Resistance to Pyrethroids in the Malaria Vector Anopheles albimanus in Two Important Villages in the Soconusco Region of Chiapas, Mexico, 2022
Abstract
:1. Introduction
2. Materials and Methods
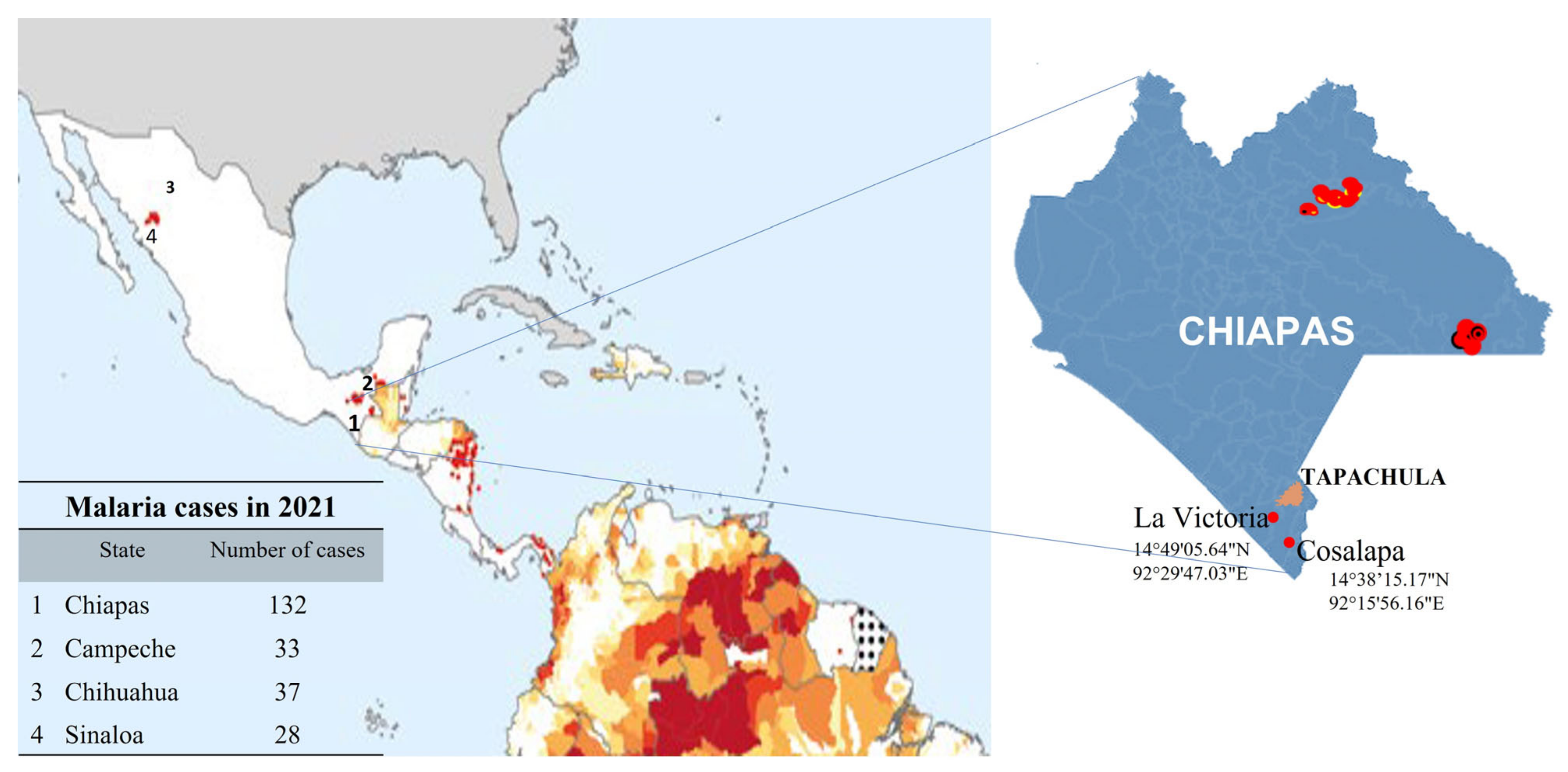
2.1. Collection of Mosquitoes from the Soconusco Region
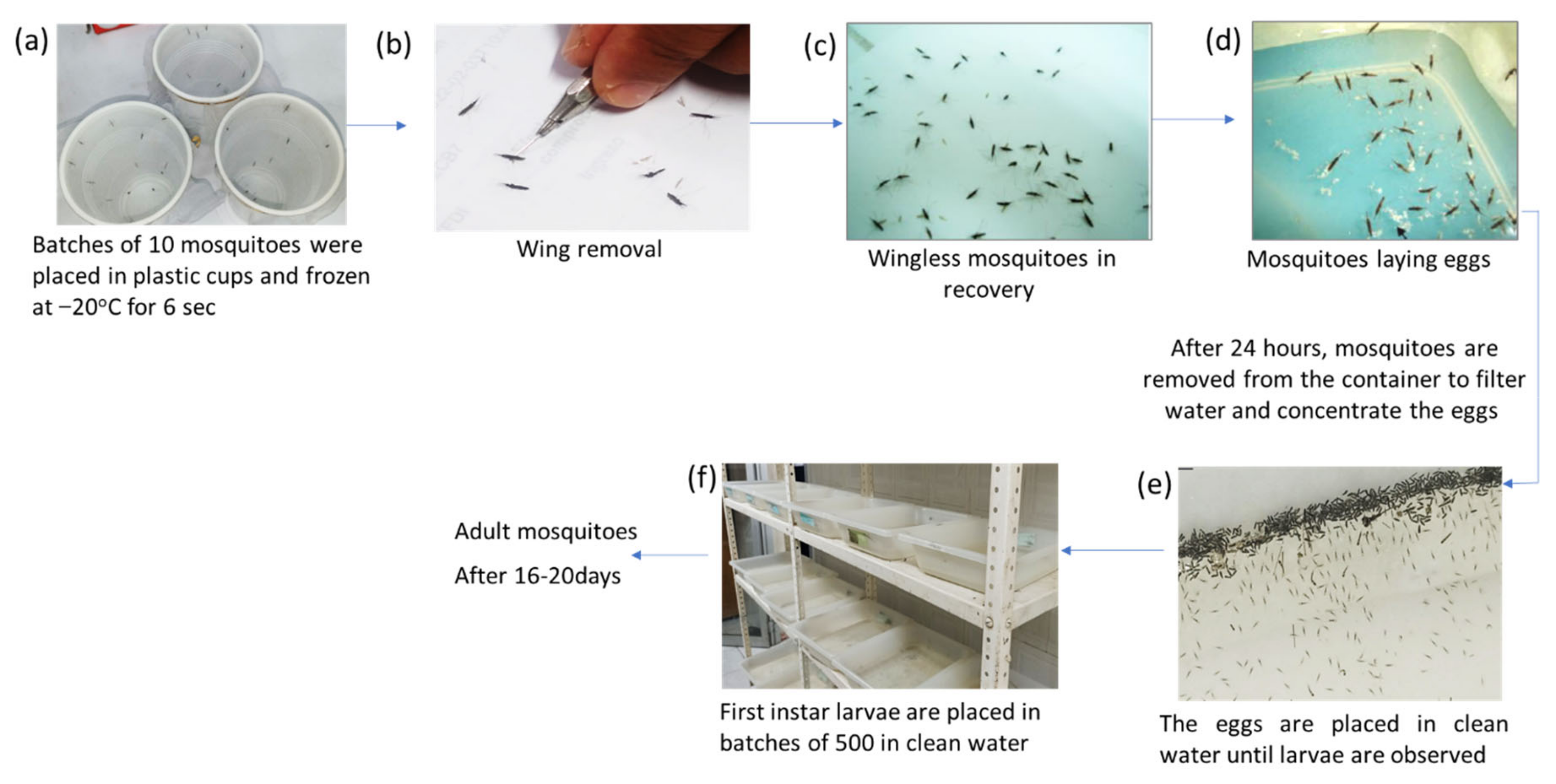
2.2. Forced Oviposition of Blood-Fed Mosquitoes
2.3. Insecticide Susceptibility Bioassays
2.3.1. WHO Tubes Tests
2.3.2. CDC Bottle Tests
Determination of the Diagnostic Concentration of the Insecticides
Mortality Percentages
2.4. Biochemical Assays
2.4.1. α- and β-Esterase Assays
2.4.2. ρNPA-Esterases Assay
2.4.3. Glutathione-S-Transferases Assay
2.4.4. Cytochrome P450 Assay
2.4.5. Protein Assay
3. Results
3.1. Insecticide Susceptibility Assays
3.1.1. WHO Tube Tests
3.1.2. CDC Bottle Tests
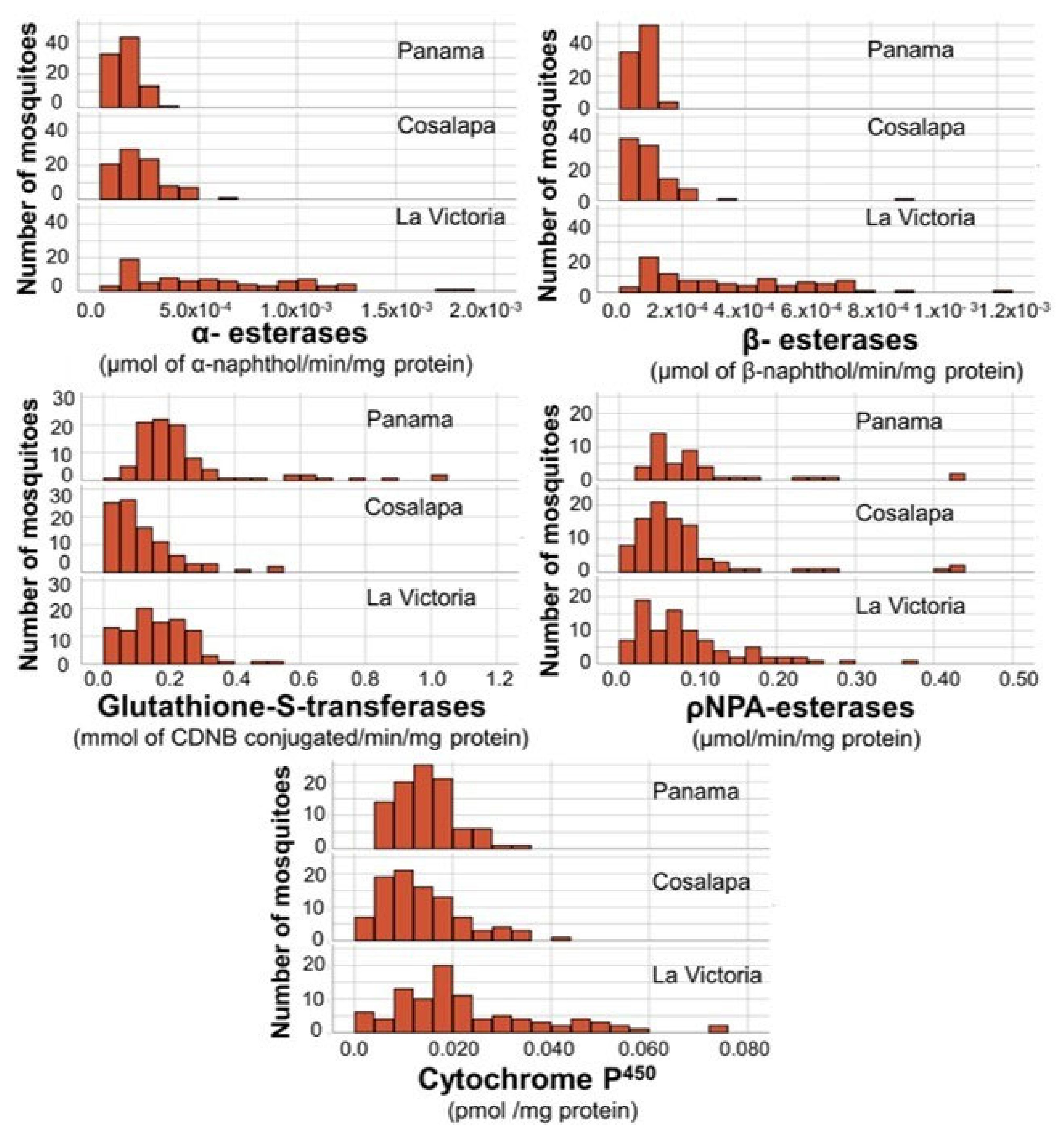
3.2. Biochemical Assays
3.2.1. α-Esterases and β-Esterases
3.2.2. Cytochromes P450
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Newsroom. Fact Sheets. Malaria. 8 December 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 4 February 2023).
- Secretaría de Salud. Dirección General de Epidemiología Boletines Epidemiológicos Históricos. Available online: https://www.gob.mx/salud/acciones-y-programas/historico-boletin-epidemiologico (accessed on 18 November 2022).
- Boletín Epidemiológico. Secretaría de Salud. Sistema Nacional de Vigilancia Epidemiológica. Sistema Único de Información. Dirección General de Epidemiología. 2021; Número 52, Volume 38. 2021. Available online: https://www.google.com/url?sa=t&source=web&rct=j&url=https://www.gob.mx/cms/uploads/attachment/file/693135/sem52.pdf&ved=2ahUKEwj5tMikgrD9AhV-mmoFHY36AugQFnoECBMQAQ&usg=AOvVaw0pshNKIX5_N11IzdoXcKwD (accessed on 18 November 2022).
- Secretaria de Salud. Prensa. 486. México Refuerza Acciones para la Eliminación del Paludismo en 2025. Available online: https://www.gob.mx/salud/prensa/486-mexico-refuerza-acciones-para-la-eliminacion-del-paludismo-en-2025?idiom=es (accessed on 5 February 2023).
- OECDiLibrary. Organisation for Economic Cooperation and Development. International Migration Outlook 2020. Mexico. Available online: https://www.oecd-ilibrary.org/sites/eb37dd8f-en/index.html?itemId=/content/component/eb37dd8f-en (accessed on 4 February 2023).
- Betanzos-Reyes, A.F.; González-Cerón, L.; Rodríguez, M.H.; Torres-Monzón, J.A. Seroepidemiología del paludismo en un grupo de migrantes en tránsito (Chiapas, 2008). Salud Publ. Mex. 2012, 54, 523–529. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0036-36342012000500009&lng=es (accessed on 4 February 2023). [CrossRef] [Green Version]
- Banco Interamericano de Desarrollo. Comunicados de Prensa. Noticias. 27 Julio 2020. Available online: https://www.iadb.org/es/noticias/guatemala-avanza-en-la-eliminacion-de-la-malaria-para-2025-con-apoyo-del-bid# (accessed on 4 February 2023).
- WHO. World Malaria Report; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240064898 (accessed on 4 February 2023).
- Solis-Santoyo, F.; Rodriguez, A.D.; Penilla-Navarro, R.P.; Sanchez, D.; Castillo-Vera, A.; Lopez-Solis, A.D.; Vazquez-Lopez, E.D.; Lozano-Fuentes, S.; Black IV, W.C.; Saavedra-Rodriguez, K. Insecticide resistance in Aedes aegypti from Tapachula, Mexico: Spatial variation and response to historical insecticide use. PLoS Negl. Trop. 2021, 15, e0009746. [Google Scholar] [CrossRef]
- Villarreal-Treviño, C.; Ríos-Delgado, J.; Penilla-Navarro, R.P.; Rodríguez, A.D.; López, J.H.; Nettel-Cruz, J.A.; Moo-Llanes, D.A.; Fuentes-Maldonado, G. Composition and abundance of anopheline species according to habitat diversity in México. Salud Publ. Mex. 2020, 62, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Treviño, C. Flujo de Genes de la Metapoblación de Anopheles albimanus (Diptera:Culicidae) a Pequeña Escala en el Ámbito Microgeográfico de Chiapas y a Gran Escala en al Ámbito Macrogeográfico de México Tesis Doctorado. 2001. Available online: http://eprints.uanl.mx/id/eprint/5121 (accessed on 4 February 2023).
- López-Solís Alma, D.; Solís-Santoyo, F.; Penilla-Navarro, R. Patricia y Rodríguez-Ramírez Américo, D. Resistencia a Insecticidas en los Mosquitos Vectores de Paludismo en Chiapas, Oaxaca y Tabasco, México. Entomol. Mex. 2005, 4, 768–772. [Google Scholar]
- Penilla-Navarro, P.; Domínguez-Constantino, I.A.; López-Solís, A.D.; Solís-Santoyo, F.; Rodríguez-Ramírez, A.D. Diagnóstico de los mecanismos de resistencia a insecticidas en los mosquitos vectores de paludismo en Chiapas, Oaxaca y Tabasco, México. Entomol. Mex. 2005, 4, 763–767. [Google Scholar]
- Penilla, R.P.; Rodriguez, A.D.; Hemingway, J.; Torres, J.L.; Arredondo-Jimenez, J.I.; Rodriguez, M.H. Resistance management strategies in malaria vector mosquito control. Baseline data for a large-scale field trial against Anopheles albimanus in Mexico. Med. Vet. Entomol. 1998, 12, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, R.C.; Strickman, D.; Litwak, T.R. Illustrated key to the female anopheline mosquitoes of Central America and Mexico. J. Am. Mosq. Control Assoc. 1990, 6, 7–34. [Google Scholar]
- Dzul, F.A.; Penilla, R.P.; Rodríguez, A.D. Susceptibilidad y Mecanismos de Resistencia a Insecticidas en Anopheles albimanus del sur de la Península de Yucatán, México. Salud Publ. Mex. 2007, 49, 302–311. [Google Scholar] [CrossRef] [Green Version]
- WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; WHO: Geneva, Switzerland, 2016; Available online: https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf (accessed on 4 February 2023).
- CDC. Centers for Disease Control and Prevention. Guideline for Evaluation of Insecticide Resistance in Vectors Using the CDC Bottle Bioassay. 1st Edition. Georgia. 2010. Available online: https://www.cdc.gov/malaria/resources/pdf/fsp/ir_manual/ir_cdc_bioassay_en.pdf (accessed on 4 February 2023).
- Lozano-Fuentes, S.; Saavedra-Rodriguez, K.; Black, I.V.; William, C.; Eisen, L. QCal: A software application for the calculation of dose-response curves in insecticide resistance bioassays. J. Am. Mosq. Control Assoc. 2012, 28, 59–61. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. Available online: https://www.sciencedirect.com/science/article/abs/pii/0003269776905273 (accessed on 4 February 2023). [CrossRef]
- Brogdon, W.G.; McAllister, J.C.; M Corwin, A.M.; Cordon-Rosales, C. Independent selection of multiple mechanisms for pyrethroid resistance in Guatemalan Anopheles albimanus (Diptera: Culicidae). J. Econ. Entomol. 1999, 92, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, L.; Rovira, J.; García, A.; Torres, R. Determinación de la resistencia a insecticidas organofosforados, carbamatos y piretroides en tres poblaciones de Anopheles albimanus (Diptera: Culicidae) de Panamá. Biomédica 2011, 31, 419–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, F.; Córdova, O.; Alvarado, A. Determinación de la resistencia a insecticidas en Aedes aegypti, Anopheles albimanus y Lutzomyia peruensis procedentes del Norte Peruano. Rev. Peru. Med. Exp. Salud Publica 2006, 23, 259–264. [Google Scholar]
- Lol, J.C.; Castellanos, M.E.; Liebman, K.A.; Lenhart, A.; Pennington, P.M.; Padilla, N.R. Molecular evidence for historical presence of knock-down resistance in Anopheles albimanus, a key malaria vector in Latin America. Parasites Vectors 2013, 6, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemingway, J.; Penilla, R.P.; Rodriguez, A.D.; James, B.M.; Edge, W.; Rogers, H.; Rodriguez, M.H. Resistance management strategies in malaria vector mosquito control. A large-scale field trial in southern Mexico. J. Pestic. Sci. 1997, 51, 375–382. [Google Scholar] [CrossRef]
- Penilla, R.P.; Rodríguez, A.D.; Hemingway, J.; Trejo, A.; López, A.D.; Rodríguez, M.H. Cytochrome P450-based resistance mechanism and pyrethroid resistance in the field Anopheles albimanus resistance management trial. Pest. Biochem. Physiol. 2007, 89, 111–117. Available online: http://www.sciencedirect.com/science/article/B6WP8. (accessed on 4 February 2023). [CrossRef]
- López-Solis, A.D.; Janich, A.; Solis-Santoyo, F.; Ordóñez-González, J.G.; Fuentes-Maldonado, G.; Saavedra-Rodríguez, K.; Villarreal-Treviño, C.; Black IV, W.C.; Rodriguez, A.D.; Penilla-Navarro, R.P. Evaluation of Commercial Aerosol Insecticides for Control of Aedes aegypti Susceptible or Resistant to Pyrethroids. Salud Publ. Mex. 2023. Available online: https://saludpublica.mx/index.php/spm/issue/view/517 (accessed on 20 February 2023).
- Domínguez-García, D.I.; Rosario-Cruz, R.; Almazán-García, C.; Saltijeral-Oaxaca, J.A.; De la Fuente, J. Boophilus microplus: Aspectos biológicos y moleculares de la resistencia a los acaricidas y su impacto en la salud animal. Trop. Subtrop. Agroecosystems 2010, 12, 181–192. [Google Scholar]
- Diabate, A.; Baldet, T.; Chandre, F.; Akoobeto, M.; Guiguemde, T.R.; Darriet, F.; Brengues, C.; Guillet, P.; Hemingway, J.; Small, G.J.; et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am. J. Trop. Med. Hyg. 2002, 67, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DGSV-Dirección General de Sanidad Vegetal. Ficha Técnica, Chaetanaphothrips Signipennis (Bagnall, 1914) (Thysanoptera: Thripidae) Mancha Roja del Banano; Centro Nacional de Referencia Fitosanitaria: Tecámac, México, 2019. [Google Scholar]
- Ferreira, M.U.; de Oliveira, T.C. Challenges for Plasmodium vivax malaria elimination in the genomics era. Pathog. Glob. Health 2015, 109, 89–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzáles Cerón, L.; Penilla-Navarro, P. Paludismo: Atención, control y participación Comunitaria. In Síntesis Sobre Políticas de Salud. Propuestas Basadas en Evidencias; Rivera-Donmarco, J., Barrientos-Gutiérrez, T., Oropeza, C., Eds.; Salud Pública de México, Instituto Nacional de Salud Pública: Cuernavaca, México, 2021; pp. 30–49. ISBN 978-607-511-216-9. [Google Scholar]
- Conrado-Cruz, J. El Estado Tiene la Cifra más Baja en Casos de Paludismo. Cuarto Poder. 2022. Available online: https://www.cuartopoder.mx/chiapas/el-estado-tiene-la-cifra-mas-baja-en-casos-de-paludismo/428033/ (accessed on 17 November 2022).


| Insecticide | Concentrations in μg/mL/Bottle | Number of Mosquitoes | Lethal Concentration 99 (LC99) | Diagnostic Concentration (2LC99) |
|---|---|---|---|---|
| Deltamethrin | 0.05, 0.075, 0.1, 0.15, 0.2 | 308 | 0.35 | 0.7 |
| Permethrin | 0.05, 0.1, 0.2, 0.4, 0.8, 1.2, 1.6 | 408 | 5.96 | 12 |
| Malathion | 0.5, 0.75, 1, 2, 3 | 253 | 7.2 | 14.4 |
| Chlorpyrifos | 0.05, 0.1, 0.2, 0.4, 0.8 | 360 | 0.99 | 2 |
| WHO Susceptibility Test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyrethroids | Organophosphate | Carbamate | ||||||||||
| Deltamethrin | Permethrin | Malathion | Bendiocarb | |||||||||
| 0.05% | 0.75% | 5% | 0.10% | |||||||||
| Villages | n | dead | Mortality % | n | dead | Mortality % | n | dead | Mortality % | n | dead | Mortality % |
| Reference * | 201 | 201 | 100 | 199 | 199 | 100 | 210 | 210 | 100 | 201 | 201 | 100 |
| Cosalapa | 185 | 139 | 75 | 150 | 134 | 89 | 201 | 201 | 100 | 185 | 185 | 100 |
| La Victoria | 190 | 133 | 70 | 180 | 153 | 85 | 188 | 188 | 100 | 195 | 195 | 100 |
| CDC susceptibility test | ||||||||||||
| Pyrethroids | Organophosphates | |||||||||||
| Deltamethrin | Permethrin | Malathion | Chlorpyrifos | |||||||||
| 0.7 µg/Bottle | 12 µg/Bottle | 14.4 µg/Bottle | 2 µg/Bottle | |||||||||
| Villages | n | dead | Mortality % | n | dead | Mortality % | n | dead | Mortality % | n | dead | Mortality % |
| Reference * | 201 | 201 | 100 | 205 | 205 | 100 | 206 | 206 | 100 | 189 | 189 | 100 |
| Cosalapa | 101 | 82 | 81 | 115 | 90 | 78 | 112 | 112 | 100 | 100 | 100 | 100 |
| La Victoria | 122 | 104 | 85 | 103 | 91 | 88 | 101 | 101 | 100 | 101 | 101 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solis-Santoyo, F.; Villarreal-Treviño, C.; López-Solis, A.D.; González-Cerón, L.; Rodríguez-Ramos, J.C.; Vera-Maloof, F.Z.; Danis-Lozano, R.; Penilla-Navarro, R.P. Resistance to Pyrethroids in the Malaria Vector Anopheles albimanus in Two Important Villages in the Soconusco Region of Chiapas, Mexico, 2022. Int. J. Environ. Res. Public Health 2023, 20, 4258. https://doi.org/10.3390/ijerph20054258
Solis-Santoyo F, Villarreal-Treviño C, López-Solis AD, González-Cerón L, Rodríguez-Ramos JC, Vera-Maloof FZ, Danis-Lozano R, Penilla-Navarro RP. Resistance to Pyrethroids in the Malaria Vector Anopheles albimanus in Two Important Villages in the Soconusco Region of Chiapas, Mexico, 2022. International Journal of Environmental Research and Public Health. 2023; 20(5):4258. https://doi.org/10.3390/ijerph20054258
Chicago/Turabian StyleSolis-Santoyo, Francisco, Cuauhtémoc Villarreal-Treviño, Alma D. López-Solis, Lilia González-Cerón, José Cruz Rodríguez-Ramos, Farah Z. Vera-Maloof, Rogelio Danis-Lozano, and Rosa Patricia Penilla-Navarro. 2023. "Resistance to Pyrethroids in the Malaria Vector Anopheles albimanus in Two Important Villages in the Soconusco Region of Chiapas, Mexico, 2022" International Journal of Environmental Research and Public Health 20, no. 5: 4258. https://doi.org/10.3390/ijerph20054258
APA StyleSolis-Santoyo, F., Villarreal-Treviño, C., López-Solis, A. D., González-Cerón, L., Rodríguez-Ramos, J. C., Vera-Maloof, F. Z., Danis-Lozano, R., & Penilla-Navarro, R. P. (2023). Resistance to Pyrethroids in the Malaria Vector Anopheles albimanus in Two Important Villages in the Soconusco Region of Chiapas, Mexico, 2022. International Journal of Environmental Research and Public Health, 20(5), 4258. https://doi.org/10.3390/ijerph20054258








