | 0.0799 | 0.120 | 0.179 | 0.231 | 0.292 | 0.382 | 0.656 |
| HQ | 0.0282 | 0.0846 | 0.138 | 0.212 | 0.274 | 0.345 | 0.440 | 0.719 | |
| Cu | EDI | 6.35 | 5.51 | 7.76 | 10.659 | 13.0 | 15.3 | 18.6 | 27.7 |
| HQ | 0.159 | 0.138 | 0.194 | 0.266 | 0.324 | 0.382 | 0.466 | 0.692 | |
| HI | 1.13 | 1.09 | 1.35 | 1.62 | 1.79 | 1.94 | 2.17 | 2.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Xu, X.; Wang, L.; Han, J.; Katuwal, H.B.; Jiao, S.; Qiu, G. Human Dietary Exposure to Heavy Metals via Rice in Nepal. Int. J. Environ. Res. Public Health 2023, 20, 4134. https://doi.org/10.3390/ijerph20054134
Shao Y, Xu X, Wang L, Han J, Katuwal HB, Jiao S, Qiu G. Human Dietary Exposure to Heavy Metals via Rice in Nepal. International Journal of Environmental Research and Public Health. 2023; 20(5):4134. https://doi.org/10.3390/ijerph20054134
Chicago/Turabian StyleShao, Yuxiao, Xiaohang Xu, Le Wang, Jialiang Han, Hem Bahadur Katuwal, Shulin Jiao, and Guangle Qiu. 2023. "Human Dietary Exposure to Heavy Metals via Rice in Nepal" International Journal of Environmental Research and Public Health 20, no. 5: 4134. https://doi.org/10.3390/ijerph20054134
APA StyleShao, Y., Xu, X., Wang, L., Han, J., Katuwal, H. B., Jiao, S., & Qiu, G. (2023). Human Dietary Exposure to Heavy Metals via Rice in Nepal. International Journal of Environmental Research and Public Health, 20(5), 4134. https://doi.org/10.3390/ijerph20054134










