Solvent Extraction for Separation of Indonesian Oil Sands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Experimental Methods
2.3. Analyses
3. Results
3.1. Analysis of Indonesian Oil Sands
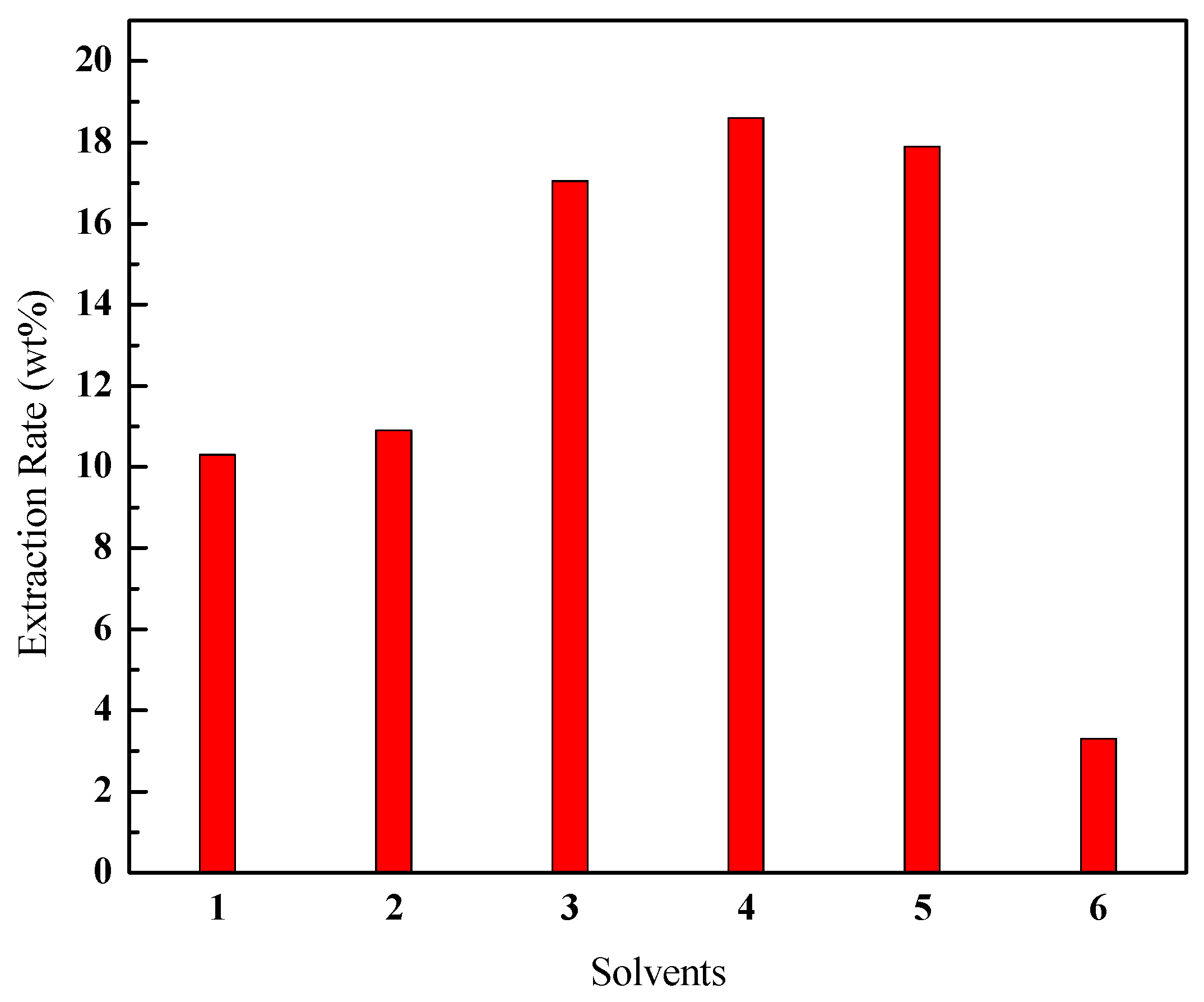
3.2. Effect of Different Organic Solvents on the Extraction Rate of Bitumen
3.3. Study of Operating Conditions
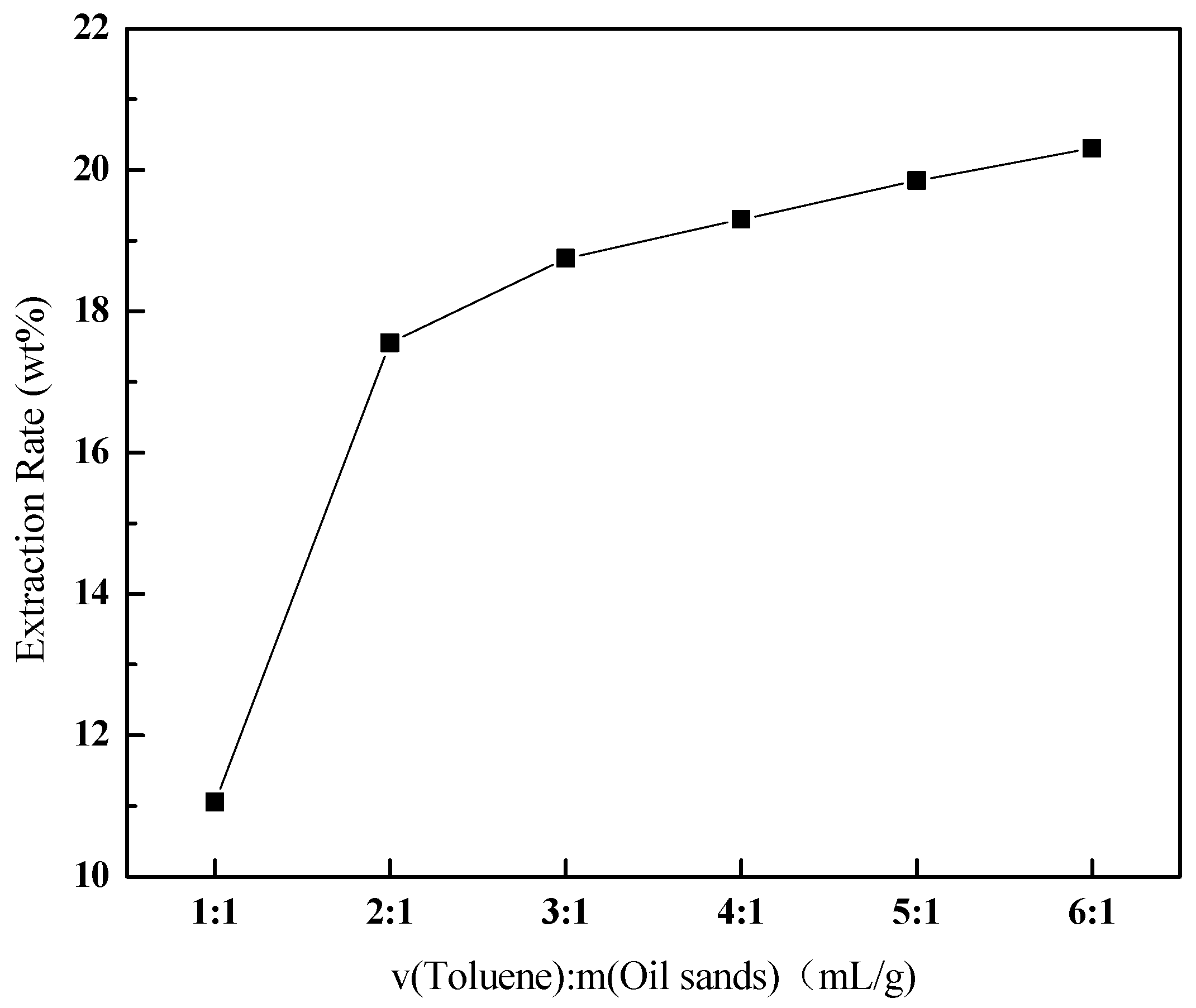
3.3.1. Effect of the Ratio of Toluene to Oil Sands
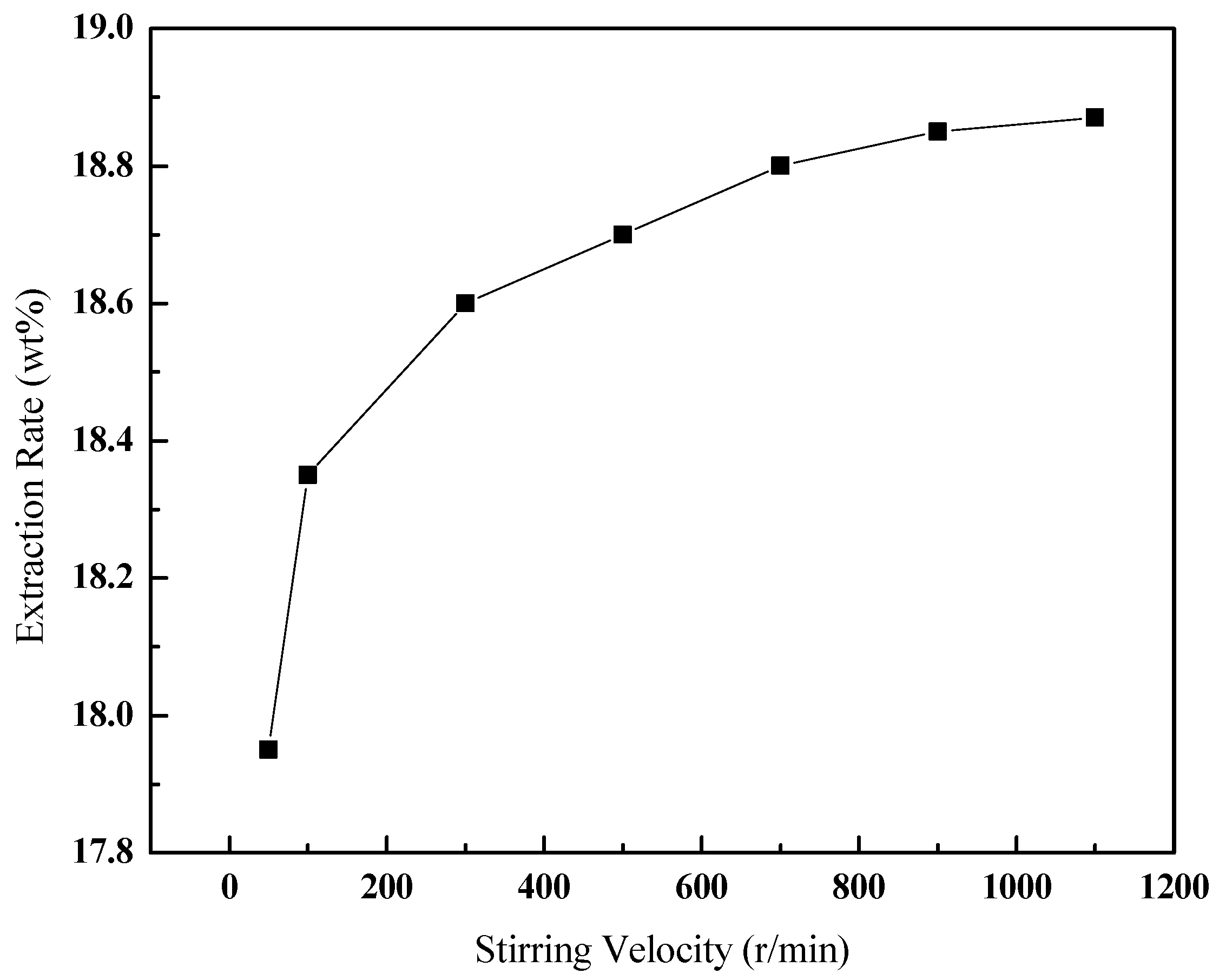
3.3.2. Effect of the Stirring Velocity
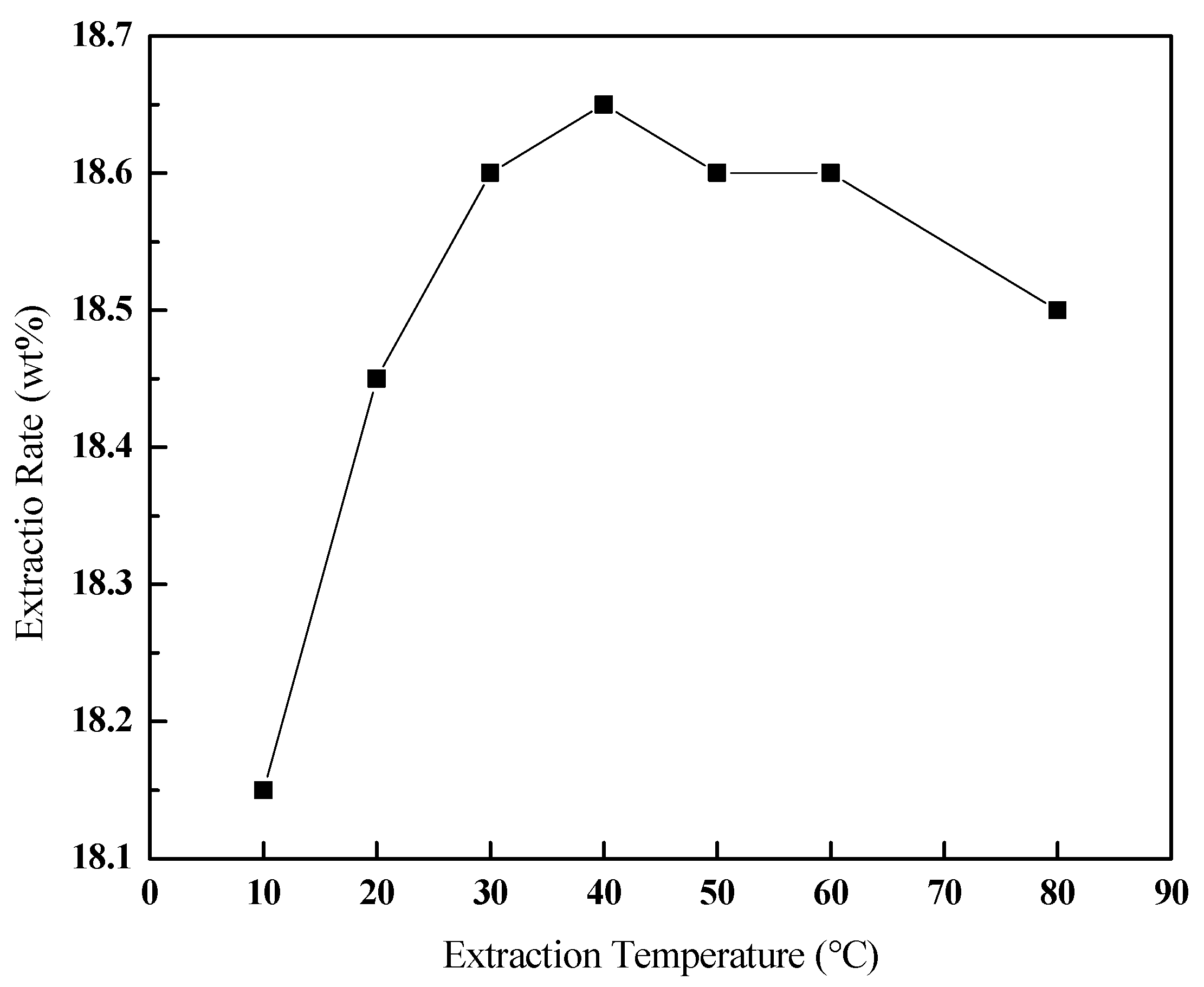
3.3.3. Effect of the Extraction Temperature
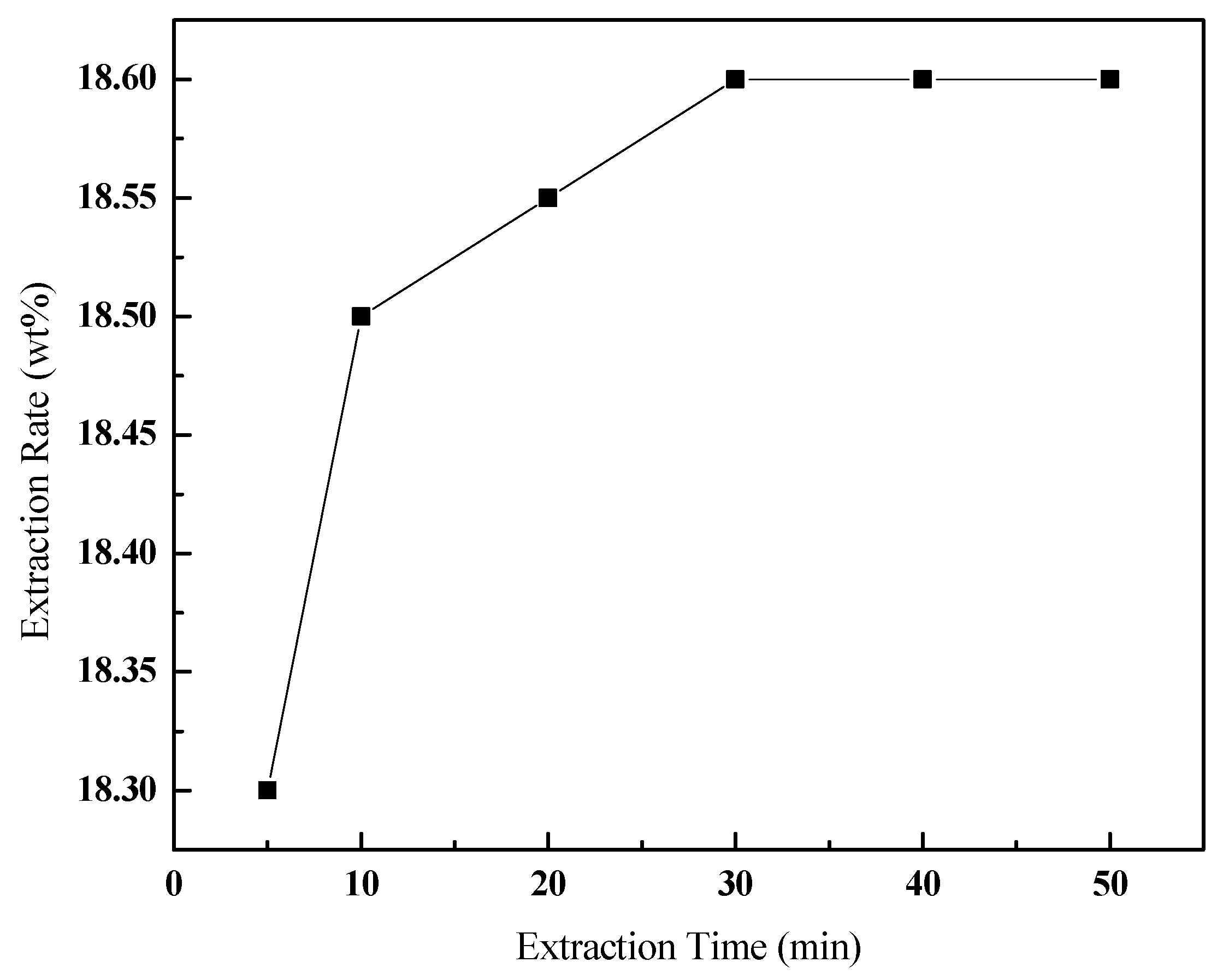
3.3.4. Effect of the Extraction Time
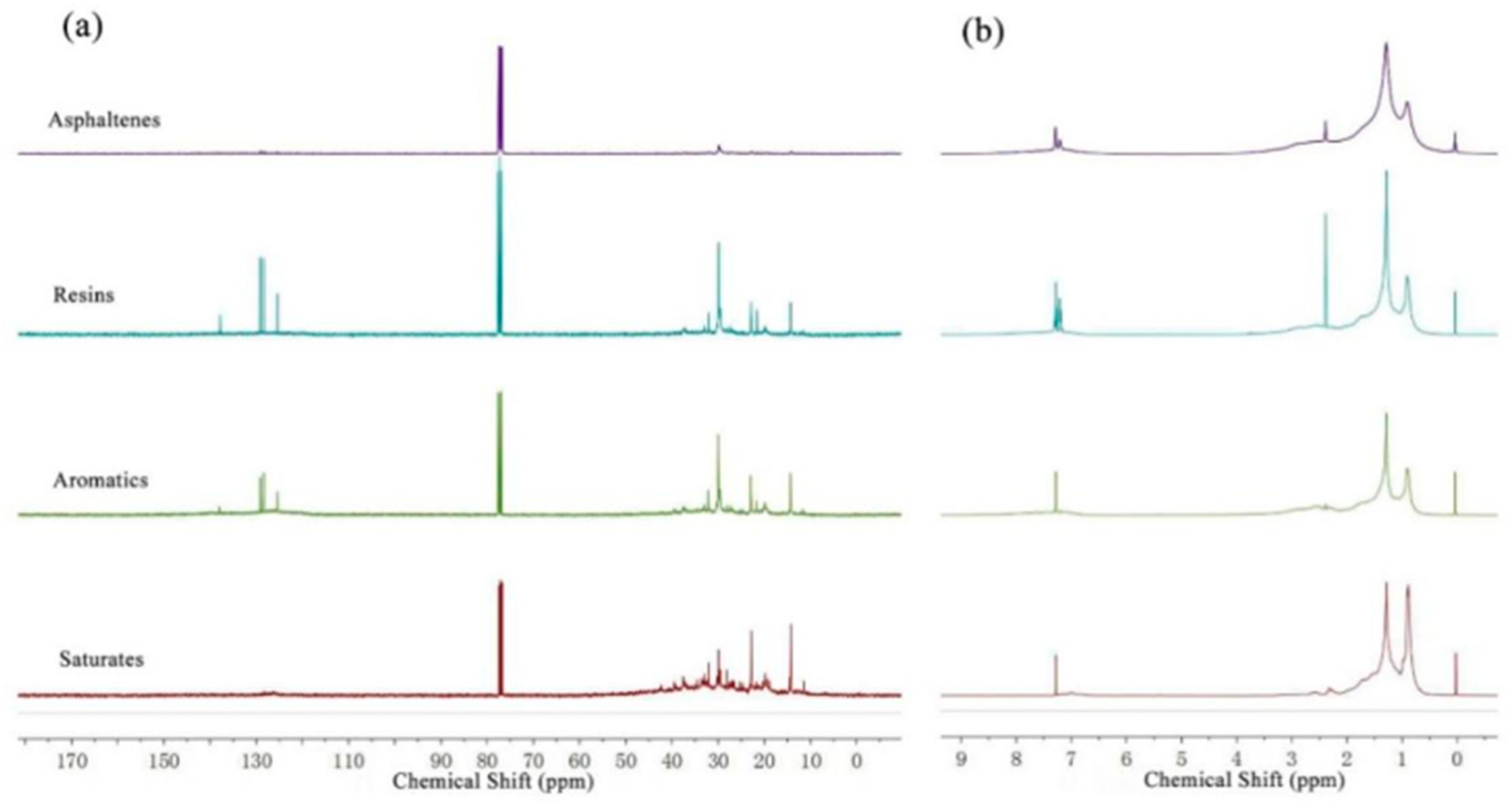
3.4. Analysis of Bitumen Obtained under Suitable Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Ma, M.; Niu, L.; Pang, C.; Guan, X. Determination of Oil Yield of Oil Sands. J. Zhengzhou Univ. (Nat. Sci. Ed.) 2014, 46, 76–79. (In Chinese) [Google Scholar]
- Aguilera, F.; Mendez, J.; Pasaro, E.; Laffon, B. Review on the Effects of Exposure to Spilled Oils on Human Health. J. Appl. Toxicol. 2010, 30, 291–301. [Google Scholar] [CrossRef]
- Lim, M.W.; Lau, E.V.; Poh, P.E.; Chong, W. Interaction studies between high-density oil and sand particles in oil flotation technology. J. Pet. Sci. Eng. 2015, 131, 114–121. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, C.D.; Shim, N.; Han, M. Total Petroleum Hydrocarbon Removal from Oil-sand by Injecting Charged Nano-bubbles. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100653. [Google Scholar]
- Li, R. Research Progress in Water-Based Bitumen Extraction from Oil Sands. J. Chem. Ind. Eng. 2011, 62, 2406–2412. [Google Scholar]
- Wang, T.; Zhang, C.; Zhao, R.; Zhu, C.; Yang, C.; Liu, C. Solvent Extraction of Bitumen from Oil Sands. Energy Fuels 2014, 28, 2297–2304. [Google Scholar] [CrossRef]
- Joshi, V.A.; Kundu, D. Ionic liquid promoted extraction of bitumen from oil sand: A review. J. Pet. Sci. Eng. 2021, 199, 108232. [Google Scholar] [CrossRef]
- Lin, F.; He, L.; Hou, J.; Masliyah, J.; Xu, Z. Role of Ethyl Cellulose in Bitumen Extraction from Oil Sands Ores Using an Aqueous-nonaqeous Hybrid Process. Energy Fuels 2016, 30, 121–129. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Jia, Y.; Yuan, W.; Song, X. High-efficiency Extraction of Bitumen from Oil Sands Using Mixture of Ionic Liquid [Emim][BF4] and Dichloromethane. China Pet. Process. Pe. 2021, 23, 132–138. [Google Scholar]
- Yang, H.; Wang, Y.; Ding, M.; Hu, B.; Ren, S. Water-Assisted Solvent Extraction of Bitumen from Oil Sands. Ind. Eng. Chem. Res. 2012, 51, 3032–3038. [Google Scholar] [CrossRef]
- Lin, F.; Xu, Y.; Nelson, R. Determination and Understanding of Solvent Losses to Tailings from a Laboratory Hybrid Bitumen Extraction Process. Energy Fuels 2018, 32, 9202–9210. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Wu, G.; He, L.; Li, H.; Sui, H. Ionic Liquid Enhanced Solvent Extraction for Bitumen Recovery from Oil Sands. Energy Fuels 2011, 25, 5224–5231. [Google Scholar] [CrossRef]
- Khammar, M.; Xu, Y. Batch Solvent Extraction of Bitumen from Oil Sand. Part 1: Theoretical Basis. Energy Fuels 2017, 31, 4616–4625. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Huang, J.; Cui, X.; Tan, X.; Liu, Q.; Zeng, H. Heterogeneous Distribution of Adsorbed Bitumen on Fine Solids from Solvent-Based Extraction of Oil Sands Probed by AFM. Energy Fuels 2017, 31, 8833–8842. [Google Scholar] [CrossRef]
- Abramov, O.V.; Abramov, V.O.; Myasnikov, S.K.; Mullakaev, M.S. Extraction of bitumen, crude oil and its products from tar sand and contaminated sandy soil under effect of ultrasound. Ultrason. Sonochem. 2009, 16, 408–416. [Google Scholar] [CrossRef]
- Nikakhtari, H.; Vagi, L.; Choi, P.; Liu, Q.; Gray, M.R. Solvent Screening for Non-aqueous Extraction of Alberta Oil Sands. Can. J. Chem. Eng. 2013, 91, 1153–1160. [Google Scholar] [CrossRef]
- Zubaidy, E.A.H.; Abouelnasr, D.M. Fuel recovery from waste oily sludge using solvent extraction. Process. Saf. Environ. Prot. 2010, 88, 318–326. [Google Scholar] [CrossRef]
- Chang, J.; Ong, C.; Shi, Y.; Yuan, J.; Ahmed, Z.; Wang, P. Smart Sand by Surface Engineering: Toward Controllable Oil/Water Separation. Ind. Eng. Chem. Res. 2021, 60, 9475–9481. [Google Scholar] [CrossRef]
- Alharbi, H.A.; Morandi, G.D.; Jones, P.D.; Wiseman, S.B.; Giesy, J.P. Comparison of the Effects of Extraction Techniques on Mass Spectrometry Profiles of Dissolved Organic Compounds in Oil Sand Process-Affected Water. Energy Fuels 2019, 33, 7001–7008. [Google Scholar] [CrossRef]
- Pal, K.; Branco, L.D.P.N.; Heintz, A.; Choi, P.; Liu, Q.; Seidl, P.R.; Gray, M.R. Performance of Solvent Mixtures for Non-aqueous Extraction of Alberta Oil Sands. Energy Fuels 2015, 29, 2261–2267. [Google Scholar] [CrossRef]
- Han, D.Y.; Cao, Z.B.; Xu, X.Q. Study on Solvent Extraction of Mongolia Outcrop Oil Sands. Energy Sources A Recover. Util. Environ. Eff. 2013, 35, 1368–1374. [Google Scholar] [CrossRef]
- Wu, J.; Dabros, T. Process for Solvent Extraction of Bitumen from Oil Sand. Energy Fuels 2012, 26, 1002–1008. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Ma, G.-D.; Li, Y.; Cao, Z.-B.; Li, D.-D. The Solvent Extraction Investigation on Kazakhstan Oil Sand. J. Liaoning Shihua Univ. 2012, 32, 6–8. (In Chinese) [Google Scholar]
- Han, D.Y.; Luo, K.Y.; Shi, W.; Qiao, H.Y.; Cao, Z.B. Enhanced recovery of bitumen from Indonesian oil sands using organic and aqueous double phase extraction system. Energy Sources A Recover. Util. Environ. Eff. 2017, 39, 1679–1685. [Google Scholar] [CrossRef]
- Xu, X.Q.; Wang, H.Y.; Shen, Z.B.; Zheng, D.W.; Ge, Z.X.; Cao, Z.B. Method of Determining Oil Content in the Oil Sands and Soil. Sci. Technol. Chem. Ind. 2008, 16, 1–4. [Google Scholar]
- Zhang, L.; Zhang, L.; Xu, Z.; Guo, X.; Xu, C.; Zhao, S. Viscosity Mixing Rule and Viscosity-Temperature Relationship Estimation for Oil Sand Bitumen Vacuum Residue and Fractions. Energy Fuels 2019, 33, 206–214. [Google Scholar] [CrossRef]
- Woods, J.; Kung, J.; Kingston, D.; Kotlyar, L.; Sparks, B.; McCracken, T. Canadian Crudes: A Comparative Study of SARA Fractions from a Modified HPLC Separation Technique. Oil Gas Sci. Technol. Rev. IFP 2008, 63, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Shi, Q.; Zhao, C.; Zhang, N.; Chung, K.H.; Xu, C.; Zhao, S. Hindered Stepwise Aggregation Model for Molecular Weight Determination of Heavy Petroleum Fractions by Vapor Pressure Osmometry (VPO). Energy Fuels 2013, 27, 1331–1336. [Google Scholar] [CrossRef]
- Woods, J.R.; Kung, J.; Kingston, D.; McCracken, T.; Kotlyar, L.S.; Sparks, B.D.; Mercier, P.H.J.; Ng, S.; Moran, K. The Comparison of Bitumens from Oil Sands with Different Recovery Profiles. Pet. Sci. Technol. 2012, 30, 2285–2293. [Google Scholar] [CrossRef]
- He, L.; Li, X.G.; Du, Y.L.; Wu, G.Z.; Li, H.; Sui, H. Parameters of Solvent Extraction for Bitumen Recovery from Oil Sands. Adv. Mater. Res. 2012, 347-353, 3728–3731. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, C.; Liu, Q.; Masliyah, J.; Xu, Z. Biodiesel-assisted Ambient Aqueous Bitumen Extraction from Athabasca Oil Sands. Energy Fuels 2018, 32, 6565–6576. [Google Scholar] [CrossRef]
- Dong, Z.J. Detaching Oil Sand at Za Lai Te Qi in Inner Mongolia. J. Petrochem. Univ. 2005, 18, 18–19. (In Chinese) [Google Scholar]
- Wang, S.Q.; Liu, J.; Zhang, L. Interaction Forces between Asphaltene Surfaces in Organic Solvents. Langmuir 2010, 26, 283–290. [Google Scholar] [CrossRef] [PubMed]


| Property | Indonesian Samples | Syncrude Canada Ltd. | Neimenggu China | |
|---|---|---|---|---|
| Oil Sands | Bitumen | 24.93 | 8~15 | 12~20 |
| Solids | 74.30 | 83~88 | 80~87 | |
| Water | 0.77 | 2.5~9 | 0.5~1 | |
| Bitumen | Saturates | 23.56 | 16~21 | 34.1 |
| Aromatics | 23.08 | 26~36 | 13.2 | |
| Resins | 21.61 | 24~41 | 37.7 | |
| Asphaltenes | 31.75 | 15~24 | 12.8 | |
| Property | Bitumen | Saturates | Aromatics | Resins | Asphaltenes | |
|---|---|---|---|---|---|---|
| MWn | 802 | 443 | 573 | 981 | 1984 | |
| Elemental Analysis | C/% | 80.86 | 86.16 | 81.56 | 80.19 | 76.62 |
| H/% | 8.84 | 11.427 | 8.594 | 8.438 | 7.234 | |
| O/% | 3.071 | 1.983 | 3.014 | 3.143 | 4.328 | |
| N/% | 0.43 | 0.07 | 0.17 | 0.70 | 0.77 | |
| S/% | 6.275 | 0.351 | 6.043 | 6.751 | 9.762 | |
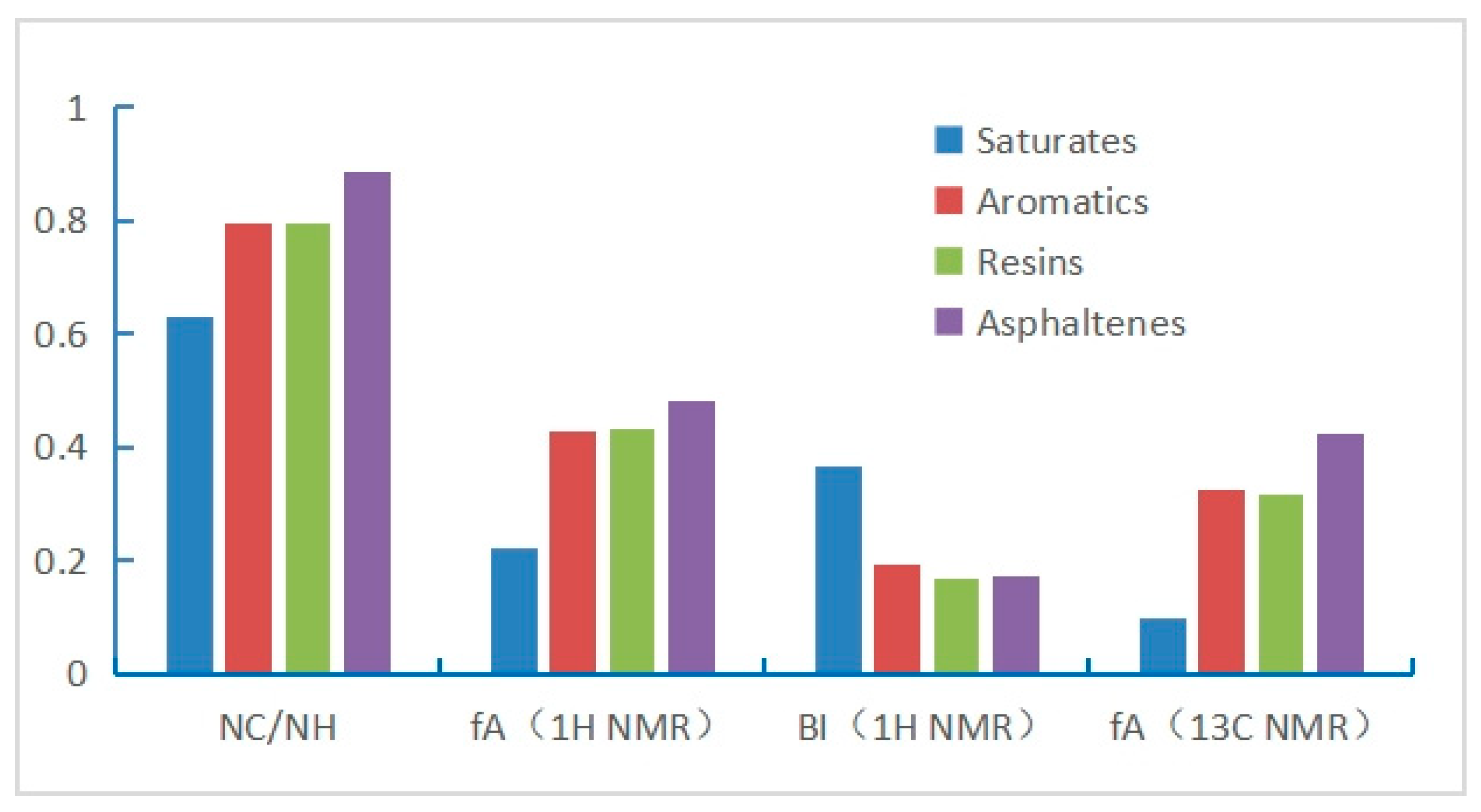
| 1H NMR | HA | 0.091 | 0.018 | 0.091 | 0.094 | 0.083 |
| Hα | 0.156 | 0.048 | 0.195 | 0.177 | 0.160 | |
| Hβ | 0.533 | 0.586 | 0.509 | 0.546 | 0.568 | |
| Hγ | 0.220 | 0.348 | 0.205 | 0.183 | 0.189 | |
| 13C NMR | AA/% | 31.746 | 9.891 | 32.680 | 31.546 | 42.553 |
| AS/% | 68.254 | 90.109 | 67.320 | 68.454 | 57.447 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Zhu, Q.; Zhao, C.; Zhou, W.; Wang, C. Solvent Extraction for Separation of Indonesian Oil Sands. Int. J. Environ. Res. Public Health 2023, 20, 4527. https://doi.org/10.3390/ijerph20054527
Cui W, Zhu Q, Zhao C, Zhou W, Wang C. Solvent Extraction for Separation of Indonesian Oil Sands. International Journal of Environmental Research and Public Health. 2023; 20(5):4527. https://doi.org/10.3390/ijerph20054527
Chicago/Turabian StyleCui, Wenlong, Qingqing Zhu, Chenze Zhao, Weiyou Zhou, and Cheli Wang. 2023. "Solvent Extraction for Separation of Indonesian Oil Sands" International Journal of Environmental Research and Public Health 20, no. 5: 4527. https://doi.org/10.3390/ijerph20054527




