Challenges in Drug Surveillance: Strengthening the Analysis of New Psychoactive Substances by Harmonizing Drug Checking Services in Proficiency Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria for Inclusion
- Origin: Substances purchased from online (clear net) markets (or) received as ‘unknown’.
- Chemical and pharmacological features:
- ○
- All compounds belonging to the most prevalent categories of NPS reported to EMCDDA, i.e., cannabinoids, cathinones, opioids, tryptamines, phenethylamines and benzodiazepines [2].
- ○
2.2. Proficiency Testing
3. Results
3.1. Batch 1
3.2. Batch 2
3.3. Batch 3
4. Discussion
5. Conclusions
- (i)
- Each laboratory can extend its GC and/or LC library with retention data from characterized samples or reference standards to distinguish between stereo-isomers. Based on this, the screening methods should ‘evolve’ to separate structural isomers. As an alternative, laboratories can use reference standards or confirmed samples to create a UV library, to identify isomers with classic LC-DAD analysis or, taking it even further, invest in an LC-MS where a DAD is put in series before the MS detector.
- (ii)
- Since most participants make use of GC-MS as the first choice technique for the screening of NPS, a uniform standardized method could be proposed allowing the exchange of retention data for certain molecules. An ‘internal’ standard molecule could then be chosen in order to inject with all samples, allowing to work with relative retention times and correct for any retention shifts due to small technical differences (instrument, column age) and environmental influences.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Monitoring Centre for Drug and Drug Addiction. EMCDDA Operating Guidelines for the European Union Early Warning System on New Psychoactive Substances; Publications Office of the European Union: Luxembourg, 2019; p. 27. [Google Scholar]
- European Monitoring Centre for Drug and Drug Addiction. European Drug Report 2022: Trends and Developments; Publications Office of the European Union: Luxembourg, 2022; p. 60. Available online: https://www.emcdda.europa.eu/publications/edr/trends-developments/2022_en (accessed on 11 October 2022).
- Pirona, A.; Bo, A.; Hedrich, D.; Ferri, M.; van Gelder, N.; Giraudon, I.; Montanari, L.; Simon, R.; Mounteney, J. New psychoactive substances: Current health-related practices and challenges in responding to use and harms in Europe. Int. J. Drug Policy 2017, 40, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Tewari, A.; Rao, R. New Psychoactive Substances: Issues and Challenges. 2016. Available online: https://www.jmhhb.org/article.asp?issn=0971-8990 (accessed on 27 October 2022).
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.M.; Thomas, K.V.; Farrell, M.; Griffiths, P. New psychoactive substances: Challenges for drug surveillance, control, and public health responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [CrossRef]
- Mounteney, J.; Griffiths, P.; Sedefov, R.; Noor, A.; Vicente, J.; Simon, R. The drug situation in Europe: An overview of data available on illicit drugs and new psychoactive substances from European monitoring in 2015. Addiction 2016, 111, 34–48. [Google Scholar] [CrossRef]
- Sumnall, H.R.; Evans-Brown, M.; McVeigh, J. Social, policy, and public health perspectives on new psychoactive substances. Drug Test. Anal. 2011, 3, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Betzler, F.; Helbig, J.; Viohl, L.; Ernst, F.; Roediger, L.; Gutwinski, S.; Ströhle, A.; Köhler, S. Drug Checking and Its Potential Impact on Substance Use. Eur. Addict. Res. 2021, 27, 25–32. [Google Scholar] [CrossRef]
- Oomen, P.E.; Schori, D.; Tögel-Lins, K.; Acreman, D.; Chenorhokian, S.; Luf, A.; Karden, A.; Paulos, C.; Fornero, E.; Gerace, E.; et al. Cannabis adulterated with the synthetic cannabinoid receptor agonist MDMB-4en-PINACA and the role of European drug checking services. Int. J. Drug Policy 2022, 100, 103493. [Google Scholar] [CrossRef]
- Butterfield, R.J.; Barratt, M.J.; Ezard, N.; Day, R.O. Drug checking to improve monitoring of new psychoactive substances in Australia. Med. J. Aust. 2016, 204, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Campos-Mañas, M.C.; Van Wichelen, N.; Covaci, A.; van Nuijs, A.L.; Ort, C.; Béen, F.; Castiglioni, S.; Hernández, F.; Bijlsma, L. Analytical investigation of cannabis biomarkers in raw urban wastewater to refine consumption estimates. Water Res. 2022, 223, 119020. [Google Scholar] [CrossRef]
- Juntti-Patinen, L.; Neuvonen, P.J. Drug-related deaths in a university central hospital. Eur. J. Clin. Pharmacol. 2002, 58, 479–482. [Google Scholar]
- van Nuijs, A.L.; Castiglioni, S.; Tarcomnicu, I.; Postigo, C.; de Alda, M.L.; Neels, H.; Zuccato, E.; Barcelo, D.; Covaci, A. Illicit drug consumption estimations derived from wastewater analysis: A critical review. Sci. Total Environ. 2011, 409, 3564–3577. [Google Scholar] [CrossRef]
- Kolbe, V.; Rentsch, D.; Boy, D.; Schmidt, B.; Kegler, R.; Büttner, A. The adulterated XANAX pill: A fatal intoxication with etizolam and caffeine. Int. J. Leg. Med. 2020, 134, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Caudevilla, F.; Ventura, M.; Fornís, I.; Barratt, M.J.; Vidal, C.; Gil Lladanosa, C.; Quintana, P.; Muñoz, A.; Calzada, N. Results of an international drug testing service for cryptomarket users. Int. J. Drug Policy 2016, 35, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef] [PubMed]
- Bergh, M.S.S.; Øiestad, Å.M.L.; Baumann, M.H.; Bogen, I.L. Selectivity and sensitivity of urine fentanyl test strips to detect fentanyl analogues in illicit drugs. Int. J. Drug Policy 2021, 90, 103065. [Google Scholar] [CrossRef]
- Marshall, Z.; Dechman, M.K.; Minichiello, A.; Alcock, L.; Harris, G.E. Peering into the literature: A systematic review of the roles of people who inject drugs in harm reduction initiatives. Drug Alcohol. Depend. 2015, 151, 1–14. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drug and Drug Addiction (EMCDDA). EMCDDA: Health and Social Responses to Drug Problems: A European Guide; Publications Office of the European Union: Luxembourg, 2017; p. 188. [Google Scholar]
- Brunt, T.M.; Nagy, C.; Bücheli, A.; Martins, D.; Ugarte, M.; Beduwe, C.; Vilamala, M.V. Drug testing in Europe: Monitoring results of the Trans European Drug Information (TEDI) project. Drug Test Anal. 2017, 9, 188–198. [Google Scholar] [CrossRef]
- Vrolijk, R.Q.; Measham, F.; Quesada, A.; Luf, A.; Schori, D.; Radley, S.; Acreman, D.; Smith, J.; Verdenik, M.; Martins, D.; et al. Size matters: Comparing the MDMA content and weight of ecstasy tablets submitted to European drug checking services in 2012–2021. Drugs Habits Soc. Policy, 2022; ahead of printing. [Google Scholar] [CrossRef]
- Graziano, S.; Anzillotti, L.; Mannocchi, G.; Pichini, S.; Busardò, F.P. Screening methods for rapid determination of new psychoactive substances (NPS) in conventional and non-conventional biological matrices. J. Pharm. Biomed. Anal. 2019, 163, 170–179. [Google Scholar] [CrossRef]
- Vaiano, F.; Busardò, F.P.; Palumbo, D.; Kyriakou, C.; Fioravanti, A.; Catalani, V.; Mari, F.; Bertol, E. A novel screening method for 64 new psychoactive substances and 5 amphetamines in blood by LC-MS/MS and application to real cases. J. Pharm. Biomed Anal. 2016, 129, 441–449. [Google Scholar] [CrossRef] [PubMed]
- NPS Discovery. Recommended Scope for NPS Testing in the United States—Based on Trends Observed in Q1 2021. 2021. Available online: https://www.cfsre.org/nps-discovery/public-alerts/recommended-scope-for-nps-testing-in-the-united-states-q1-2021 (accessed on 18 October 2022).
- NPS Discovery. Recommended Scope for NPS Testing in the United States—Based on Trends Observed in Q2 2021. 2021. Available online: https://www.cfsre.org/images/scoperecommendations/Q2-2022-NPS-Scope-Recommendations-NPS-Discovery-072522.pdf (accessed on 18 October 2022).
- NPS Discovery. Recommended Scope for NPS Testing in the United States—Based on Trends Observed in Q3 2021. 2021. Available online: https://www.cfsre.org/images/scoperecommendations/Q3-2021-NPS-Scope-Recommendations-NPS-Discovery-110221.pdf (accessed on 18 October 2022).
- NPS Discovery. Recommended Scope for NPS Testing in the United States—Based on Trends Observed in Q4 2021. 2021. Available online: https://www.cfsre.org/images/scoperecommendations/Q4-2021-NPS-Scope-Recommendations-NPS-Discovery-021722.pdf (accessed on 18 October 2022).
- Deconinck, E.; Duchateau, C.; Balcaen, M.; Gremeaux, L.; Courselle, P. Chemometrics and infrared spectroscopy—A winning team for the analysis of illicit drug products. Rev. Anal. Chem. 2022, 41, 228–255. [Google Scholar] [CrossRef]
- Bumbrah, G.S.; Sharma, R.M. Raman spectroscopy—Basic principle, instrumentation and selected applications for the characterization of drugs of abuse. Egypt. J. Forensic. Sci. 2016, 6, 209–215. [Google Scholar] [CrossRef]
- Beaulieu, T.; Wood, E.; Tobias, S.; Lysyshyn, M.; Patel, P.; Matthews, J.; Ti, L. Is expected substance type associated with timing of drug checking service utilization? A cross-sectional study. Harm. Reduct. J. 2021, 18, 66. [Google Scholar] [CrossRef]
- Blanckaert, P.; Cannaert, A.; Van Uytfanghe, K.; Hulpia, F.; Deconinck, E.; Van Calenbergh, S.; Stove, C. Report on a novel emerging class of highly potent benzimidazole NPS opioids: Chemical and in vitro functional characterization of isotonitazene. Drug Test. Anal. 2020, 12, 422–430. [Google Scholar] [CrossRef]
- Blanckaert, P.; Balcaen, M.; Vanhee, C.; Risseeuw, M.; Canfyn, M.; Desmedt, B.; Van Calenbergh, S.; Deconinck, E. Analytical characterization of “etonitazepyne,” a new pyrrolidinyl-containing 2-benzylbenzimidazole opioid sold online. Drug Test. Anal. 2021, 13, 1627–1634. [Google Scholar] [CrossRef]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Blanckaert, P.; Dowling, G.; Grill, M.; Schwelm, H.M.; Auwärter, V.; Chapman, S.J. Separating the wheat from the chaff: Observations on the analysis of lysergamides LSD, MIPLA, and LAMPA. Drug Test. Anal. 2022, 14, 545–556. [Google Scholar] [CrossRef]
- Favretto, D.; Pascali, J.P.; Tagliaro, F. New challenges and innovation in forensic toxicology: Focus on the “New Psychoactive Substances”. J. Chromatogr. A 2013, 1287, 84–95. [Google Scholar] [CrossRef]
- Nordmeier, F.; Richter, L.H.J.; Schmidt, P.H.; Schaefer, N.; Meyer, M.R. Studies on the in vitro and in vivo metabolism of the synthetic opioids U-51754, U-47931E, and methoxyacetylfentanyl using hyphenated high-resolution mass spectrometry. Sci. Rep. 2019, 9, 13774. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.F.; Mohr, A.L.A.; Papsun, D.M.; Logan, B.K. Analysis of the Illicit Opioid U-48800 and Related Compounds by LC–MS-MS and Case Series of Fatalities Involving U-48800. J. Anal. Toxicol. 2022, 46, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.N.P.; Benedito, L.E.C.; de Souza, M.P.; Maldaner, A.O.; de Oliveira, A.L. Quantitative NMR as a tool for analysis of new psychoactive substances. Forensic Chem. 2020, 21, 100282. [Google Scholar] [CrossRef]
- Castaing-Cordier, T.; Ladroue, V.; Besacier, F.; Bulete, A.; Jacquemin, D.; Giraudeau, P.; Farjon, J. High-field and benchtop NMR spectroscopy for the characterization of new psychoactive substances. Forensic Sci. Int. 2021, 321, 110718. [Google Scholar] [CrossRef] [PubMed]
- Lobo Vicente, J.; Chassaigne, H.; Holland, M.V.; Reniero, F.; Kolář, K.; Tirendi, S.; Vandecasteele, L.; Vinckier, I.; Guillou, C. Systematic analytical characterization of new psychoactive substances: A case study. Forensic Sci. Int. 2016, 265, 107–115. [Google Scholar] [CrossRef]
- Quintas, A.; Ferreira, C.; Santiago, R.; Ribeiro, A.C.; Martins, D.; Cunha, M.; Dias, M. Utopioid comprehensive structural characterization combining MS, NMR and bioinformatic tools: A new strategy. Forensic Sci. Int. Submitted.
- Giorgetti, A.; Mogler, L.; Halter, S.; Haschimi, B.; Alt, A.; Rentsch, D.; Schmidt, B.; Thoma, V.; Vogt, S.; Auwärter, V. Four cases of death involving the novel synthetic cannabinoid 5F-Cumyl-PEGACLONE. Forensic Toxicol. 2020, 38, 314–326. [Google Scholar] [CrossRef]
- Angerer, V.; Mogler, L.; Steitz, J.-P.; Bisel, P.; Hess, C.; Schoeder, C.T.; Müller, C.E.; Huppertz, L.M.; Westphal, F.; Schäper, J.; et al. Structural characterization and pharmacological evaluation of the new synthetic cannabinoid CUMYL-PEGACLONE. Drug Test Anal. 2018, 10, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Tocco, G.; Papsun, D.M.; Mohr, A.L.; Fogarty, M.F.; Krotulski, A.J. U-47700 and Its Analogs: Non-Fentanyl Synthetic Opioids Impacting the Recreational Drug Market. Brain Sci. 2020, 10, 895. [Google Scholar] [CrossRef]
- United Nations. Convention on Psychotropic Substances 1971, Including Final Act and Resolutions, as Agreed by the 1971 United Nations Conference for the Adoption of a Protocol on Psychotropic Substances, and the Schedules Annexed to the Convention; United Nations: New York, NY, USA, 1971. [Google Scholar]
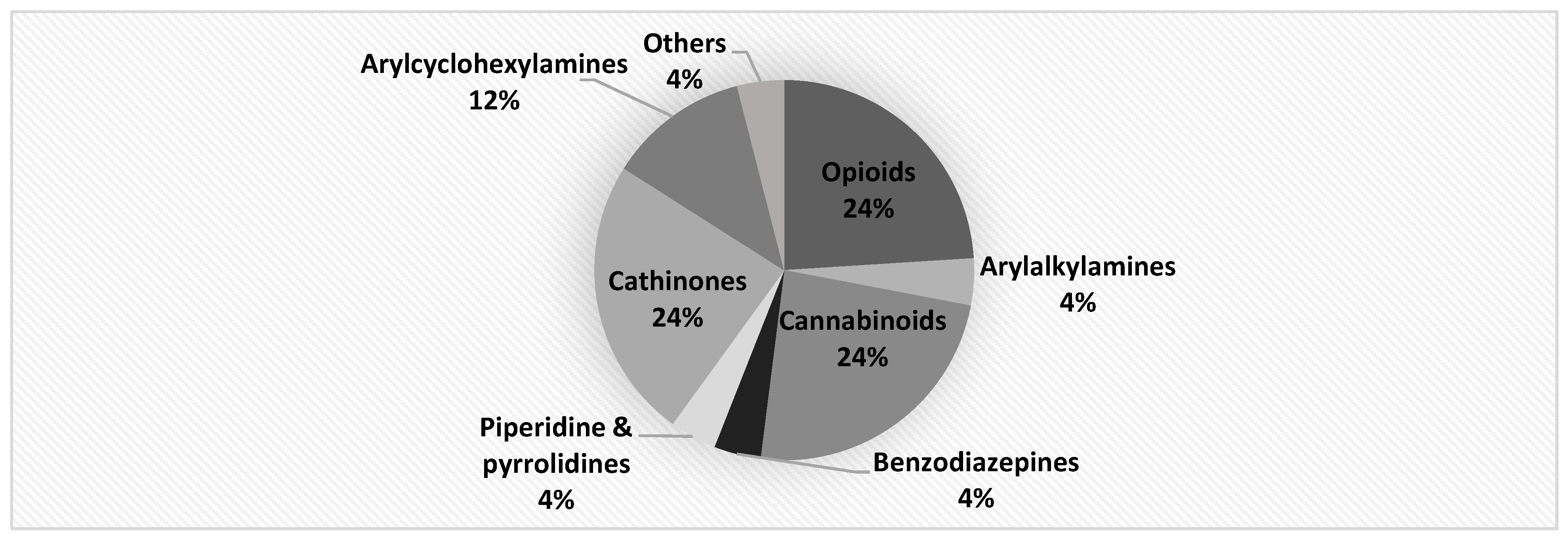
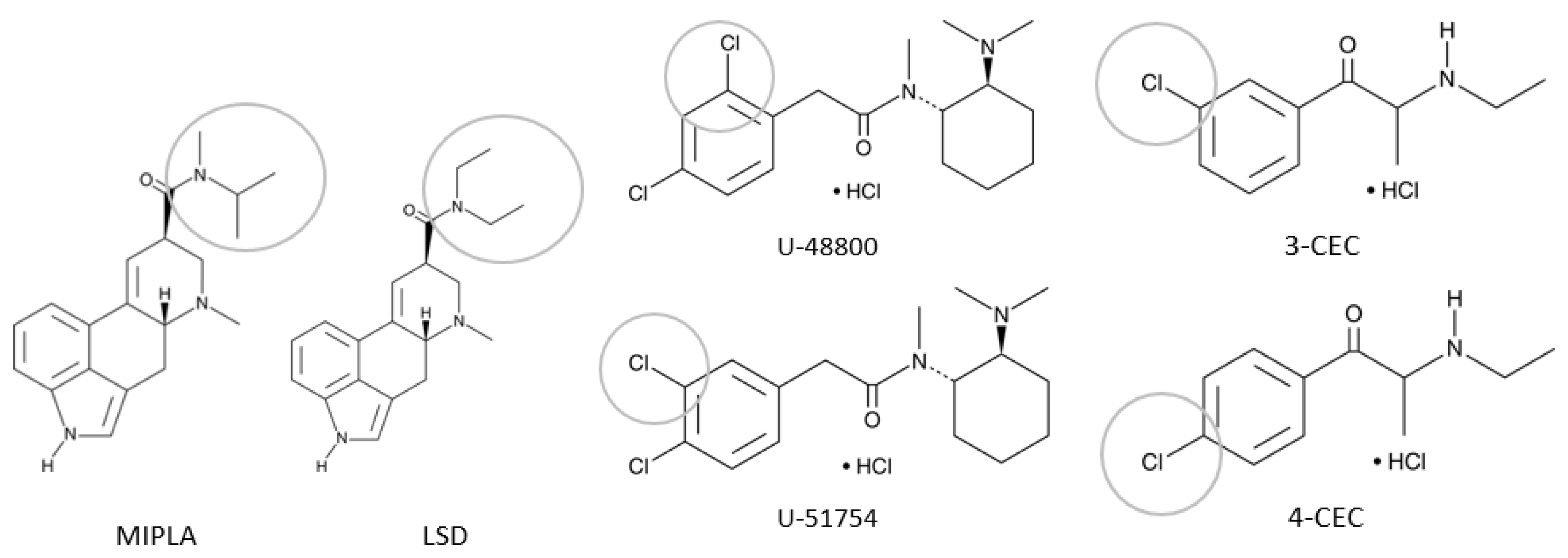
| Laboratory | Technique |
|---|---|
| L1 | GC-MS LC-MS * |
| L2 | GC-MS FTIR * |
| L3 | LC-MS MALDI-HR-MS FTIR * LC-DAD ° |
| L4 | GC-MS |
| Bought/Received as: | L1 | L2 | L3 | L4 | |
|---|---|---|---|---|---|
| Batch 1 | |||||
| 1A | BOH-2C-B | BOH-2C-B | BOH-2C-B | BOH-2C-B * | BOH-2C-B |
| 1B | CUMYL-PeGACLONE (or SGT-151) | Furanyl UF-17 | Furanyl UF-17 | Furanyl UF-17 * | U-47109 |
| Batch 2 | |||||
| 2A | CUMYL-5F-P7AICA (or SGT-263) | 5C-AKB48 * | 5C-AKB48 CUMYL-4CN-BINACA | 5C-AKB48 *° | 5C-AKB48 CUMYL-4CN-BINACA |
| 2B | 4F-MDMB-BINACA (or 4F-ADB) | 4F-MDMB-BINACA * | 4F-MDMB-BINACA | 4F-MDMB-BINACA *° | 4F-MDMB-BINACA |
| 2C | -MIPLA | MIPLA * | LSD * | MIPLA *° | MIPLA |
| 2D | Brorphine | Brorphine | Brorphine | Brorphine *° | Brorphine |
| 2E | Butonitazene | Butonitazene | Isotonitazene | Butonitazene * | Butonitazene |
| 2F | Etonitazepyne | Etonitazepyne * | Unknown | Etonitazepyne * | Etonitazepyne |
| Batch 3 | |||||
| 3A | 5-Cl-ADB-A (or MDMB-4-en-PINACA) | MDMB-4-en-PINACA * 4F-MDMB-BINACA * | MDMB-4-en-PINACA * 4F-MDMB-BINACA * | MDMB-4-en-PINACA *° 4F-MDMB-BINACA *° | MDMB-4-en-PINACA |
| 3B | Flualprazolam | Flualprazolam | Flualprazolam * | Flualprazolam *° | Flualprazolam |
| 3C | Isopropylphenidate | Isopropylphenidate | Isopropylphenidate * | Isopropylphenidate *° | Isopropylphenidate |
| 3D | Unknown | 4-CEC 4-Cl-α-PVP 4-chloropentedrone 4-CMC | 4-CEC * 4-Cl-α-PVP * 4-chloropentedrone * | 4-CEC ° 4-Cl-α-PVP ° 4-chloropentedrone ° 4-CMC ° | x-CEC 4-Cl-α-PVP 4-chloropentedrone x-CMC |
| 3E | Unknown | U-48800 or U-51754 * | U-48800 * | U-48800 *° | U-48800 or U-51754 |
| 3F | Unknown | 4-CEC | 4-CEC * | 4-CEC *° | x-CEC |
| 3G | Unknown | Bk-DMBDB | Bk-DMBDB * | Bk-DMBDB *° | Bk-DMBDB |
| 3H | BC-66 | 4F-MDMB-BICA | 4F-MDMB-BICA * | 4F-MDMB-BICA *° | 4F-MDMB-BICA |
| 3I | 3F-PCP | 3F-PCP | 3F-PCP * | 3F-PCP *° | 3F-PCP |
| 3J | Fluonitazene | Fluonitazene * | Fluonitazene * | Fluonitazene *° | Fluonitazene |
| 3K | Hydroxetamine | Hydroxetamine * | Unknown * | Hydroxetamine *° | Hydroxetamine |
| 3L | Deoxymethoxetamine | Deoxymethoxetamine * | Unknown * | Deoxymethoxetamine *° | Deoxymethoxetamine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcaen, M.; Ventura, M.; Gil, C.; Luf, A.; Martins, D.; Cunha, M.; Tögel-Lins, K.; Wolf, D.; Blanckaert, P.; Deconinck, E. Challenges in Drug Surveillance: Strengthening the Analysis of New Psychoactive Substances by Harmonizing Drug Checking Services in Proficiency Testing. Int. J. Environ. Res. Public Health 2023, 20, 4628. https://doi.org/10.3390/ijerph20054628
Balcaen M, Ventura M, Gil C, Luf A, Martins D, Cunha M, Tögel-Lins K, Wolf D, Blanckaert P, Deconinck E. Challenges in Drug Surveillance: Strengthening the Analysis of New Psychoactive Substances by Harmonizing Drug Checking Services in Proficiency Testing. International Journal of Environmental Research and Public Health. 2023; 20(5):4628. https://doi.org/10.3390/ijerph20054628
Chicago/Turabian StyleBalcaen, Margot, Mireia Ventura, Cristina Gil, Anton Luf, Daniel Martins, Mar Cunha, Karsten Tögel-Lins, Danny Wolf, Peter Blanckaert, and Eric Deconinck. 2023. "Challenges in Drug Surveillance: Strengthening the Analysis of New Psychoactive Substances by Harmonizing Drug Checking Services in Proficiency Testing" International Journal of Environmental Research and Public Health 20, no. 5: 4628. https://doi.org/10.3390/ijerph20054628
APA StyleBalcaen, M., Ventura, M., Gil, C., Luf, A., Martins, D., Cunha, M., Tögel-Lins, K., Wolf, D., Blanckaert, P., & Deconinck, E. (2023). Challenges in Drug Surveillance: Strengthening the Analysis of New Psychoactive Substances by Harmonizing Drug Checking Services in Proficiency Testing. International Journal of Environmental Research and Public Health, 20(5), 4628. https://doi.org/10.3390/ijerph20054628






