Which Factors Influence Running Gait in Children and Adolescents? A Narrative Review
Abstract
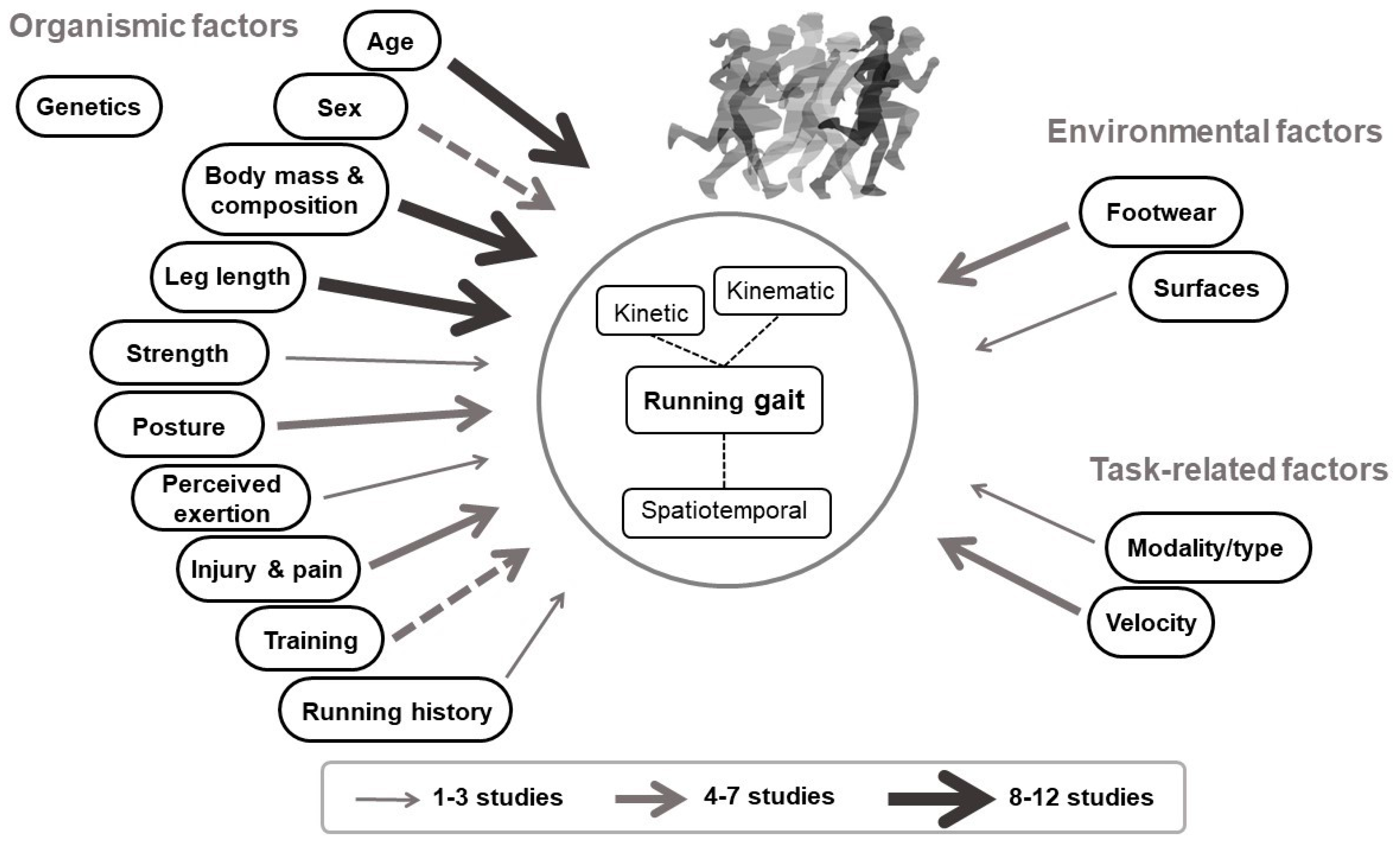
1. Introduction
2. Organismic Factors
2.1. Genetics
2.2. Biological Age
2.3. Sex
2.4. Body Mass and Composition
2.5. Leg Length
2.6. Strength
2.7. Posture
2.8. Perceived Exertion
2.9. Injury and Pain
2.10. Running History, Lifestyle, and Training
3. Environmental Factors
3.1. Footwear
3.2. Running Surface and Incline
4. Task-Related Factors
4.1. Running Modality or Type
4.2. Running Velocity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FFS | Forefoot strike |
| FSA | Foot strike angle |
| FSP | Foot strike pattern |
| JIA | Juvenile idiopathic arthritis |
| MFS | Midfoot strike |
| PE | Perceived exertion |
| PHV | Peak height velocity |
| RFS | Rearfoot strike |
| ROM | Range of motion |
References
- Scheer, V.; Hoffman, M.D. Too Much Too Early? An Analysis of Worldwide Childhood Ultramarathon Participation and Attrition in Adulthood. J. Sports Med. Phys. Fit. 2019, 59, 1363–1368. [Google Scholar] [CrossRef]
- Krabak, B.J.; Snitily, B.; Milani, C.J.E. Running Injuries During Adolescence and Childhood. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Scheer, V.; Hoffman, M.D. Should Children Be Running Ultramarathons? Curr. Sports Med. Rep. 2018, 17, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Phinyomark, A.; Osis, S.; Hettinga, B.A.; Ferber, R. Kinematic Gait Patterns in Healthy Runners: A Hierarchical Cluster Analysis. J. Biomech. 2015, 48, 3897–3904. [Google Scholar] [CrossRef]
- Dugan, S.A.; Bhat, K.P. Biomechanics and Analysis of Running Gait. Phys. Med. Rehabil. Clin. N. Am. 2005, 16, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Novacheck, T.F. The Biomechanics of Running. Gait Posture 1998, 7, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Thordarson, D.B. Running Biomechanics. Clin. Sports Med. 1997, 16, 239–247. [Google Scholar] [CrossRef] [PubMed]
- van Oeveren, B.T.; de Ruiter, C.J.; Beek, P.J.; van Dieën, J.H. The Biomechanics of Running and Running Styles: A Synthesis. Sports Biomech. 2021, 1–39. [Google Scholar] [CrossRef]
- Chia, L.C.; Licari, M.K.; Guelfi, K.J.; Reid, S.L. A Comparison of Running Kinematics and Kinetics in Children with and without Developmental Coordination Disorder. Gait Posture 2013, 38, 264–269. [Google Scholar] [CrossRef]
- Diamond, N.; Downs, J.; Morris, S. “The Problem with Running”—Comparing the Propulsion Strategy of Children with Developmental Coordination Disorder and Typically Developing Children. Gait Posture 2014, 39, 547–552. [Google Scholar] [CrossRef]
- Rethwilm, R.; Böhm, H.; Haase, M.; Perchthaler, D.; Dussa, C.U.; Federolf, P. Dynamic Stability in Cerebral Palsy during Walking and Running: Predictors and Regulation Strategies. Gait Posture 2021, 84, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.M.; Daffertshofer, A.; Dominici, N. The Development of Mature Gait Patterns in Children during Walking and Running. Eur. J. Appl. Physiol. 2021, 121, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Fortney, V.L. The Kinematics and Kinetics of the Running Pattern of Two-, Four-, and Six-Year-Old Children. Res. Q. Exerc. Sport 1983, 54, 126–135. [Google Scholar] [CrossRef]
- Lye, J.; Parkinson, S.; Diamond, N.; Downs, J.; Morris, S. Propulsion Strategy in the Gait of Primary School Children; the Effect of Age and Speed. Hum. Mov. Sci. 2016, 50, 54–61. [Google Scholar] [CrossRef]
- Schepens, B.; Willems, P.A.; Cavagna, G.A. The Mechanics of Running in Children. J. Physiol. 1998, 509 Pt 3, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Whitall, J. A Developmental Study of the Interlimb Coordination in Running and Galloping. J. Mot. Behav. 1989, 21, 409–428. [Google Scholar] [CrossRef]
- Williams, S.; Netto, K.; Kennedy, R.; Turner-Bryndzej, J.; Campbell, R.; Rosalie, S.M. Biomechanical Correlates of Running Performance in Active Children. J. Sci. Med. Sport 2019, 22, 65–69. [Google Scholar] [CrossRef]
- Glazier, P.S. Towards a Grand Unified Theory of Sports Performance. Hum. Mov. Sci. 2017, 56, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Thomis, M.A.; Towne, B. Genetic Determinants of Prepubertal and Pubertal Growth and Development. Food Nutr. Bull. 2006, 27, S257–S278. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.; Van Mechelen, W. (Eds.) Oxford Textbook of Children’s Sport and Exercise Medicine, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2017; ISBN 978-0-19-875767-2. [Google Scholar]
- Kung, S.M.; Fink, P.W.; Legg, S.J.; Ali, A.; Shultz, S.P. Age-Dependent Variability in Spatiotemporal Gait Parameters and the Walk-to-Run Transition. Hum. Mov. Sci. 2019, 66, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Latorre Román, P.Á.; Redondo Balboa, F.; Párraga Montilla, J.; Soto Hermoso, V.M.; Consuegra González, P.J.; García Pinillos, F. Analysis of Foot Strike Pattern, Rearfoot Dynamic and Foot Rotation over Childhood. A Cross-Sectional Study. J. Sports Sci. 2019, 37, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.W.; Oliver, J.L.; Hughes, M.G.; Cronin, J.B.; Lloyd, R.S. Maximal Sprint Speed in Boys of Increasing Maturity. Pediatr. Exerc. Sci. 2015, 27, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.W.; Oliver, J.L.; Hughes, M.G.; Lloyd, R.S.; Cronin, J.B. Influence of Age, Maturity, and Body Size on the Spatiotemporal Determinants of Maximal Sprint Speed in Boys. J. Strength Cond. Res. 2017, 31, 1009–1016. [Google Scholar] [CrossRef]
- Nagahara, R.; Takai, Y.; Haramura, M.; Mizutani, M.; Matsuo, A.; Kanehisa, H.; Fukunaga, T. Age-Related Differences in Spatiotemporal Variables and Ground Reaction Forces During Sprinting in Boys. Pediatr. Exerc. Sci. 2018, 30, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, R.; Haramura, M.; Takai, Y.; Oliver, J.L.; Wichitaksorn, N.; Sommerfield, L.M.; Cronin, J.B. Age-Related Differences in Kinematics and Kinetics of Sprinting in Young Female. Scand. J. Med. Sci. Sports 2019, 29, 800–807. [Google Scholar] [CrossRef]
- Krabak, B.J.; Tenforde, A.S.; Davis, I.S.; Fredericson, M.; Harrast, M.A.; d’Hemecourt, P.; Luke, A.C.; Roberts, W.O. Youth Distance Running: Strategies for Training and Injury Reduction. Curr. Sports Med. Rep. 2019, 18, 53–59. [Google Scholar] [CrossRef]
- Malina, R.M.; Bouchard, C.; Bar-Or, O. Growth, Maturation, and Physical Activity, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2004; ISBN 978-0-88011-882-8. [Google Scholar]
- Raudsepp, L.; Pääsuke, M. Gender Differences in Fundamental Movement Patterns, Motor Performances, and Strength Measurements of Prepubertal Children. Pediatr. Exerc. Sci. 1995, 7, 294–304. [Google Scholar] [CrossRef]
- Luedke, L.E.; Heiderscheit, B.C.; Williams, D.S.B.; Rauh, M.J. Factors Associated With Self-Selected Step Rate in High School Cross Country Runners. J. Strength Cond. Res. 2021, 35, 1141–1148. [Google Scholar] [CrossRef]
- Baxter-Jones, A. Growth and Maturation. In Oxford Textbook of Children’s Sport and Exercise Medicine; Armstrong, N., van Mechelen, W., Eds.; Oxford University Press: Oxford, UK, 2017; pp. 13–23. [Google Scholar]
- Handelsman, D.J. Sex Differences in Athletic Performance Emerge Coinciding with the Onset of Male Puberty. Clin. Endocrinol. 2017, 87, 68–72. [Google Scholar] [CrossRef]
- DiStefano, L.J.; Martinez, J.C.; Crowley, E.; Matteau, E.; Kerner, M.S.; Boling, M.C.; Nguyen, A.-D.; Trojian, T.H. Maturation and Sex Differences in Neuromuscular Characteristics of Youth Athletes. J. Strength Cond. Res. 2015, 29, 2465–2473. [Google Scholar] [CrossRef]
- Holden, S.; Doherty, C.; Boreham, C.; Delahunt, E. Sex Differences in Sagittal Plane Control Emerge during Adolescent Growth: A Prospective Investigation. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Bruening, D.A.; Baird, A.R.; Weaver, K.J.; Rasmussen, A.T. Whole Body Kinematic Sex Differences Persist across Non-Dimensional Gait Speeds. PLoS ONE 2020, 15, e0237449. [Google Scholar] [CrossRef]
- Ferber, R.; Davis, I.M.; Williams, D.S. Gender Differences in Lower Extremity Mechanics during Running. Clin. Biomech. (Bristol Avon) 2003, 18, 350–357. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Ogawa, H.; Shimizu, N.; Kanehisa, H.; Yanai, T.; Kawakami, Y. Gender Differences in Hip and Ankle Joint Kinematics on Knee Abduction during Running. Eur. J. Sport Sci. 2014, 14 (Suppl. S1), S302–S309. [Google Scholar] [CrossRef] [PubMed]
- Barber-Westin, S.D.; Noyes, F.R.; Galloway, M. Jump-Land Characteristics and Muscle Strength Development in Young Athletes: A Gender Comparison of 1140 Athletes 9 to 17 Years of Age. Am. J. Sports Med. 2006, 34, 375–384. [Google Scholar] [CrossRef]
- De Ste Croix, M.B.A.; Armstrong, N.; Welsman, J.R.; Sharpe, P. Longitudinal Changes in Isokinetic Leg Strength in 10–14-Year-Olds. Ann. Hum. Biol. 2002, 29, 50–62. [Google Scholar] [CrossRef]
- Grimston, S.K.; Nigg, B.M.; Hanley, D.A.; Engsberg, J.R. Differences in Ankle Joint Complex Range of Motion as a Function of Age. Foot Ankle 1993, 14, 215–222. [Google Scholar] [CrossRef]
- Latorre Román, P.Á.; Balboa, F.R.; Pinillos, F.G. Foot Strike Pattern in Children during Shod-Unshod Running. Gait Posture 2017, 58, 220–222. [Google Scholar] [CrossRef]
- Lieberman, D.E.; Castillo, E.R.; Otarola-Castillo, E.; Sang, M.K.; Sigei, T.K.; Ojiambo, R.; Okutoyi, P.; Pitsiladis, Y. Variation in Foot Strike Patterns among Habitually Barefoot and Shod Runners in Kenya. PLoS ONE 2015, 10, e0131354. [Google Scholar] [CrossRef]
- Meyers, R.W.; Oliver, J.L.; Hughes, M.G.; Lloyd, R.S.; Cronin, J.B. The Influence of Maturation on Sprint Performance in Boys over a 21-Month Period. Med. Sci. Sports Exerc. 2016, 48, 2555–2562. [Google Scholar] [CrossRef]
- Rubinstein, M.; Eliakim, A.; Steinberg, N.; Nemet, D.; Ayalon, M.; Zeev, A.; Pantanowitz, M.; Brosh, T. Biomechanical Characteristics of Overweight and Obese Children during Five Different Walking and Running Velocities. Footwear Sci. 2017, 9, 149–159. [Google Scholar] [CrossRef]
- Mesquita, P.R.; Neri, S.G.R.; Lima, R.M.; Carpes, F.P.; de David, A.C. Childhood Obesity Is Associated with Altered Plantar Pressure Distribution during Running. Gait Posture 2018, 62, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, N.; Rubinstein, M.; Nemet, D.; Ayalon, M.; Zeev, A.; Pantanowitz, M.; Brosh, T.; Eliakim, A. Effects of a Program for Improving Biomechanical Characteristics during Walking and Running in Children Who Are Obese. Pediatr Phys. Ther. 2017, 29, 330–340. [Google Scholar] [CrossRef]
- Steinberg, N.; Nemet, D.; Pantanowitz, M.; Eliakim, A. Gait Pattern, Impact to the Skeleton and Postural Balance in Overweight and Obese Children: A Review. Sports 2018, 6, 75. [Google Scholar] [CrossRef]
- Rumpf, M.C.; Cronin, J.B.; Oliver, J.; Hughes, M. Kinematics and Kinetics of Maximum Running Speed in Youth Across Maturity. Pediatr. Exerc. Sci. 2015, 27, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Beunen, G.; Malina, R.M. Growth and Physical Performance Relative to the Timing of the Adolescent Spurt. Exerc. Sport Sci. Rev. 1988, 16, 503–540. [Google Scholar] [CrossRef]
- John, C.; Rahlf, A.L.; Hamacher, D.; Zech, A. Influence of Biological Maturity on Static and Dynamic Postural Control among Male Youth Soccer Players. Gait Posture 2019, 68, 18–22. [Google Scholar] [CrossRef]
- Allen, D.J.; Heisler, H.; Mooney, J.; Kring, R. The Effect of Step Rate Manipulation on Foot Strike Pattern of Long Distance Runners. Int. J. Sports Phys. Ther. 2016, 11, 54–63. [Google Scholar]
- Garcia, M.C.; Taylor-Haas, J.A.; Ford, K.R.; Long, J.T. Assessment of Waveform Similarity in Youth Long-Distance Runners. Gait Posture 2020, 77, 105–111. [Google Scholar] [CrossRef]
- Wild, C.Y.; Steele, J.R.; Munro, B.J. Insufficient Hamstring Strength Compromises Landing Technique in Adolescent Girls. Med. Sci. Sports Exerc. 2013, 45, 497–505. [Google Scholar] [CrossRef]
- Quatman-Yates, C.C.; Quatman, C.E.; Meszaros, A.J.; Paterno, M.V.; Hewett, T.E. A Systematic Review of Sensorimotor Function during Adolescence: A Developmental Stage of Increased Motor Awkwardness? Br. J. Sports Med. 2012, 46, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, I.M.; Hollander, K.; Brockmann, B.; Hamacher, D.; Sehner, S.; Stücker, R.; Zech, A.; Babin, K. P 060—The Impact of Postural Weakness on Running Kinematics in Healthy Children—Results of the Barefoot LIFE-Study. Gait Posture 2018, 65, 331–332. [Google Scholar] [CrossRef]
- Verbecque, E.; Johnson, C.; Rameckers, E.; Thijs, A.; van der Veer, I.; Meyns, P.; Smits-Engelsman, B.; Klingels, K. Balance Control in Individuals with Developmental Coordination Disorder: A Systematic Review and Meta-Analysis. Gait Posture 2021, 83, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Marro, T.; Paolucci, S.; Morelli, D. Stability and Harmony of Gait in Children with Cerebral Palsy. Res. Dev. Disabil. 2012, 33, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Piitulainen, H.; Kulmala, J.-P.; Mäenpää, H.; Rantalainen, T. The Gait Is Less Stable in Children with Cerebral Palsy in Normal and Dual-Task Gait Compared to Typically Developed Peers. J. Biomech. 2021, 117, 110244. [Google Scholar] [CrossRef]
- Deschamps, K.; Staes, F.; Peerlinck, K.; Van Geet, K.; Hermans, C.; Lobet, S. Postural Control of Typical Developing Boys during the Transition from Double-Leg Stance to Single-Leg Stance. Eur. J. Pediatr. 2017, 176, 273–278. [Google Scholar] [CrossRef]
- Cherng, R.-J.; Lee, H.-Y.; Su, F.-C. Frequency Spectral Characteristics of Standing Balance in Children and Young Adults. Med. Eng. Phys. 2003, 25, 509–515. [Google Scholar] [CrossRef]
- Ford, K.R.; Shapiro, R.; Myer, G.D.; Van Den Bogert, A.J.; Hewett, T.E. Longitudinal Sex Differences during Landing in Knee Abduction in Young Athletes. Med. Sci. Sports Exerc. 2010, 42, 1923–1931. [Google Scholar] [CrossRef]
- Hewett, T.E.; Myer, G.D.; Ford, K.R. Decrease in Neuromuscular Control about the Knee with Maturation in Female Athletes. J. Bone Jt. Surg. Am. 2004, 86, 1601–1608. [Google Scholar] [CrossRef]
- Groslambert, A.; Mahon, A.D. Perceived Exertion: Influence of Age and Cognitive Development. Sports Med. 2006, 36, 911–928. [Google Scholar] [CrossRef]
- Kasai, D.; Parfitt, G.; Tarca, B.; Eston, R.; Tsiros, M.D. The Use of Ratings of Perceived Exertion in Children and Adolescents: A Scoping Review. Sports Med. 2021, 51, 33–50. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Aaron, D.J.; Utter, A.C.; Nagle, E. Omni Scale Rating of Perceived Exertion at Ventilatory Breakpoint by Direct Observation of Children’s Kinematics. Percept Mot. Skills 2007, 104, 975–984. [Google Scholar] [CrossRef]
- Kung, S.M.; Fink, P.W.; Legg, S.J.; Ali, A.; Shultz, S.P. Age-Related Differences in Perceived Exertion While Walking and Running Near the Preferred Transition Speed. Pediatr. Exerc. Sci. 2020, 32, 227–232. [Google Scholar] [CrossRef]
- Brown, T.; Moran, M. Pediatric Sports-Related Injuries. Clin. Pediatr. 2019, 58, 199–212. [Google Scholar] [CrossRef]
- Emery, C.A. Injury Prevention and Future Research. Med. Sport Sci. 2005, 49, 170–191. [Google Scholar] [CrossRef]
- McSweeney, S.C.; Grävare Silbernagel, K.; Gruber, A.H.; Heiderscheit, B.C.; Krabak, B.J.; Rauh, M.J.; Tenforde, A.S.; Wearing, S.C.; Zech, A.; Hollander, K. Adolescent Running Biomechanics—Implications for Injury Prevention and Rehabilitation. Front. Sports Act. Living 2021, 3, 689846. [Google Scholar] [CrossRef]
- Paterno, M.V.; Taylor-Haas, J.A.; Myer, G.D.; Hewett, T.E. Prevention of Overuse Sports Injuries in the Young Athlete. Orthop. Clin. N. Am. 2013, 44, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Lachniet, P.B.; Taylor-Haas, J.A.; Paterno, M.V.; DiCesare, C.A.; Ford, K.R. Altered sagittal plane hip biomechanics in adolescent male distance runners with a history of lower extremity injury. Int. J. Sports Phys. Ther. 2018, 13, 441–452. [Google Scholar] [CrossRef]
- Hartmann, M.; Kreuzpointner, F.; Haefner, R.; Michels, H.; Schwirtz, A.; Haas, J.P. Effects of Juvenile Idiopathic Arthritis on Kinematics and Kinetics of the Lower Extremities Call for Consequences in Physical Activities Recommendations. Int. J. Pediatr. 2010, 2010, 835984. [Google Scholar] [CrossRef] [PubMed]
- Kuntze, G.; Nesbitt, C.; Nettel-Aguirre, A.; Esau, S.; Scholz, R.; Brooks, J.; Twilt, M.; Toomey, C.; Mosher, D.; Ronsky, J.L.; et al. Gait Adaptations in Youth with Juvenile Idiopathic Arthritis. Arthritis Care Res. 2020, 72, 917–924. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, S.; Reed, L.F.; Wearing, S.C. Vertical Ground Reaction Forces during Gait in Children with and without Calcaneal Apophysitis. Gait Posture 2019, 71, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Wellenkotter, J.; Kernozek, T.W.; Meardon, S.; Suchomel, T. The Effects of Running Cadence Manipulation on Plantar Loading in Healthy Runners. Int. J. Sports Med. 2014, 35, 779–784. [Google Scholar] [CrossRef]
- El Tumi, H.; Johnson, M.I.; Dantas, P.B.F.; Maynard, M.J.; Tashani, O.A. Age-Related Changes in Pain Sensitivity in Healthy Humans: A Systematic Review with Meta-Analysis. Eur. J. Pain 2017, 21, 955–964. [Google Scholar] [CrossRef]
- Pontzer, H.; Suchman, K.; Raichlen, D.A.; Wood, B.M.; Mabulla, A.Z.P.; Marlowe, F.W. Foot Strike Patterns and Hind Limb Joint Angles during Running in Hadza Hunter-Gatherers. J. Sport Health Sci. 2014, 3, 95–101. [Google Scholar] [CrossRef]
- Bassett, D.R.; John, D.; Conger, S.A.; Fitzhugh, E.C.; Coe, D.P. Trends in Physical Activity and Sedentary Behaviors of United States Youth. J. Phys. Act. Health 2015, 12, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, N.H.; Steptoe, A.; Boniface, D.R.; Wardle, J. Trends in Physical Activity and Sedentary Behaviour in Adolescence: Ethnic and Socioeconomic Differences. Br. J. Sports Med. 2007, 41, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, O.; Goldberg, B. Trainability of the Prepubescent Child. Phys. Sportsmed. 1989, 17, 64–82. [Google Scholar] [CrossRef]
- Matos, N.; Winsley, R.J. Trainability of Young Athletes and Overtraining. J. Sports Sci. Med. 2007, 6, 353–367. [Google Scholar]
- Shephard, R.J. Effectiveness of Training Programmes for Prepubescent Children. Sports Med. 1992, 13, 194–213. [Google Scholar] [CrossRef]
- Rumpf, M.C.; Cronin, J.B.; Mohamad, I.N.; Mohamad, S.; Oliver, J.L.; Hughes, M.G. The Effect of Resisted Sprint Training on Maximum Sprint Kinetics and Kinematics in Youth. Eur. J. Sport Sci. 2015, 15, 374–381. [Google Scholar] [CrossRef]
- Sheerin, K.R.; Hume, P.A.; Whatman, C. Effects of a Lower Limb Functional Exercise Programme Aimed at Minimising Knee Valgus Angle on Running Kinematics in Youth Athletes. Phys. Ther. Sport 2012, 13, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Petray, C.K.; Krahenbuhl, G.S. Running Training, Instruction on Running Technique, and Running Economy in 10-Year-Old Males. Res. Q. Exerc. Sport 1985, 56, 251–255. [Google Scholar] [CrossRef]
- Clermont, C.A.; Phinyomark, A.; Osis, S.T.; Ferber, R. Classification of Higher- and Lower-Mileage Runners Based on Running Kinematics. J. Sport Health Sci. 2019, 8, 249–257. [Google Scholar] [CrossRef]
- Wegener, C.; Hunt, A.E.; Vanwanseele, B.; Burns, J.; Smith, R.M. Effect of Children’s Shoes on Gait: A Systematic Review and Meta-Analysis. J. Foot Ankle Res. 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Hollander, K.; Riebe, D.; Campe, S.; Braumann, K.-M.; Zech, A. Effects of Footwear on Treadmill Running Biomechanics in Preadolescent Children. Gait Posture 2014, 40, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Mullen, S.; Toby, E.B. Adolescent Runners: The Effect of Training Shoes on Running Kinematics. J. Pediatr. Orthop. 2013, 33, 453–457. [Google Scholar] [CrossRef]
- Hollander, K.; Stebbins, J.; Albertsen, I.M.; Hamacher, D.; Babin, K.; Hacke, C.; Zech, A. Arch Index and Running Biomechanics in Children Aged 10–14 Years. Gait Posture 2018, 61, 210–214. [Google Scholar] [CrossRef]
- Shih, Y.; Lin, K.-L.; Shiang, T.-Y. Is the Foot Striking Pattern More Important than Barefoot or Shod Conditions in Running? Gait Posture 2013, 38, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.H.; Silvernail, J.F.; Brueggemann, P.; Rohr, E.; Hamill, J. Footfall Patterns during Barefoot Running on Harder and Softer Surfaces. Footwear Sci. 2013, 5, 39–44. [Google Scholar] [CrossRef]
- Kerdok, A.E.; Biewener, A.A.; McMahon, T.A.; Weyand, P.G.; Herr, H.M. Energetics and Mechanics of Human Running on Surfaces of Different Stiffnesses. J. Appl. Physiol. 2002, 92, 469–478. [Google Scholar] [CrossRef]
- Riley, P.O.; Dicharry, J.; Franz, J.; Della Croce, U.; Wilder, R.P.; Kerrigan, D.C. A Kinematics and Kinetic Comparison of Overground and Treadmill Running. Med. Sci. Sports Exerc. 2008, 40, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Van Hooren, B.; Fuller, J.T.; Buckley, J.D.; Miller, J.R.; Sewell, K.; Rao, G.; Barton, C.; Bishop, C.; Willy, R.W. Is Motorized Treadmill Running Biomechanically Comparable to Overground Running? A Systematic Review and Meta-Analysis of Cross-Over Studies. Sports Med. 2020, 50, 785–813. [Google Scholar] [CrossRef]
- Rozumalski, A.; Novacheck, T.F.; Griffith, C.J.; Walt, K.; Schwartz, M.H. Treadmill vs. Overground Running Gait during Childhood: A Qualitative and Quantitative Analysis. Gait Posture 2015, 41, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Vernillo, G.; Giandolini, M.; Edwards, W.B.; Morin, J.-B.; Samozino, P.; Horvais, N.; Millet, G.Y. Biomechanics and Physiology of Uphill and Downhill Running. Sports Med. 2017, 47, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef]
- Bontemps, B.; Piponnier, E.; Chalchat, E.; Blazevich, A.J.; Julian, V.; Bocock, O.; Duclos, M.; Martin, V.; Ratel, S. Children Exhibit a More Comparable Neuromuscular Fatigue Profile to Endurance Athletes Than Untrained Adults. Front. Physiol. 2019, 10, 119. [Google Scholar] [CrossRef]
- Patikas, D.A.; Williams, C.A.; Ratel, S. Exercise-Induced Fatigue in Young People: Advances and Future Perspectives. Eur. J. Appl. Physiol. 2018, 118, 899–910. [Google Scholar] [CrossRef]
- Piponnier, E.; Martin, V.; Bontemps, B.; Chalchat, E.; Julian, V.; Bocock, O.; Duclos, M.; Ratel, S. Child-Adult Differences in Neuromuscular Fatigue Are Muscle-Dependent. J. Appl. Physiol. 2018, 125, 1246–1256. [Google Scholar] [CrossRef]
- Piponnier, E.; Martin, V.; Bourdier, P.; Biancarelli, B.; Kluka, V.; Garcia-Vicencio, S.; Jegu, A.-G.; Cardenoux, C.; Morio, C.; Coudeyre, E.; et al. Maturation-Related Changes in the Development and Etiology of Neuromuscular Fatigue. Eur. J. Appl. Physiol. 2019, 119, 2545–2555. [Google Scholar] [CrossRef]
- Ratel, S.; Martin, V. Is There a Progressive Withdrawal of Physiological Protections against High-Intensity Exercise-Induced Fatigue during Puberty? Sports 2015, 3, 346–357. [Google Scholar] [CrossRef]
- Ratel, S.; Williams, C.A.; Oliver, J.; Armstrong, N. Effects of Age and Recovery Duration on Performance during Multiple Treadmill Sprints. Int. J. Sports Med. 2006, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- García-Pinillos, F.; Molina-Molina, A.; Párraga-Montilla, J.A.; Latorre-Román, P.A. Kinematic Alterations after Two High-Intensity Intermittent Training Protocols in Endurance Runners. J. Sport Health Sci. 2019, 8, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A. (Ed.) Human Muscle Fatigue; 1. Publ.; Routledge: London, UK, 2009; ISBN 978-0-203-88548-2. [Google Scholar]
- Martin, V.; Kerhervé, H.; Messonnier, L.A.; Banfi, J.-C.; Geyssant, A.; Bonnefoy, R.; Féasson, L.; Millet, G.Y. Central and Peripheral Contributions to Neuromuscular Fatigue Induced by a 24-h Treadmill Run. J. Appl. Physiol. 2010, 108, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Goodall, S.; Stone, M.; Howatson, G.; Gibson, A.S.C.; Ansley, L. Central and Peripheral Fatigue in Male Cyclists after 4-, 20-, and 40-Km Time Trials. Med. Sci. Sport. Exerc. 2015, 47, 537. [Google Scholar] [CrossRef]
- Fourchet, F.; Girard, O.; Kelly, L.; Horobeanu, C.; Millet, G.P. Changes in Leg Spring Behaviour, Plantar Loading and Foot Mobility Magnitude Induced by an Exhaustive Treadmill Run in Adolescent Middle-Distance Runners. J. Sci. Med. Sport 2015, 18, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Fourchet, F.; Kelly, L.; Horobeanu, C.; Loepelt, H.; Taiar, R.; Millet, G. High-Intensity Running and Plantar-Flexor Fatigability and Plantar-Pressure Distribution in Adolescent Runners. J. Athl. Train. 2015, 50, 117–125. [Google Scholar] [CrossRef]
- Jewell, C.; Boyer, K.A.; Hamill, J. Do Footfall Patterns in Forefoot Runners Change over an Exhaustive Run? J. Sport. Sci. 2017, 35, 74–80. [Google Scholar] [CrossRef]
- Orendurff, M.S.; Kobayashi, T.; Tulchin-Francis, K.; Tullock, A.M.H.; Villarosa, C.; Chan, C.; Kraus, E.; Strike, S. A Little Bit Faster: Lower Extremity Joint Kinematics and Kinetics as Recreational Runners Achieve Faster Speeds. J. Biomech. 2018, 71, 167–175. [Google Scholar] [CrossRef]
- Schache, A.G.; Blanch, P.D.; Dorn, T.W.; Brown, N.A.T.; Rosemond, D.; Pandy, M.G. Effect of Running Speed on Lower Limb Joint Kinetics. Med. Sci. Sports Exerc. 2011, 43, 1260–1271. [Google Scholar] [CrossRef]
- Forrester, S.E.; Townend, J. The Effect of Running Velocity on Footstrike Angle--a Curve-Clustering Approach. Gait Posture 2015, 41, 26–32. [Google Scholar] [CrossRef]
- Miyamoto, A.; Takeshita, T.; Yanagiya, T. Differences in Sprinting Performance and Kinematics between Preadolescent Boys Who Are Fore/Mid and Rear Foot Strikers. PLoS ONE 2018, 13, e0205906. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudlow, A.; Galantine, P.; Vercruyssen, F.; Peyrot, N.; Raymond, J.-J.; Duché, P. Which Factors Influence Running Gait in Children and Adolescents? A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 4621. https://doi.org/10.3390/ijerph20054621
Sudlow A, Galantine P, Vercruyssen F, Peyrot N, Raymond J-J, Duché P. Which Factors Influence Running Gait in Children and Adolescents? A Narrative Review. International Journal of Environmental Research and Public Health. 2023; 20(5):4621. https://doi.org/10.3390/ijerph20054621
Chicago/Turabian StyleSudlow, Anthony, Paul Galantine, Fabrice Vercruyssen, Nicolas Peyrot, Jean-Jacques Raymond, and Pascale Duché. 2023. "Which Factors Influence Running Gait in Children and Adolescents? A Narrative Review" International Journal of Environmental Research and Public Health 20, no. 5: 4621. https://doi.org/10.3390/ijerph20054621
APA StyleSudlow, A., Galantine, P., Vercruyssen, F., Peyrot, N., Raymond, J.-J., & Duché, P. (2023). Which Factors Influence Running Gait in Children and Adolescents? A Narrative Review. International Journal of Environmental Research and Public Health, 20(5), 4621. https://doi.org/10.3390/ijerph20054621






