The Management of Obstructive Sleep Apnea Patients during the COVID-19 Pandemic as a Public Health Problem—Interactions with Sleep Efficacy and Mental Health
Abstract
1. Introduction
- (1)
- To evaluate how patients managed their sleep apnea during the COVID-19 pandemic, considering their symptomatology and comorbidities and the higher risk of severe disease with a fatal outcome;
- (2)
- To establish if CPAP adherence has increased/decreased after the onset of the pandemic;
- (3)
- To compare the stress level in OSA patients with their pre-pandemic levels and to observe if its modification is related to their individual characteristics (age, gender, and BMI) or to comorbidities and apnea severity.
2. Materials and Methods
2.1. Study Design and Setting
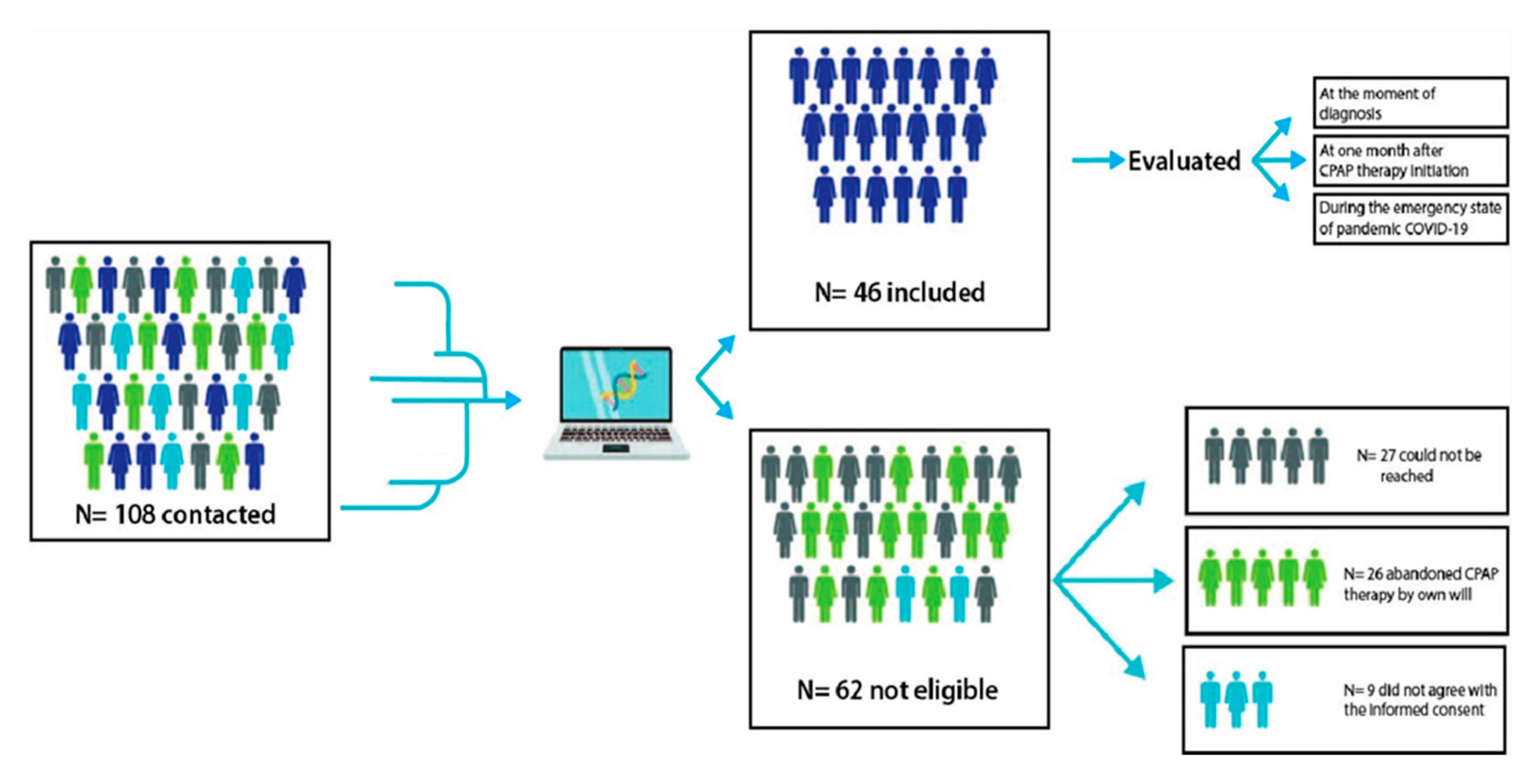
2.2. Participants
2.3. Variables
2.4. Data Sources
2.5. Statistical Analysis
3. Results
3.1. General Characteristics at Baseline of the Included Patients
3.2. The Sleep Parameters at the Baseline
3.3. CPAP Compliance Parameters at One Month after Diagnosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Health Organization. Novel Coronavirus (2019-nCoV): Situation Report, 19. Available online: https://apps.who.int/iris/handle/10665/330988 (accessed on 29 November 2020).
- Rauf, A.; Abu-Izneid, T.; Olatunde, A.; Ahmed Khalil, A.; Alhumaydhi, F.A.; Tufail, T.; Shariati, M.A.; Rebezov, M.; Almarhoon, Z.M.; Mabkhot, Y.N.; et al. COVID-19 Pandemic: Epidemiology, Etiology, Conventional and Non-Conventional Therapies. Int. J. Environ. Res. Public Health 2020, 17, 8155. [Google Scholar] [CrossRef]
- Dhar, C.S.; Oommen, A.M. Epidemiology of COVID-19. J. Dig. Endosc. 2020, 11, 3–7. [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Prettner, K.; Kuhn, M.; Bloom, D.E. The economic burden of COVID-19 in the United States: Estimates and projections under an infection-based herd immunity approach. J. Econ. Ageing 2021, 20, 100328. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Dipalma, G.; Vimercati, L.; Catucci, O. Immunity Profiling of COVID-19 Infection, Dynamic Variations of Lymphocyte Subsets, a Comparative Analysis on Four Different Groups. Microorganisms 2021, 9, 2036. [Google Scholar] [CrossRef]
- Patano, A.; Cirulli, N.; Beretta, M.; Plantamura, P.; Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Marinelli, G.; Scarano, A.; et al. Education Technology in Orthodontics and Paediatric Dentistry during the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6056. [Google Scholar] [CrossRef]
- Spagnolo, L.; Vimercati, L.; Caputi, A.; Benevento, M.; De Maria, L.; Ferorelli, D.; Solarino, B. Role and Tasks of the Occupational Physician during the COVID-19 Pandemic. Medicina 2021, 57, 479. [Google Scholar]
- Centers of Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html (accessed on 30 November 2020).
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between Long COVID and Overweight. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Park, J.G.; Ramar, K.; Olson, E.J. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin. Proc. 2011, 86, 549–555. [Google Scholar] [CrossRef]
- Centers for Medicare & Medicaid Services. Available online: https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/CPAP (accessed on 10 August 2021).
- Eisele, H.J.; Markart, P.; Schulz, R. Obstructive Sleep Apnea, Oxidative Stress, and Cardiovascular Disease: Evidence from Human Studies. Oxid. Med. Cell Longev. 2015, 2015, 608438. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef]
- Jean-Louis, G.; Zizi, F.; Brown, D.; Ogedegbe, G.; Borer, J.; McFarlane, S. Obstructive sleep apnea and cardiovascular disease: Evidence and underlying mechanisms. Minerva Pneumol. 2009, 48, 277–293. [Google Scholar]
- Devouassoux, G.; Lévy, P.; Rossini, E.; Pin, I.; Fior-Gozlan, M.; Henry, M.; Seigneurin, D.; Pépin, J.L. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J. Allergy Clin. Immunol. 2007, 119, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Strohl, K.P.; Brown, D.B.; Collop, N.; George, C.; Grunstein, R.; Han, F.; Kline, L.; Malhotra, A.; Pack, A.; Phillips, B.; et al. An official American Thoracic Society Clinical Practice Guideline: Sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am. J. Respir. Crit. Care Med. 2013, 187, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Nobili, L.; Beelke, M.; De Carli, F.; Ferrillo, F. The contributing role of sleepiness in highway vehicle accidents. Sleep 2001, 15, 203–206. [Google Scholar]
- McNicholas, W.T.; Rodenstein, D. Sleep apnoea and driving risk: The need for regulation. Eur. Respir. Rev. 2015, 24, 602–606. [Google Scholar] [CrossRef]
- Tregear, S.; Reston, J.; Schoelles, K.; Phillips, B. Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis. J. Clin. Sleep Med. 2009, 5, 573–581. [Google Scholar] [CrossRef]
- Di Milia, L.; Smolensky, M.H.; Costa, G.; Howarth, H.D.; Ohayon, M.M.; Philip, P. Demographic factors, fatigue, and driving accidents: An examination of the published literature. Accid. Anal. Prev. 2011, 43, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Stepanski, E.; Lamphere, J.; Badia, P.; Zorick, F.; Roth, T. Sleep fragmentation and daytime sleepiness. Sleep 1984, 7, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Pitidis, A.; Giustini, M.; Taggi, F.; Sanna, A. Motor vehicle accidents and obstructive sleep apnea syndrome: A methodology to calculate the related burden of injuries. Chron Respir. Dis. 2015, 12, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Sassani, A.; Findley, L.J.; Kryger, M.; Goldlust, E.; George, C.; Davidson, T.M. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep 2004, 27, 453–458. [Google Scholar] [CrossRef]
- Tregear, S.; Reston, J.; Schoelles, K.; Phillips, B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: Systematic review and metaanalysis. Sleep 2010, 33, 1373–1380. [Google Scholar] [CrossRef]
- European Union. Commission Directive 2014/85/EU of 1 July 2014, Amending Directive 2006/126/EC of the European Parliament and of the Council on Driving Licences; OJ L 194, 2.7.2014; European Union: Brussels, Belgium, 2014; pp. 10–13. [Google Scholar]
- Bonsignore, M.R.; Randerath, W.; Schiza, S.; Verbraecken, J.; Elliott, M.W.; Riha, R.; Barbe, F.; Bouloukaki, I.; Castrogiovanni, A.; Deleanu, O.; et al. European Respiratory Society Statement on Sleep Apnoea, Sleepiness and Driving Risk. Eur. Respir. J. 2020, 57, 2001272. [Google Scholar] [CrossRef]
- Romania Ministery Of Heath, Romania, Bucharest. ORDER for the approval of the Minimum Standards for Physical and Mental Fitness required for driving a car, no. 1162 from 31 August 2010. Available online: https://legislatie.just.ro/Public/DetaliiDocument/122023 (accessed on 11 October 2022).
- Santabarbara, J.; Lasheras, I.; Lipnicki, D.M.; Bueno-Notivol, J.; Perez-Moreno, M.; López-Antón, R.; De la Cámara, C.; Lobo, A.; Gracia-García, P. Prevalence of anxiety in the COVID-19 pandemic: An updated meta-analysis of community-based studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110207. [Google Scholar] [CrossRef]
- Marroquín, B.; Vine, V.; Morgan, R. Mental health during the COVID-19 pandemic: Effects of stay-at-home policies, social distancing behavior, and social resources. Psychiatry Res. 2020, 293, 113419. [Google Scholar] [CrossRef]
- Lee, S.A.; Han, S.H.; Ryu, H.U. Anxiety and its relationship to quality of life independent of depression in patients with obstructive sleep apnea. J. Psychosom Res. 2015, 79, 32–36. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Bhat, S.; Chokroverty, S. Sleep disorders and COVID-19. Sleep Med. 2021, 91, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Glowacz, F.; Schmits, E. Psychological distress during the COVID-19 lockdown: The young adults most at risk. Psychiatry Res. 2020, 293, 113486. [Google Scholar] [CrossRef]
- Garbarino, S.; Bardwell, W.A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A S.Systematic Review and Meta-Analysis. Behav. Sleep Med. 2020, 18, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Yu, B.Y.; Cheung, D.S.T.; Cheung, T.; Lam, S.C.; Chung, K.F.; Ho, F.Y.; Yeung, W.F. Sleep and Mood Disturbances during the COVID-19 Outbreak in an Urban Chinese Population in Hong Kong: A Longitudinal Study of the Second and Third Waves of the Outbreak. Int. J. Environ. Res. Public Health 2021, 18, 8444. [Google Scholar] [CrossRef]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef]
- Vingilis, E.; Beirness, D.; Boase, P.; Byrne, P.; Johnson, J.; Jonah, B.; Mann, R.E.; Rapoport, M.J.; Seeley, J.; Wickens, C.M.; et al. Coronavirus disease 2019: What could be the effects on Road safety? Accid. Anal. Prev. 2020, 144, 105687. [Google Scholar] [CrossRef] [PubMed]
- Toronto Star. Available online: https://www.thestar.com/politics/provincial/2020/04/23/lcbo-reporting-its-sales-have-gone-up-during-the-covid-19-crisis.html (accessed on 26 December 2020).
- CTV News Kitchener. Available online: https://kitchener.ctvnews.ca/alcohol-sales-on-the-rise-during-covid-19-pandemic-1.4920002 (accessed on 23 November 2021).
- Liu, N.; Zhang, F.; Wei, C.; Jia, Y.; Shang, Z.; Sun, L.; Wu, L.; Sun, Z.; Zhou, Y.; Wang, Y.; et al. Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: Gender differences matter. Psychiatry Res. 2020, 287, 112921. [Google Scholar] [CrossRef] [PubMed]
- Wickens, C.M.; Smart, R.G.; Mann, R.E. The impact of depression on driver performance. Int. J. Ment. Health Add. 2014, 12, 524–537. [Google Scholar] [CrossRef]
- Levenstein, S.; Prantera, C.; Varvo, V.; Scribano, M.L.; Berto, E.; Luzi, C.; Andreoli, A. Development of the Perceived Stress Questionnaire: A new tool for psychosomatic research. J. Psychosom. Res. 1993, 37, 19–32. [Google Scholar] [CrossRef]
- Masa, J.F.; Jiménez, A.; Durán, J.; Capote, F.; Monasterio, C.; Mayos, M.; Terán, J.; Hernández, L.; Barbé, F.; Maimó, A.; et al. Alternative methods of titrating continuous positive airway pressure: A large multicenter study. Am. J. Respir. Crit. Care Med. 2004, 170, 218–224. [Google Scholar] [CrossRef]
- World Medical Association. Ethics Unit. Declaration of Helsinki 2007. Available online: www.wma.net/e/ethicsunit/helsinki.htm (accessed on 13 October 2022).
- Wolkove, N.; Baltzan, M.; Kamel, H.; Dabrusin, R.; Palayew, M. Long-Term Compliance with Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnea. Can. Respir. J. 2008, 15, 365–369. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.M.; Isacco, C.G.; et al. SARS-CoV-2 Disease Adjuvant Therapies and Supplements Breakthrough for the Infection Prevention. Microorganisms 2021, 9, 525. [Google Scholar] [CrossRef]
- Charitos, I.A.; Ballini, A.; Bottalico, L.; Cantore, S.; Passarelli, P.C.; Inchingolo, F.; D’Addona, A.; Santacroce, L. Special features of SARS-CoV-2 in daily practice. World J. Clin. Cases 2020, 8, 3920–3933. [Google Scholar] [CrossRef] [PubMed]
- Bordea, I.R.; Xhajanka, E.; Candrea, S.; Bran, S.; Onișor, F.; Inchingolo, A.D.; Malcangi, G.; Pham, V.H.; Inchingolo, A.M.; Scarano, A.; et al. Coronavirus (SARS-CoV-2) Pandemic: Future Challenges for Dental Practitioners. Microorganisms 2020, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Dalvi, S.; Sălăgean, T.; Pop, I.D.; Bordea, I.R.; Benedicenti, S. Understanding COVID-19 Pandemic: Molecular Mechanisms and Potential Therapeutic Strategies. An Evidence-Based Review. J. Inflamm. Res. 2021, 14, 13–56. [Google Scholar] [CrossRef] [PubMed]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar]
- Lin, C.M.; Davidson, T.M.; Ancoli-Israel, S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med. Rev. 2008, 12, 481–496. [Google Scholar] [CrossRef]
- Thurnheer, R.; Wraith, P.K.; Douglas, N.J. Influence of age and gender on upper airway resistance in NREM and REM sleep. J. Appl. Physiol. 2001, 90, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Budin, C.E.; Ciumarnean, L.; Maierean, A.; Rajnoveanu, R.; Bordea, R.I. Therapeutic alternatives with CPAP in obstructive sleep apnea. J. Mind Med. Sci. 2019, 6, 181–189. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef]
- Gold, M.S.; Sehayek, D.; Gabrielli, S.; Zhang, X.; Christine, M.C.; Moshe, B.S. COVID-19 and comorbidities: A systematic review and meta-analysis. Postgrad. Med. 2020, 132, 749–755. [Google Scholar] [CrossRef]
- Charitos, I.A.; Del Prete, R.; Inchingolo, F.; Mosca, A.; Carretta, D. What we have learned for the future about COVID-19 and healthcare management of it? Acta Biomed. 2021, 91, e2020126. [Google Scholar]
- Celik, M.; Sarıkaya, Y.; Acar, M.; Kalenderoğlu, A.; Doğan, S.; Kaskalan, E.; Karataş, M. Effect of Continuous Positive Airway Pressure Treatment on Depression, Anxiety and Perceived Stress Levels in Patients with Obstructive Sleep Apnea Syndrome. Turk. Psikiyatr. Derg Winter 2016, 27, 244–250. [Google Scholar]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Sadeghniiat-Haghighi, K.; Akbarpour, S.; Samadi, S.; Rahimi, B.; Alemohammad, Z.B. The effect of apnea management on novel coronavirus infection: A study on patients with obstructive sleep apnea. Sleep Health 2021, 7, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 10, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.; Libman, E.; Baltzan, M.; Fichten, C.; Bailes, S. Impact of the COVID-19 pandemic on obstructive sleep apnea: Recommendations for symptom management. J. Clin. Sleep Med. 2021, 17, 429–434. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions between obesity and obstructive sleep apnea: Implications for treatment. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef]
- Alexescu, T.G.; Bordea, I.R.; Cozma, A.; Rajnoveanu, R.; Buzoianu, A.D.; Nemes, R.M.; Tudorache, S.I.; Boca, B.M.; Todea, D.A. Metabolic Profile and the Risk of Early Atherosclerosis in Patients with Obesity and Overweight. Rev. Chim. 2019, 70, 3627–3633. [Google Scholar] [CrossRef]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar]
- Garvey, J.F.; Pengo, M.F.; Drakatos, P.; Kent, B.D. Epidemiological aspects of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 920–929. [Google Scholar]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.M.; Isacco, C.G.; et al. SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs. Microorganisms 2021, 9, 793. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. Available online: https://aasm.org/resources/pdf/sleep-apnea-economic-crisis.pdf (accessed on 13 November 2021).
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- The New York Times. Available online: https://www.nytimes.com/2020/03/01/nyregion/new-york-coronvirus-confirmed.html (accessed on 13 November 2021).
- Grubac, Z.; Sutulovic, N.; Ademovic, A.; Velimirovic, M.; Rasic-Markovic, A.; Macut, D.; Petronijevic, N.; Stanojlovic, O.; Hrncic, D. Short-term sleep fragmentation enhances anxiety-related behavior: The role of hormonal alterations. PLoS ONE 2019, 14, e0218920. [Google Scholar] [CrossRef]
- Grubač, Ž.; Šutulović, N.; Šuvakov, S.; Jerotić, D.; Puškaš, N.; Macut, D.; Rašić-Marković, A.; Simić, T.; Stanojlović, O.; Hrnčić, D. Anxiogenic Potential of Experimental Sleep Fragmentation Is Duration-Dependent and Mediated via Oxidative Stress State. Oxid. Med. Cell Longev. 2021, 2021, 2262913. [Google Scholar] [CrossRef] [PubMed]
- NYS COVID-19 Tracker. Available online: https://COVID19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Map (accessed on 13 November 2021).
- Thorpy, M.; Figuera-Losada, M.; Ahmed, I.; Monderer, R.; Petrisko, M.; Martin, C.; Akhtar, J.; Thorpy, J.; Haines, C. Management of sleep apnea in New York City during the COVID-19 pandemic. Sleep Med. 2020, 74, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- United States Census Bureau. Available online: https://www.census.gov/quickfacts/fact/table/bronxcountybronxboroughnewyork/ (accessed on 13 November 2021).
- Bordea, I.R.; Candrea, S.; Salagean, T.; Pop, I.D.; Lucaciu, O.; Ilea, A.; Manole, M.; Băbțan, A.M.; Sirbu, A.; Hanna, R. Impact of COVID-19 Pandemic on Healthcare Professionals and Oral Care Operational Services: A systematic review. Microorganism 2021, 2021, 453–463. [Google Scholar] [CrossRef]
- Vista Health Solutions. Available online: https://www.nyhealthinsurer.com/new-york-bronx-health-insurance/ (accessed on 13 November 2021).
- Sattar, N.; McInnes, I.B.; McMurray, J.J.V. Obesity a risk factor for severe COVID-19 infection: Multiple potential mechanisms. Circulation 2020, 120, 047659. [Google Scholar] [CrossRef]
- Maierean, A.; Ciumarnean, L.; Alexescu, T.G.; Domokos, B.; Rajnoveanu, R.; Arghir, O.; Todea, D.; Buzoianu, A.D.; Dogaru, G.; Bordea, R.I. Complementary therapeutic approaches in asthma. Balneo Res. J. 2019, 10, 204–212. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M. Lille Intensive Care COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020, 8, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Santilli, M.; Manciocchi, E.; D’Addazio, G.; Di Maria, E.; D’Attilio, M.; Femminella, B.; Sinjari, B. Prevalence of Obstructive Sleep Apnea Syndrome: A Single-Center Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 10277. [Google Scholar] [CrossRef] [PubMed]
- Todea, D.; Rosca, L.; Herescu, A. Obstructive Sleep Apnea Syndrome—A Matter of Public Health. Transylv. Rev. Adm. Sci. 2021, 8, 186–201. [Google Scholar]

| VARIABLES | |
|---|---|
| Category | Variables Included |
| Personal information | Sex |
| Age | |
| Environment | |
| Smoker status | |
| Anthropometric measures | Weight |
| Height | |
| BMI | |
| Neck circumference | |
| Abdominal circumference | |
| Associated diseases | Hypertension, chronic ischemic cardiopathy, myocardial infarction, dyslipidemia, cardiac failure |
| Diabetes mellitus | |
| Asthma, COPD | |
| Sleep parameters collected at the diagnosis | AHI, ODI, minimum SaO2, average SaO2 |
| Nocturnal and diurnal symptomatology | |
Epworth sleepiness scale
| Table S2, Supplementary Materials |
CPAP Parameters
| Average time of use, compliance above 4 h, residual AHI), PSQ score (at baseline and during the pandemic) |
PSQ Score
| Table S1, Supplementary Materials |
| Criteria | Response | Value (n = 46, % n) |
|---|---|---|
| Place of residence | rural | 23 (50%) |
| urban | 23 (50%) | |
| Sex | M | 37 (80.4%) |
| F | 9 (19.6%) | |
| Age (years) | min | 37 |
| max | 76 | |
| median | 56.69 ± 10.9 | |
| Weight at diagnosis (kilograms) | min | 73.00 |
| max | 168.00 | |
| median | 110 (95.75–125.5) | |
| Height (centimeters) | min | 158.00 |
| max | 188.00 | |
| median | 171.6 ± 6.7 | |
| BMI at diagnosis (kg/m2) | min | 28.00 |
| max | 58.50 | |
| median | 36.33 (33.42–41.21) | |
| Neck circumference (centimeters) | min | 38.00 |
| max | 56.00 | |
| median | 47.4 ± 4.3 | |
| Abdominal circumference (centimeters) | min | 93 |
| max | 158 | |
| median | 124.5 (115.75–141.00) | |
| Smoker status | non-smoker | 13 (28.3%) |
| smoker | 33 (71.7%) | |
| Perceived Stress Questionnaire | min | 30 |
| max | 82 | |
| median | 54 | |
| 25th–75th percentiles | 41.75–66.25 | |
| Associated diseases | hypertension | 36 (78.3%) |
| chronic ischemic cardiopathy | 0(0%) | |
| myocardial infarction | 2 (4.3%) | |
| dyslipidemia | 22 (47.8%) | |
| cardiac failure | 5 (10.9%) | |
| diabetes mellitus | 11(23.9%) | |
| asthma | 2 (4.3%) | |
| COPD | 8 (17.4%) |
| AHI (Events/Hour of Sleep) | |
|---|---|
| min | 23.4 |
| max | 132.0 |
| mean | 63.75 |
| standard deviation | 39.9–81.2 |
| DESATURATION INDEX (events/hour of sleep) | |
| min | 23.7 |
| max | 127.5 |
| mean | 59.6 |
| standard deviation | 38.8–79.1 |
| MINIMUM SAO2 (%) | |
| min | 35 |
| max | 87 |
| mean | 65 |
| standard deviation | 60–74.3 |
| AVERAGE SAO2 (%) | |
| min | 70 |
| max | 94 |
| mean | 87.5 |
| standard deviation | 81.8–90.3 |
| EPWORTH SCALE | |
| min | 3 |
| max | 23 |
| median | 16 |
| 25th–75th percentiles | 12–19.25 |
| SYMPTOMS | |
| Snoring | 44 (95.7%) |
| Witnessed apnea | 30 (65.2%) |
| Nightmare | 7 (15.2%) |
| Nocturia | 36 (78.3%) |
| Ravished bed | 34 (73.9%) |
| Chocking in sleep | 21 (45.7%) |
| Daytime sleepiness | 37 (80.4%) |
| Morning headaches | 21 (45.7%) |
| Morning fatigue | 31 (67.4%) |
| Influenced work capacity | 20 (43.5%) |
| One Month after Diagnosis | During the State of Emergency | ||
|---|---|---|---|
| AVERAGE TIME OF USE (minutes) | min | 164.00 | 210.00 |
| max | 481.00 | 495.00 | |
| mean | 354.5 | 399.5 | |
| 25th–75th percentiles | 288.7–389.2 | 352.5–448.0 | |
| COMPLIANCE ABOVE 4 HOURS (%) | min | 42.00 | 51.0 |
| max | 82.00 | 96.0 | |
| mean | 69.5 | 79.0 | |
| percentiles 25–75 | 54.7–76.0 | 71.7–84.0 | |
| RESIDUAL AHI (Events/hour of sleep) | min | 0.5 | 0.5 |
| max | 20.00 | 12.1 | |
| mean | 5.6 | 2.4 | |
| percentiles 25–75 | 3.35–9.3 | 1.6–7.0 |
| Parameter Correlation Coefficient-p | Compliance above 4 h One Month after Diagnosis (%) | Residual Events/ Hour of Sleep One Month after Diagnosis | Average Use/Night at One Month after Diagnosis (min) | Compliance above 4 h ES (%) | Events/ Hour of Sleep ES | Average Use/Night ES (min) |
|---|---|---|---|---|---|---|
| PSQ at the diagnosis | −0.490 | 0.233 | −0.172 | NA | NA | NA |
| 0.001 | 0.119 | 0.253 | NA | NA | NA | |
| Age | 0.153 | −0.216 | −0.104 | −0.212 | −0.087 | −0.267 |
| 0.310 ** | 0.149 | 0.493 ** | 0.158 | 0.564 *** | 0.073 | |
| AHI at the diagnosis | −0.396 | 0.318 ** | −0.095 | 0.292 | 0.036 | 0.476 ** |
| 0.006 | 0.031 | 0.531 *** | 0.049 | 0.814 *** | 0.001 | |
| Desaturation index at the diagnosis | −0.529 | 0.369 ** | −0.223 | 0.140 | 0.193 | 0.342 ** |
| 0.000 | 0.012 | 0.136 | 0.352 ** | 0.198 | 0.020 | |
| Minimum SaO2% at the diagnosis | 0.304 ** | −0.321 | −0.049 | 0.081 | −0.213 * | −0.265 |
| 0.040 ** | 0.030 | 0.747 *** | 0.593 *** | 0.155 | 0.075 | |
| Average SaO2% at the diagnosis | 0.264 | −0.152 * | 0.013 | −0.207 | 0.125 | −0.444 |
| 0.076 | 0.314 ** | 0.931 *** | 0.167 | 0.406 ** | 0.002 | |
| PSQ ES | NA | NA | NA | 0.361 ** | −0.067 | 0.436 ** |
| NA | NA | NA | 0.014 | 0.658 *** | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maierean, A.D.; Vulturar, D.M.; Chetan, I.M.; Crivii, C.-B.; Bala, C.; Vesa, S.C.; Todea, D.A. The Management of Obstructive Sleep Apnea Patients during the COVID-19 Pandemic as a Public Health Problem—Interactions with Sleep Efficacy and Mental Health. Int. J. Environ. Res. Public Health 2023, 20, 4313. https://doi.org/10.3390/ijerph20054313
Maierean AD, Vulturar DM, Chetan IM, Crivii C-B, Bala C, Vesa SC, Todea DA. The Management of Obstructive Sleep Apnea Patients during the COVID-19 Pandemic as a Public Health Problem—Interactions with Sleep Efficacy and Mental Health. International Journal of Environmental Research and Public Health. 2023; 20(5):4313. https://doi.org/10.3390/ijerph20054313
Chicago/Turabian StyleMaierean, Anca Diana, Damiana Maria Vulturar, Ioana Maria Chetan, Carmen-Bianca Crivii, Cornelia Bala, Stefan Cristian Vesa, and Doina Adina Todea. 2023. "The Management of Obstructive Sleep Apnea Patients during the COVID-19 Pandemic as a Public Health Problem—Interactions with Sleep Efficacy and Mental Health" International Journal of Environmental Research and Public Health 20, no. 5: 4313. https://doi.org/10.3390/ijerph20054313
APA StyleMaierean, A. D., Vulturar, D. M., Chetan, I. M., Crivii, C.-B., Bala, C., Vesa, S. C., & Todea, D. A. (2023). The Management of Obstructive Sleep Apnea Patients during the COVID-19 Pandemic as a Public Health Problem—Interactions with Sleep Efficacy and Mental Health. International Journal of Environmental Research and Public Health, 20(5), 4313. https://doi.org/10.3390/ijerph20054313









