Potential Exposure to Respiratory and Enteric Bacterial Pathogens among Wastewater Treatment Plant Workers, South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Site Description
2.3. Sample Collection
2.4. Microbial Cell Concentration
2.5. Total Genomic DNA Extraction and 16 S Sequencing
2.6. Data Analysis
3. Results
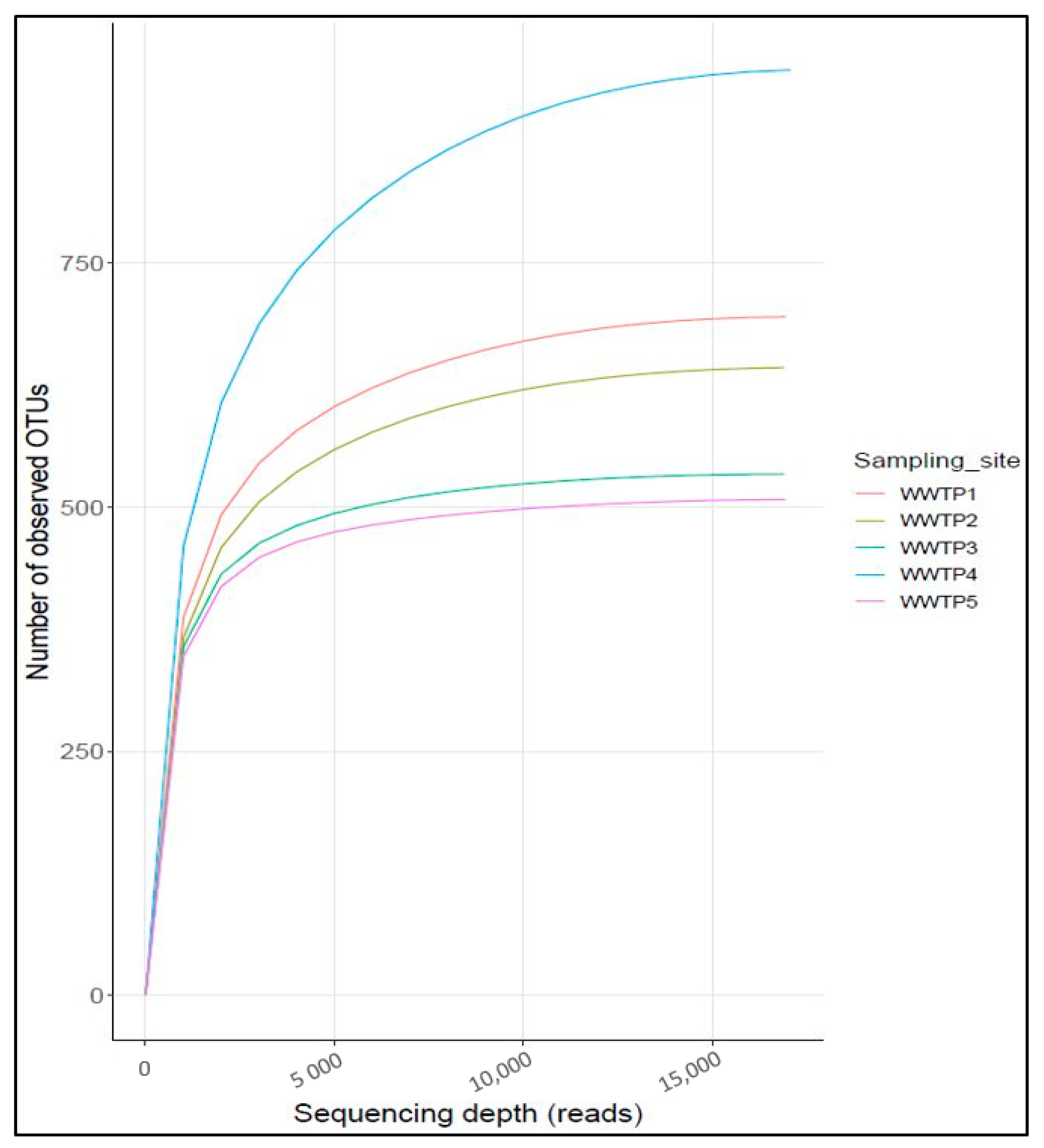
3.1. Alpha Diversity
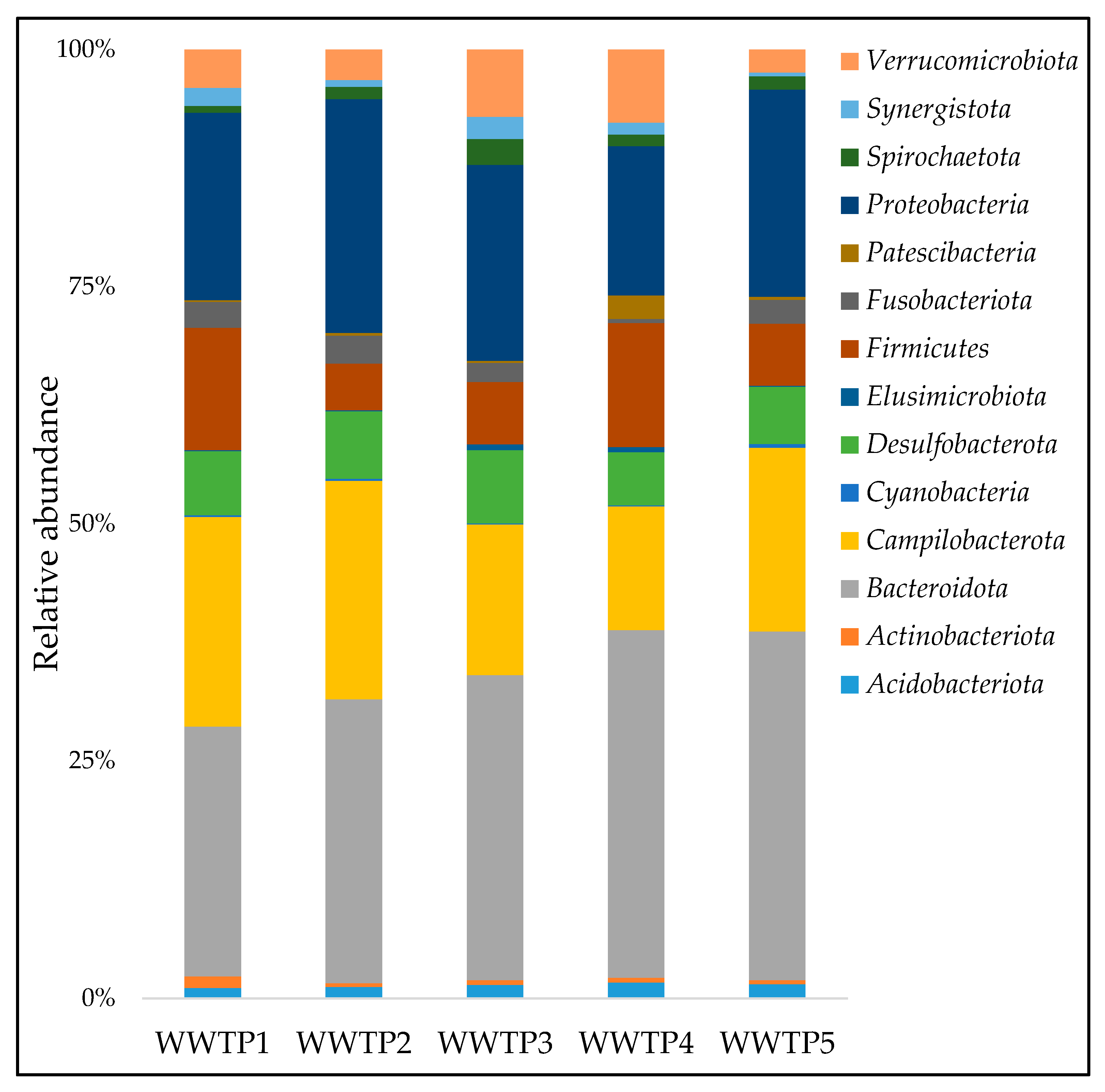
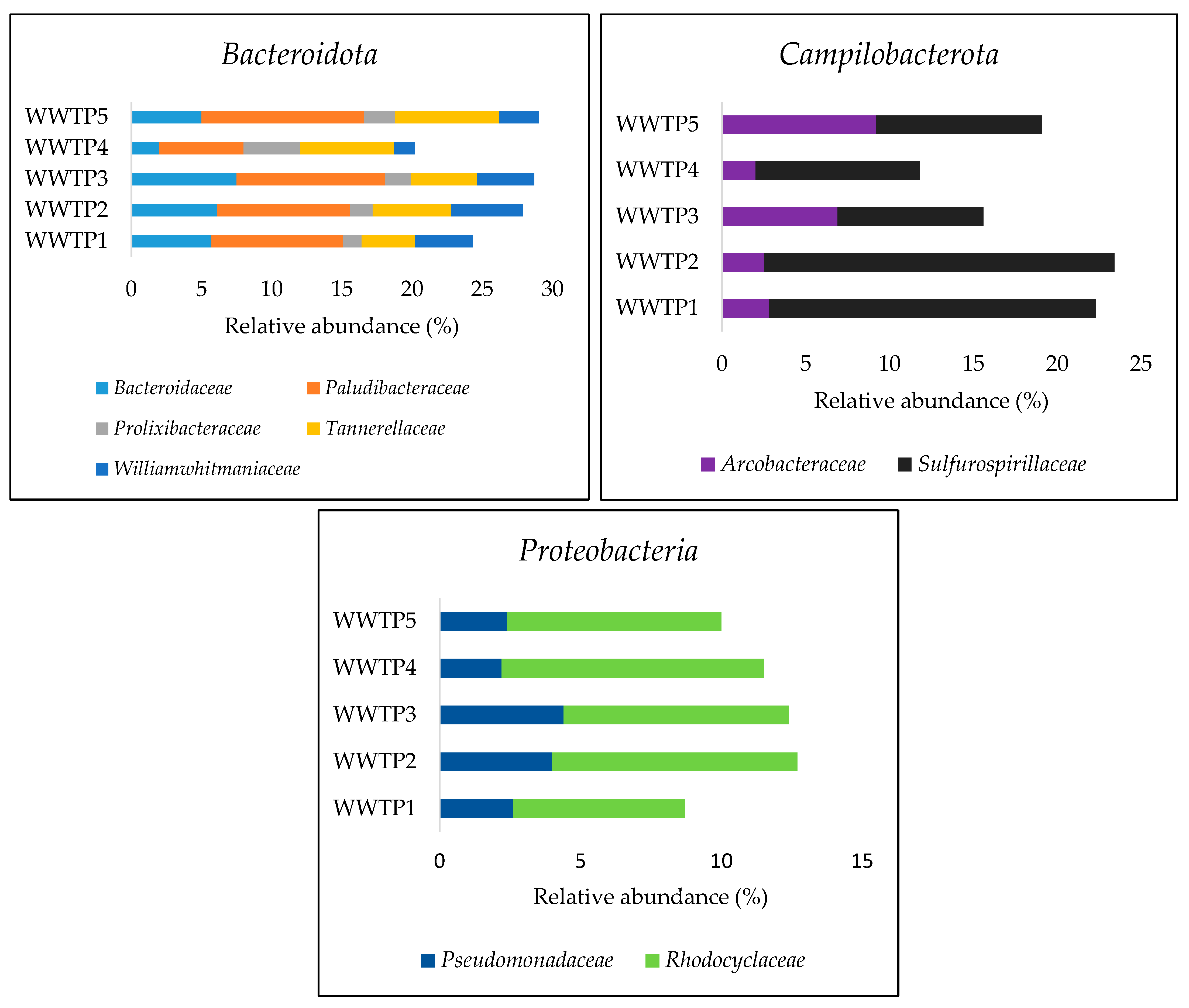
3.2. Taxonomic Comosition at Phylum, Family, and Genera Levels
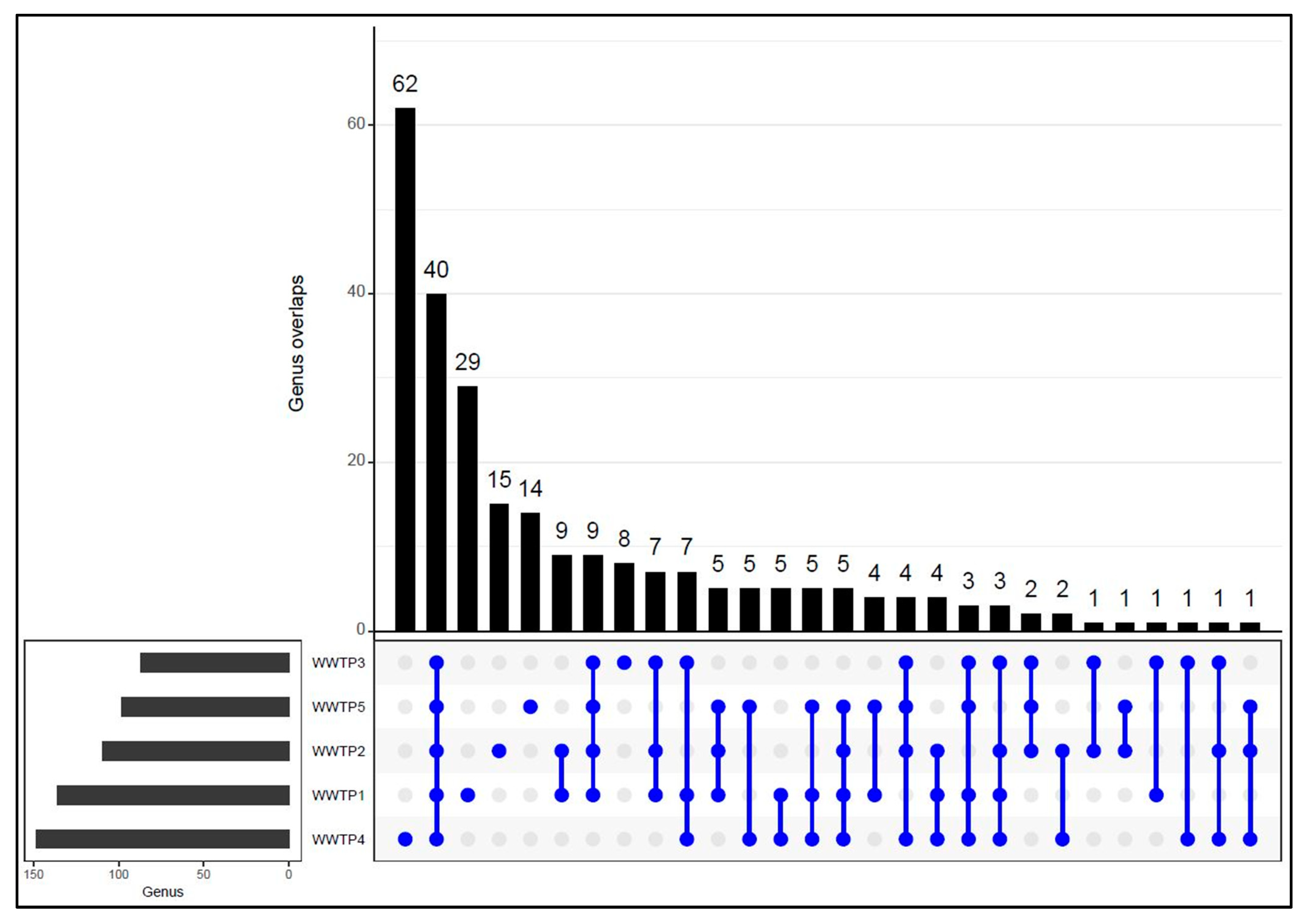
3.3. Shared and Distinct Bacterial Genera
3.4. Genera That Contain Potential Pathogenic Species in Influent Samples
Pathogenic Genera and Their Potential Health Outcomes
3.5. Risk Characterisation of Potentially Pathogenic Bacteria
4. Discussion
4.1. Bacterial Community Composition in Influent Samples
4.2. Potentially Pathogenic Genera
4.3. Risk Characterisation of Identified Potential Pathogens
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chahal, C.; Van Den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and particle associations in wastewater: Significance and implications for treatment and disinfection processes. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [CrossRef] [PubMed]
- World Bank; ILO; WaterAid; WHO. Health, Safety and Dignity of Sanitation Workers; World Bank: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Lu, R.; Frederiksen, M.W.; Uhrbrand, K.; Li, Y.; Østergaard, C.; Madsen, A.M. Wastewater treatment plant workers’ exposure and methods for risk evaluation of their exposure. Ecotoxicol. Environ. Saf. 2020, 205, 111365. [Google Scholar] [CrossRef] [PubMed]
- Van Hooste, W.; Charlier, A.M.; Rotsaert, P.; Bulterys, S.; Moens, G.; van Sprundel, M.; De Schryver, A. Work-related Helicobacter pylori infection among sewage workers in municipal wastewater treatment plants in Belgium. Occup. Environ. Med. 2010, 67, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hambach, R.; Droste, J.; François, G.; Weyler, J.; Van Soom, U.; De Schryver, A.; Vanoeteren, J.; van Sprundel, M. Work-related health symptoms among compost facility workers: A cross-sectional study. Arch. Public Health 2012, 70, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schantora, A.L.; Casjens, S.; Deckert, A.; Kampen, V.V.; Neumann, H.D.; Brüning, T.; Raulf, M.; Bünger, J.; Hoffmeyer, F. Prevalence of work-related rhino-conjunctivitis and respiratory symptoms among domestic waste collectors. Environ. Expo. Pollut. 2014, 834, 53–61. [Google Scholar] [CrossRef]
- Darboe, B.; Kao, M.Y.; Tsai, D. Respiratory symptoms among municipal waste workers in the Gambia: Types of solid waste and working conditions. Int. J. Health Promot. Educ. 2015, 53, 17–27. [Google Scholar] [CrossRef]
- Heldal, K.K.; Madso, L.; Eduard, W. Airway inflammation among compost workers exposed to actinomycetes spores. Ann. Agric. Environ. Med. 2015, 22, 253–258. [Google Scholar] [CrossRef]
- Heldal, K.K.; Austigard, Å.D.; Svendsen, K.H.; Einarsdottir, E.; Goffeng, L.O.; Sikkeland, L.I.; Nordby, K.C. Endotoxin and hydrogen sulphide exposure and effects on the airways among wastewater workers in sewage treatment plants and sewer net system. Ann. Work Expo. Health 2019, 63, 437–447. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, T. Detecting human bacterial pathogens in wastewater treatment plants by a high-throughput shotgun sequencing technique. Environ. Sci. Technol. 2013, 47, 5433–5441. [Google Scholar] [CrossRef]
- Boers, S.A.; Jansen, R.; Hays, J.P. Understanding and overcoming the pitfalls and biases of next-generation sequencing (NGS) methods for use in the routine clinical microbiological diagnostic laboratory. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1059–1070. [Google Scholar] [CrossRef] [Green Version]
- Numberger, D.; Ganzert, L.; Zoccarato, L.; Mühldorfer, K.; Sauer, S.; Grossart, H.P.; Greenwood, A.D. Characterization of bacterial communities in wastewater with enhanced taxonomic resolution by full-length 16S rRNA sequencing. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Cheng, Y.; Qian, C.; Lu, W. Bacterial community evolution along full-scale municipal wastewater treatment processes. J. Water Health 2020, 18, 665–680. [Google Scholar] [CrossRef] [PubMed]
- LaMartina, E.L.; Mohaimani, A.A.; Newton, R.J. Urban wastewater bacterial communities assemble into seasonal steady states. Microbiome 2021, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M. Analysis of microbial communities and pathogen detection in domestic sewage using metagenomic sequencing. Diversity 2020, 13, 6. [Google Scholar] [CrossRef]
- de Steenhuijsen Piters, W.A.; Sanders, E.A.; Bogaert, D. The role of the local microbial ecosystem in respiratory health and disease. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140294. [Google Scholar] [CrossRef] [Green Version]
- McLellan, S.L.; Huse, S.M.; Mueller-Spitz, S.R.; Andreishcheva, E.N.; Sogin, M. Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environ. Microbiol. 2010, 12, 378–392. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, A.L.; Souza, A.J.; Andrade, P.A.M.; Andreote, F.D.; Coscione, A.R.; Oliveira, F.C.; Regitano, J.B. Sewage sludge microbial structures and relations to their sources, treatments, and chemical attributes. Front. Microbiol. 2018, 9, 1462. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoo, K.; Kim, M.S.; Han, I.; Lee, M.; Kang, B.R.; Lee, T.K.; Park, J. The capacity of wastewater treatment plants drives bacterial community structure and its assembly. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Assress, H.A.; Selvarajan, R.; Nyoni, H.; Ntushelo, K.; Mamba, B.B.; Msagati, T.A. Diversity, co-occurrence and implications of fungal communities in wastewater treatment plants. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Osunmakinde, C.; Selvarajan, R.; Mamba, B.B.; Msagati, T.A. Profiling bacterial diversity and potential pathogens in wastewater treatment plants using high-throughput sequencing analysis. Microorganisms 2019, 7, 506. [Google Scholar] [CrossRef] [Green Version]
- World Bank; ILO; WaterAid; WHO. Improving Health, Safety, Dignity of Sanitation Workers: A Call To Action; World Bank: Washington, DC, USA, 2019; Available online: https://hdl.handle.net/10986/32640 (accessed on 12 March 2021).
- Dehghani, M.; Sorooshian, A.; Ghorbani, M.; Fazlzadeh, M.; Miri, M.; Badiee, P.; Parvizi, A.; Ansari, M.; Baghani, A.N.; Delikhoon, M. Seasonal variation in culturable bioaerosols in a wastewater treatment plant. Aerosol Air Qual. Res. 2018, 18, 2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, G.; Eble, J.E.; Gaither, M.R. A practical guide to sample preservation and pre-PCR processing of aquatic environmental DNA. Mol. Ecol. Resour. 2020, 20, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, 1. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, 61217. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.S.; Kirkegaard, R.H.; Karst, S.M.; Albertsen, M. Ampvis2: An R package to analyse and visualise 16S rRNA amplicon data. BioRxiv 2018, 299537. [Google Scholar] [CrossRef] [Green Version]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualisation of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- South Africa Occupational Health and Safety Act, 1993 Regulations for Hazardous Biological Agents. 2022. Available online: URI:/akn/za/act/gn/2022/r1887/eng@2022-03-16 (accessed on 22 November 2022).
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of wastewater on surface water quality in developing countries: A case study of South Africa. Water Qual. 2017, 10, 66561. [Google Scholar]
- Shanks, O.C.; Newton, R.J.; Kelty, C.A.; Huse, S.M.; Sogin, M.L.; McLellan, S.L. Comparison of the microbial community structures of untreated wastewaters from different geographic locales. Appl. Environ. Microbiol. 2013, 79, 2906–2913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giwa, A.S.; Ali, N.; Athar, M.A.; Wang, K. Dissecting microbial community structure in sewage treatment plant for pathogens’ detection using metagenomic sequencing technology. Arch. Microbiol. 2020, 202, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ning, D.; Zhang, B.; Li, Y.; Zhang, P.; Shan, X.; Zhang, Q.; Brown, M.R.; Li, Z.; Van Nostrand, J.D.; et al. Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat. Microbiol. 2019, 4, 1183–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, R.J.; McClary, J.S. The flux and impact of wastewater infrastructure microorganisms on human and ecosystem health. Curr. Opin. Biotechnol. 2019, 57, 145–150. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Y.; Ma, Q.; Zhang, Z.; Li, D.; Wang, J.; Shen, W.; Shen, E.; Zhou, J. Illumina MiSeq sequencing reveals diverse microbial communities of activated sludge systems stimulated by different aromatics for indigo biosynthesis from indole. PLoS ONE 2015, 10, 125732. [Google Scholar] [CrossRef]
- Begmatov, S.; Dorofeev, A.G.; Kadnikov, V.V.; Beletsky, A.V.; Pimenov, N.V.; Ravin, N.V.; Mardanov, A.V. The structure of microbial communities of activated sludge of large-scale wastewater treatment plants in the city of Moscow. Sci. Rep. 2022, 12, 3458. [Google Scholar] [CrossRef]
- Ibarbalz, F.M.; Orellana, E.; Figuerola, E.L.; Erijman, L. Shotgun metagenomic profiles have a high capacity to discriminate samples of activated sludge according to wastewater type. Appl. Environ. Microbiol. 2016, 82, 5186–5196. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, L.; Xiang, F.; Zhao, L.; Qiao, Z. Activated sludge microbial community and treatment performance of wastewater treatment plants in industrial and municipal zones. Int. J. Environ. Res. Public Health 2020, 17, 436. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.; Bijlmer, H.; Fournier, P.E.; Graves, S.; Hartzell, J.; Kersh, G.J.; Limonard, G.; Marrie, T.J.; Massung, R.F.; McQuiston, J.H.; et al. Diagnosis and management of Q fever—United States, 2013: Recommendations from CDC and the Q Fever Working Group. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2013, 62, 1–29. [Google Scholar]
- Sakamoto, K. The pathology of Mycobacterium tuberculosis infection. Vet. Pathol. 2012, 49, 423–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, W.; Staley, C.; Sidhu, J.; Sadowsky, M.; Toze, S. Amplicon-based profiling of bacteria in raw and secondary treated wastewater from treatment plants across Australia. Appl. Microbiol. Biotechnol. 2017, 101, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, T. Pathogenic bacteria in sewage treatment plants as revealed by 454 pyrosequencing. Environ. Sci. Technol. 2011, 45, 7173–7179. [Google Scholar] [CrossRef]
- Donnenberg, M.S. Pathogenic strategies of enteric bacteria. Nature 2000, 406, 768–774. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.X.; Wang, Z.; Huang, K.; Wang, Y.; Liang, W.; Tan, Y.; Liu, B.; Tang, J. Bacterial pathogens and community composition in advanced sewage treatment systems revealed by metagenomics analysis based on high-throughput sequencing. PLoS ONE 2015, 10, 125549. [Google Scholar] [CrossRef] [Green Version]
- Tong, J.; Fang, P.; Zhang, J.; Wei, Y.; Su, Y.; Zhang, Y. Microbial community evolution and fate of antibiotic resistance genes during sludge treatment in two full-scale anaerobic digestion plants with thermal hydrolysis pretreatment. Bioresour. Technol. 2019, 288, 121575. [Google Scholar] [CrossRef]
- Newton, R.J.; McLellan, S.L.; Dila, D.K.; Vineis, J.H.; Morrison, H.G.; Eren, A.M.; Sogin, M.L. Sewage reflects the microbiomes of human populations. MBio 2015, 6, e02574-14. [Google Scholar] [CrossRef] [Green Version]
- Varela, A.R.; Nunes, O.C.; Manaia, C.M. Quinolone resistant Aeromonas spp. as carriers and potential tracers of acquired antibiotic resistance in hospital and municipal wastewater. Sci. Total Environ. 2016, 542, 665–671. [Google Scholar] [CrossRef]
- Solaiman, S.; Micallef, S.A. Aeromonas spp. diversity in US mid-Atlantic surface and reclaimed water, seasonal dynamics, virulence gene patterns and attachment to lettuce. Sci. Total Environ. 2021, 779, 146472. [Google Scholar] [CrossRef]
- Figueras, M.J.; Beaz-Hidalgo, R. Aeromonas Infections in Humans; Caister Academic Press: London, UK, 2015; Volume 7, pp. 65–108. [Google Scholar]
- Fisher, J.C.; Levican, A.; Figueras, M.J.; McLellan, S.L. Population dynamics and ecology of Arcobacter in sewage. Front. Microbiol. 2014, 5, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greay, T.L.; Gofton, A.W.; Zahedi, A.; Paparini, A.; Linge, K.L.; Joll, C.A.; Ryan, U.M. Evaluation of 16S next-generation sequencing of hypervariable region 4 in wastewater samples: An unsuitable approach for bacterial enteric pathogen identification. Sci. Total Environ. 2019, 670, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Beilfuss, H.A.; Quig, D.; Block, M.A.; Schreckenberger, P.C. Definitive identification of Laribacter hongkongensis acquired in the United States. J. Clin. Microbiol. 2015, 53, 2385–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Ghaju, R.; Tanaka, Y.; Sherchand, J.B.; Haramoto, E. Identification of 16S rRNA and virulence-associated genes of Arcobacter in water samples in the Kathmandu Valley, Nepal. Pathogens 2019, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Quinn, P.J. Endotoxins: Lipopolysaccharides of Gram-Negative Bacteria. In Endotoxins: Structure, Function and Recognition; Wang, X., Quinn, P., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 53. [Google Scholar] [CrossRef]
- Liebers, V.; Raulf-Heimsoth, M.; Brüning, T. Health effects due to endotoxin inhalation. Arch. Toxicol. 2008, 82, 203–210. [Google Scholar] [CrossRef]
- Levy, K.; Smith, S.M.; Carlton, E.J. Climate change impacts on waterborne diseases: Moving toward designing interventions. Curr. Environ. Health Rep. 2018, 5, 272–282. [Google Scholar] [CrossRef]
- World Health Organization (WHO). United Nations Children’s Fund (UNICEF). Progress on Drinking Water, Sanitation and Hygiene, p. 110. 2017. Available online: https://www.unicef.org/reports/progress-on-drinking-water-sanitation-and-hygiene-2019 (accessed on 8 February 2023).

| Site | WWTP1 | WWTP2 | WWTP3 | WWTP4 | WWTP5 |
|---|---|---|---|---|---|
| Source of wastewater (%) | Mixed (domestic (90) and industrial (10) | Domestic (100) | Mixed (domestic (80) and industrial (20) | Domestic (100) | Domestic (100) |
| Population size served | 366,709 | 600,000 | 236,580 | 1,041,200 | 472,000 |
| Treatment capacity * (ML/day) | 35 | 60 | 93 | 180 | 85 |
| Treatment train | Raw influent, bar screens, grit removal chamber, primary clarifiers, surface aeration tank, secondary sedimentation | Raw influent, bar screens, grit removal chamber, primary clarifiers, surface aeration tank and trickling bio-filters, secondary sedimentation | Raw influent, bar screens, grit removal chamber, primary clarifiers, surface aeration tank, secondary sedimentation | Raw influent, bar screens, grit removal chamber, primary clarifiers, diffused aeration tank, secondary sedimentation | Raw influent, bar screens, grit removal chamber, primary clarifiers, diffused aeration tank, secondary sedimentation |
| Biological treatment | Activated sludge | Activated sludge and bio-filters | Activated sludge | Activated sludge | Activated sludge |
| Tertiary treatment | Chlorine disinfection | Chlorine disinfection | Chlorine disinfection | Chlorine disinfection | Chlorine disinfection |
| Intended reuse of treated effluent | Irrigation and housekeeping purposes | Irrigation and cooling water to a nearby power station | Housekeeping purposes | Discharged into a river | Agricultural purposes |
| Workforce | 22 | 38 | 37 | 66 | 27 |
| Sample | Total Reads | ASV | Chao1 | ACE | Shannon | Simpson |
|---|---|---|---|---|---|---|
| WWTP1 | 85,200 | 19,449 | 695 | 697 | 6.00 | 0.997 |
| WWTP2 | 75,629 | 16,334 | 643 | 646 | 5.91 | 0.996 |
| WWTP3 | 59,881 | 13,594 | 534 | 535 | 5.85 | 0.996 |
| WWTP4 | 84,585 | 20,300 | 949 | 957 | 6.32 | 0.998 |
| WWTP5 | 92,471 | 16,923 | 508 | 510 | 5.85 | 0.996 |
| Genera by Type of Infection | Relative Abundance (%) | HBA Risk Group |
|---|---|---|
| Respiratory: | ||
| Coxiella | 0.05 | 3 |
| Mycobacterium | 0.1 | 2/3 |
| Enteric: | ||
| Aeromonas | 2.8 | 2 |
| Arcobacter | 2.6 | unclassified |
| Escherichia/Shigella | 0.1 | 2/3 |
| Laribacter | 0.2 | 2 |
| Opportunistic: | ||
| Acinetobacter | 0.4 | 2 |
| Actinomyces | 0.1 | 2 |
| Atopobium | 0.05 | unclassified |
| Bacteroides | 5.1 | 2 |
| Blastomonas | 0.05 | unclassified |
| Brachybacterium | 0.05 | unclassified |
| Chryseobacterium | 0.05 | unclassified |
| Citrobacter | 0.05 | unclassified |
| Comamonas | 0.3 | unclassified |
| Dysgonomonas | 0.3 | unclassified |
| Empedobacter | 0.05 | unclassified |
| Enterobacter | 0.5 | 2 |
| Enterococcus | 0.05 | 2 |
| Erysipelothrix | 0.1 | 2 |
| Finegoldia | 0.05 | unclassified |
| Gordonia | 0.05 | unclassified |
| Klebsiella | 0.05 | 2 |
| Leptotrichia | 1.4 | unclassified |
| Leuconostoc | 0.05 | unclassified |
| Ochrobactrum | 0.05 | unclassified |
| Prevotella | 0.2 | 2 |
| Pseudomonas | 2.9 | 2/3 |
| Pseudoxanthomonas | 0.1 | unclassified |
| Roseomonas | 0.05 | unclassified |
| Shewanella | 0.5 | unclassified |
| Sphingobacterium | 0.05 | unclassified |
| Streptobacillus | 0.05 | 2 |
| Streptococcus | 0.6 | 2 |
| Synergistes | 0.1 | unclassified |
| Treponema | 0.9 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poopedi, E.; Singh, T.; Gomba, A. Potential Exposure to Respiratory and Enteric Bacterial Pathogens among Wastewater Treatment Plant Workers, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 4338. https://doi.org/10.3390/ijerph20054338
Poopedi E, Singh T, Gomba A. Potential Exposure to Respiratory and Enteric Bacterial Pathogens among Wastewater Treatment Plant Workers, South Africa. International Journal of Environmental Research and Public Health. 2023; 20(5):4338. https://doi.org/10.3390/ijerph20054338
Chicago/Turabian StylePoopedi, Evida, Tanusha Singh, and Annancietar Gomba. 2023. "Potential Exposure to Respiratory and Enteric Bacterial Pathogens among Wastewater Treatment Plant Workers, South Africa" International Journal of Environmental Research and Public Health 20, no. 5: 4338. https://doi.org/10.3390/ijerph20054338
APA StylePoopedi, E., Singh, T., & Gomba, A. (2023). Potential Exposure to Respiratory and Enteric Bacterial Pathogens among Wastewater Treatment Plant Workers, South Africa. International Journal of Environmental Research and Public Health, 20(5), 4338. https://doi.org/10.3390/ijerph20054338






