Facet Dependence of Biosynthesis of Vivianite from Iron Oxides by Geobacter sulfurreducens
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Iron Oxide Nanocrystals
2.2. Geobacter Sulfurreducens PCA Inoculation
2.3. Batch Trials for Microbial Fe(III) Reduction and Biosynthetic Vivianite
2.4. Chemical Analysis
2.5. Characterization Analysis
3. Results and Discussion
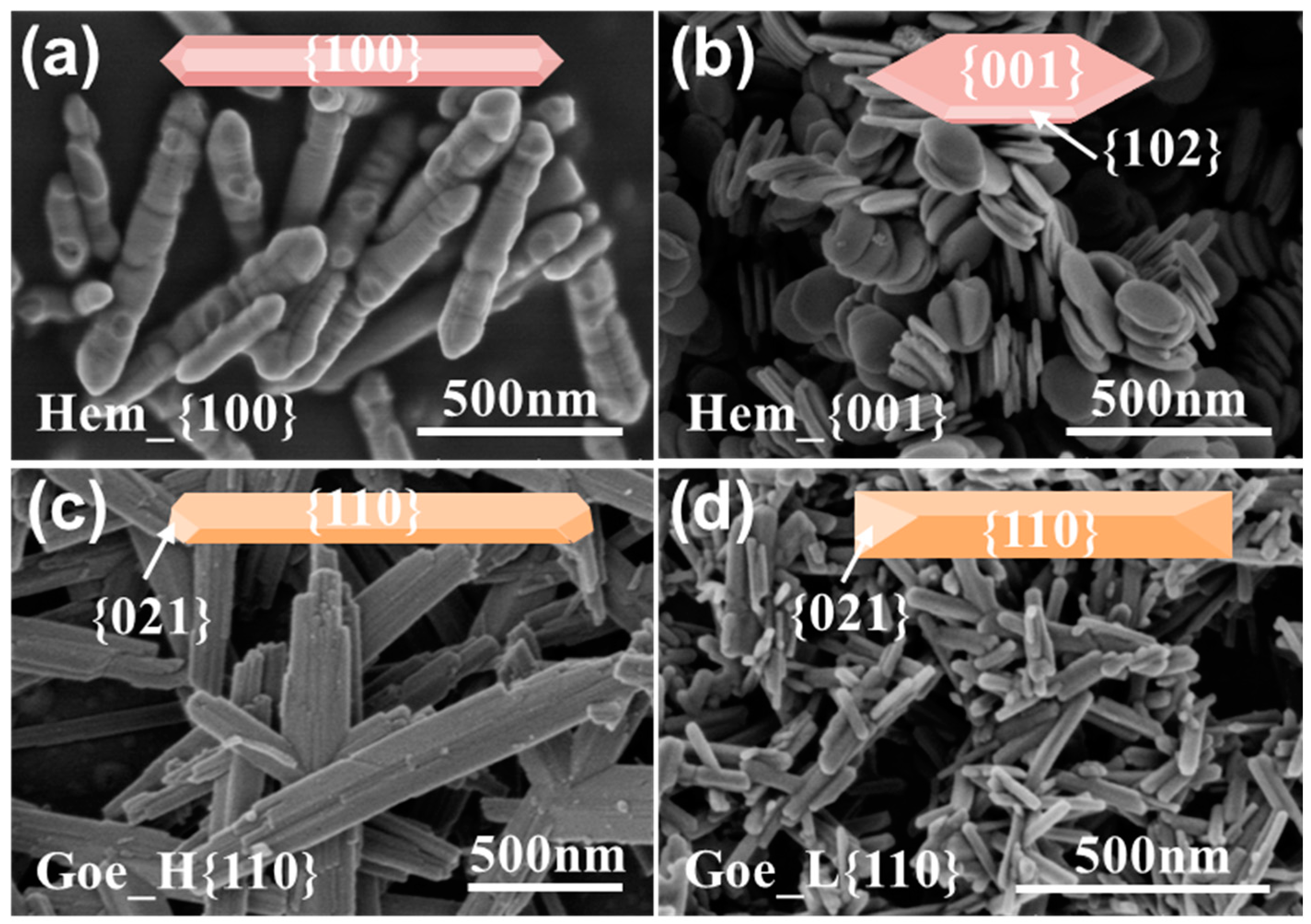
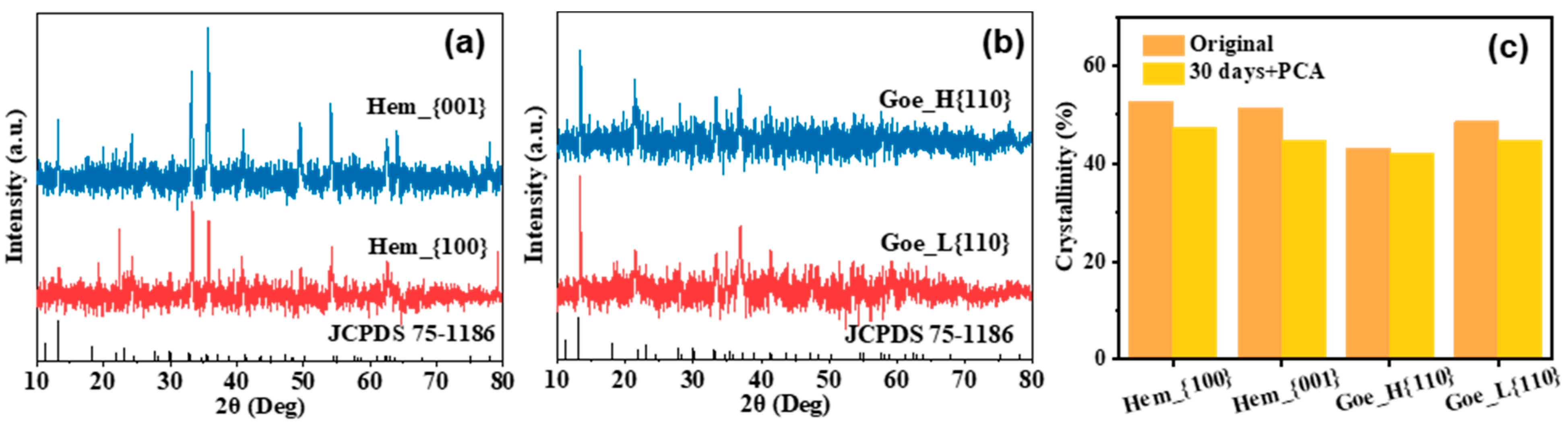
3.1. Characteristics of Iron Oxides with Different Facet Structures
3.2. Facet Dependence of Bioreduction of Iron Oxides by Geobacter sulfurreducens
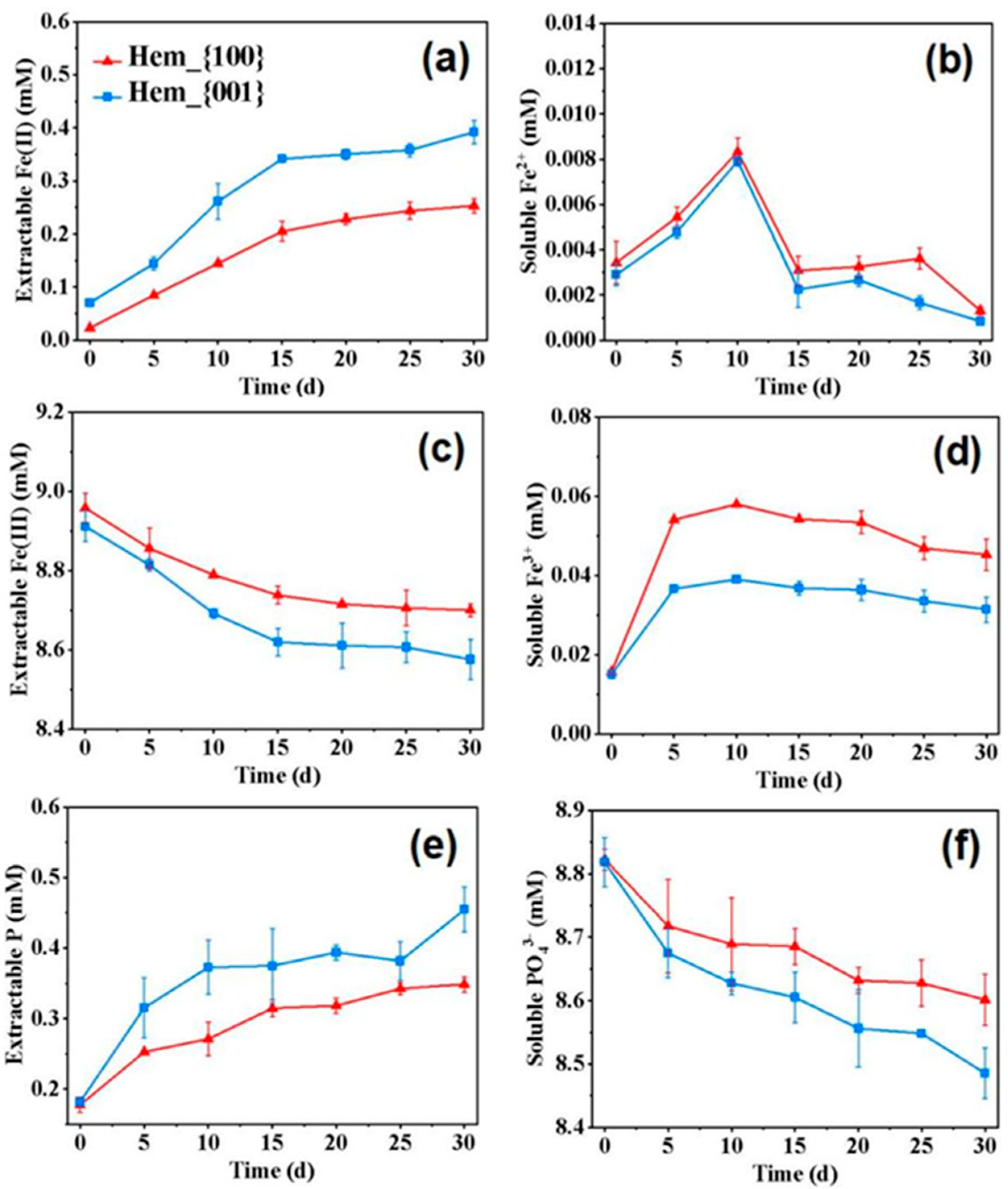
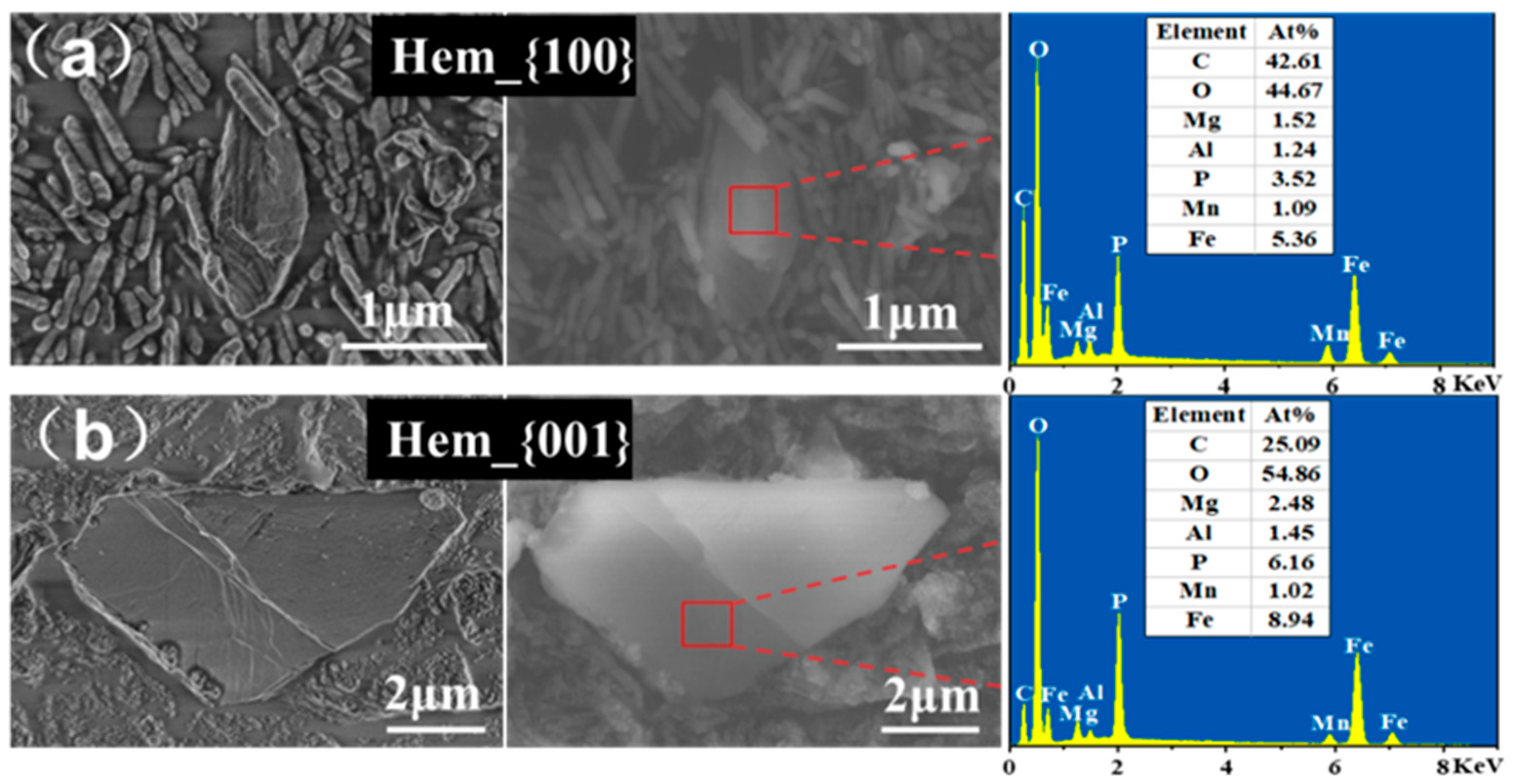
3.3. Facet-Dependent Formation of Vivianite during Dissimilatory Iron Reduction
3.4. Potential Mechanism of Facet-Dependent Vivianite Formation by G. sulfurreducens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Wang, S.; An, J.; Liang, D.; Zhao, Q.; Tian, L.; Wu, Y.; Wang, X.; Li, N. Graphite accelerate dissimilatory iron reduction and vivianite crystal enlargement. Water. Res. 2021, 189, 116663. [Google Scholar] [CrossRef]
- Frossard, E.; Condron, L.; Oberson, A.; Sinaj, S.; Fardeau, J. Processes governing phosphorus availability in temperate soils. J. Eeviron. Qual. 2000, 29, 15–23. [Google Scholar] [CrossRef]
- Wilfert, P.; Kumar, P.S.; Korving, L.; Witkamp, G.-J.; Van Loosdrecht, M.C. The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: A review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef] [PubMed]
- Kulik, D.A.; Kersten, M.; Heiser, U.; Neumann, T. Application of Gibbs Energy Minimization to ModelEarly-Diagenetic Solid-Solution Aqueous-Solution EquilibriaInvolving Authigenic Rhodochrosites in Anoxic BalticSea Sediments. Aquat. Geochem. 2000, 6, 147–199. [Google Scholar] [CrossRef]
- O’loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef]
- Tekin, D.; Yörük, S.; Kocadagistan, E. Kinetics of Bacterial Reduction of Hematite by Acidithiobacillus ferrooxidans-r4A1FC2B3. Asian. J. Chem. 2013, 25, 8875–8878. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Liu, Y.; Zhang, C.; Wan, J.; Hu, L.; Zhou, C.; Xiong, W. Efficient degradation of sulfamethazine in simulated and real wastewater at slightly basic pH values using Co-SAM-SCS/H2O2 Fenton-like system. Water. Res. 2018, 138, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Y.; An, J.; Liang, D.; Tian, L.; Zhou, L.; Wang, X.; Li, N. Geobacter autogenically secretes fulvic acid to facilitate the dissimilated iron reduction and vivianite recovery. Environ. Sci. Technol. 2020, 54, 10850–10858. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, Y.; Wang, R.; Mishra, S.B.; Duan, H.; Qu, H. Modification of sand with iron and copper derived from electroplating wastewater for efficient adsorption of phosphorus from aqueous solutions: A combinatorial approach for an effective waste minimization. J. Clean. Prod. 2018, 200, 471–477. [Google Scholar] [CrossRef]
- Lv, J.; Miao, Y.; Huang, Z.; Han, R.; Zhang, S. Facet-mediated adsorption and molecular fractionation of humic substances on hematite surfaces. Environ. Sci. Technol. 2018, 52, 11660–11669. [Google Scholar] [CrossRef]
- Li, R.-H.; Cui, J.-L.; Hu, J.-H.; Wang, W.-J.; Li, B.; Li, X.-D.; Li, X.-Y. Transformation of Fe–P complexes in bioreactors and P recovery from sludge: Investigation by XANES spectroscopy. Environ. Sci. Technol. 2020, 54, 4641–4650. [Google Scholar] [CrossRef]
- Notini, L.; Byrne, J.M.; Tomaszewski, E.J.; Latta, D.E.; Zhou, Z.; Scherer, M.M.; Kappler, A. Mineral defects enhance bioavailability of goethite toward microbial Fe (III) reduction. Environ. Sci. Technol. 2019, 53, 8883–8891. [Google Scholar] [CrossRef]
- Gilcreas, F. Standard methods for the examination of water and waste water. Am. M. J. Public. Health. 1966, 56, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, D.; Röske, I.; Hupfer, M.; Ohms, G. A simple method to distinguish between polyphosphate and other phosphate fractions of activated sludge. Water. Res. 1990, 24, 1355–1360. [Google Scholar] [CrossRef]
- Li, T.; Zhong, W.; Jing, C.; Li, X.; Zhang, T.; Jiang, C.; Chen, W. Enhanced hydrolysis of p-nitrophenyl phosphate by iron (hydr) oxide nanoparticles: Roles of exposed facets. Environ. Sci. Technol. 2020, 54, 8658–8667. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.; Shao, Z.; Tian, Y.; Chen, G.; Wang, K.; Chen, Z.; Dou, Y.; Zhang, W. In-situ preparation of Fe2O3 hierarchical arrays on stainless steel substrate for high efficient catalysis. J. Solid. State. Chem. 2017, 246, 278–283. [Google Scholar] [CrossRef]
- Hu, S.; Wu, Y.; Ding, Z. Facet-dependent reductive dissolution of hematite nanoparticles by Shewanella putrefaciens CN-32. Environ. Sci. Nano. 2020, 7, 2522–2531. [Google Scholar] [CrossRef]
- Hansel, C.M.; Benner, S.G.; Nico, P. Structural constraints of ferric (hydr)oxides on dissimilatory iron reduction and the fate of Fe(II). Geochim. Cosmochim. Acta. 2004, 68, 3217–3229. [Google Scholar] [CrossRef]
- Bonneville, S.; Behrends, T.; Cappellen, P.V. Solubility and dissimilatory reduction kinetics of iron (III) oxyhydroxides: A linear free energy relationship. Geochim. Cosmochim. Acta 2009, 73, 5273–5282. [Google Scholar] [CrossRef]
- Roden, E.E.; Zachara, J.M. Microbial reduction of crystalline iron (III) oxides: Influence of oxide surface area and potential for cell growth. Environ. Sci. Technol. 1996, 30, 1618–1628. [Google Scholar] [CrossRef]
- Bose, S.; Hochella, M.F.; Gorby, Y.A. Bioreduction of hematite nanoparticles by the dissimilatory iron reducing bacterium Shewanella oneidensis MR-1. Geochim. Cosmochim. Acta 2009, 73, 962–976. [Google Scholar] [CrossRef]
- Urrutia, M.M.; Roden, E.E.; Zachara, J.M. Influence of aqueous and solid-phase Fe (II) complexants on microbial reduction of crystalline iron (III) oxides. Environ. Sci. Technol. 1999, 33, 4022–4028. [Google Scholar] [CrossRef]
- Hu, S.; Wu, Y.; Shi, Z. Quinone-mediated dissimilatory iron reduction of hematite: Interfacial reactions on exposed {001} and {100} facets. J. Colloid Interface Sci. 2021, 583, 544–552. [Google Scholar] [CrossRef]
- Han, R.; Lv, J.; Zhang, S.; Zhang, S. Hematite facet-mediated microbial dissimilatory iron reduction and production of reactive oxygen species during aerobic oxidation. Water. Res. 2021, 195, 116988. [Google Scholar] [CrossRef] [PubMed]
- Cutting, R.; Coker, V.; Fellowes, J.; Lloyd, J.; Vaughan, D. Mineralogical and morphological constraints on the reduction of Fe (III) minerals by Geobacter sulfurreducens. Geochim. Cosmochim. Acta 2009, 73, 4004–4022. [Google Scholar] [CrossRef]
- Prot, T.; Pannekoek, W.; Belloni, C.; Dugulan, A.; Hendrikx, R.; Korving, L.; Van Loosdrecht, M. Efficient formation of vivianite without anaerobic digester: Study in excess activated sludge. J. Environ. Chem. Eng. 2022, 10, 107473. [Google Scholar] [CrossRef]
- Rustad, J.R.; Boily, J.-F. Density functional calculation of the infrared spectrum of surface hydroxyl groups on goethite (α-FeOOH). Am. Mineral. 2010, 95, 414–417. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Song, F. Facet-dependent Cr(VI) adsorption of hematite nanocrystals. Environ. Sci. Technol. 2016, 50, 1964. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Li, T. Facet-dependent adsorption and fractionation of natural organic matter on crystalline metal oxide nanoparticles. Environ. Sci. Technol. 2020, 54, 8622–8631. [Google Scholar] [CrossRef]
- Boily, J.-F.; Felmy, A.R. On the protonation of oxo-and hydroxo-groups of the goethite (α-FeOOH) surface: A FTIR spectroscopic investigation of surface O–H stretching vibrations. Geochim. Cosmochim. Acta 2008, 72, 3338–3357. [Google Scholar] [CrossRef]
- Ding, X.; Song, X.; Boily, J.-F.O. Identification of fluoride and phosphate binding sites at FeOOH surfaces. J. Phys. Chem. C 2012, 116, 21939–21947. [Google Scholar] [CrossRef]
- Shchukarev, A.; Boily, J.-F.; Felmy, A.R. XPS of fast-frozen hematite colloids in NaCl aqueous solutions: I. Evidence for the formation of multiple layers of hydrated sodium and chloride ions induced by the {001} basal plane. J. Phys. Chem. C 2007, 111, 18307–18316. [Google Scholar] [CrossRef]
- Usman, M.; Byrne, J.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S. Magnetite and green rust: Synthesis, properties, and environmental applications of mixed-valent iron minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, J.; Zhang, Q.; Aleem, M.; Fang, F.; Xue, Z.; Cao, J. Potentials and challenges of phosphorus recovery as vivianite from wastewater: A review. Chemosphere 2019, 226, 246–258. [Google Scholar] [CrossRef]
- Papassiopi, N.; Vaxevanidou, K.; Paspaliaris, I. Effectiveness of iron reducing bacteria for the removal of iron from bauxite ores. Miner. Eng. 2010, 23, 25–31. [Google Scholar] [CrossRef]
- Wen, L.; Huang, L.; Wang, Y.; Yuan, Y.; Zhou, L. Facet-engineered hematite boosts microbial electrogenesis by synergy of promoting electroactive biofilm formation and extracellular electron transfer. Sci. Total. Environ. 2022, 819, 153154. [Google Scholar] [CrossRef]
- Royer, R.A.; Burgos, W.D.; Fisher, A.S.; Jeon, B.-H.; Unz, R.F.; Dempsey, B.A. Enhancement of hematite bioreduction by natural organic matter. Environ. Sci. Technol. 2002, 36, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Royer, R.A.; Burgos, W.D.; Fisher, A.S.; Unz, R.F.; Dempsey, B.A. Enhancement of biological reduction of hematite by electron shuttling and Fe (II) complexation. Environ. Sci. Technol. 2002, 36, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, X.; Chen, J.; Liu, J.; Wu, H.; Zhan, H.; Liang, C.; Wu, M. Continuous shape and spectroscopy tuning of hematite nanocrystals. Inorg. Chem. 2010, 49, 8411–8420. [Google Scholar] [CrossRef]
- Li, Z.; Lai, X.; Wang, H.; Mao, D.; Xing, C.; Wang, D. Direct hydrothermal synthesis of single-crystalline hematite nanorods assisted by 1,2-propanediamine. Nanotechnology 2009, 20, 245603. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, S.; Wang, S.; Luo, L.; Cao, D.; Christie, P. Molecular-Scale Investigation with ESI-FT-ICR-MS on Fractionation of Dissolved Organic Matter Induced by Adsorption on Iron Oxyhydroxides. Environ. Sci. Technol. 2016, 50, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, S.; Numata, T.; Okayama, T. Shape effects of goethite particles on their photocatalytic activity in the decomposition of acetaldehyde. Catal Sci Technol. 2014, 4, 164–169. [Google Scholar] [CrossRef]
- Akın, S.Ş.; Magalhaes, D.; Kazanc, F. A study on the effects of various combustion parameters on the mineral composition of Tunçbilek fly ash. Fuel 2020, 275, 117881. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wen, L.; Zhou, L.; Yuan, Y. Facet Dependence of Biosynthesis of Vivianite from Iron Oxides by Geobacter sulfurreducens. Int. J. Environ. Res. Public Health 2023, 20, 4247. https://doi.org/10.3390/ijerph20054247
Luo X, Wen L, Zhou L, Yuan Y. Facet Dependence of Biosynthesis of Vivianite from Iron Oxides by Geobacter sulfurreducens. International Journal of Environmental Research and Public Health. 2023; 20(5):4247. https://doi.org/10.3390/ijerph20054247
Chicago/Turabian StyleLuo, Xiaoshan, Liumei Wen, Lihua Zhou, and Yong Yuan. 2023. "Facet Dependence of Biosynthesis of Vivianite from Iron Oxides by Geobacter sulfurreducens" International Journal of Environmental Research and Public Health 20, no. 5: 4247. https://doi.org/10.3390/ijerph20054247
APA StyleLuo, X., Wen, L., Zhou, L., & Yuan, Y. (2023). Facet Dependence of Biosynthesis of Vivianite from Iron Oxides by Geobacter sulfurreducens. International Journal of Environmental Research and Public Health, 20(5), 4247. https://doi.org/10.3390/ijerph20054247




