Antibacterial Activity of Epigallocatechin Gallate (EGCG) against Shigella flexneri
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strain and Antimicrobial Agents
2.2. Detection of Antimicrobial Activity in Planktonic Conditions
2.2.1. The Minimum Inhibitory Concentration Determination
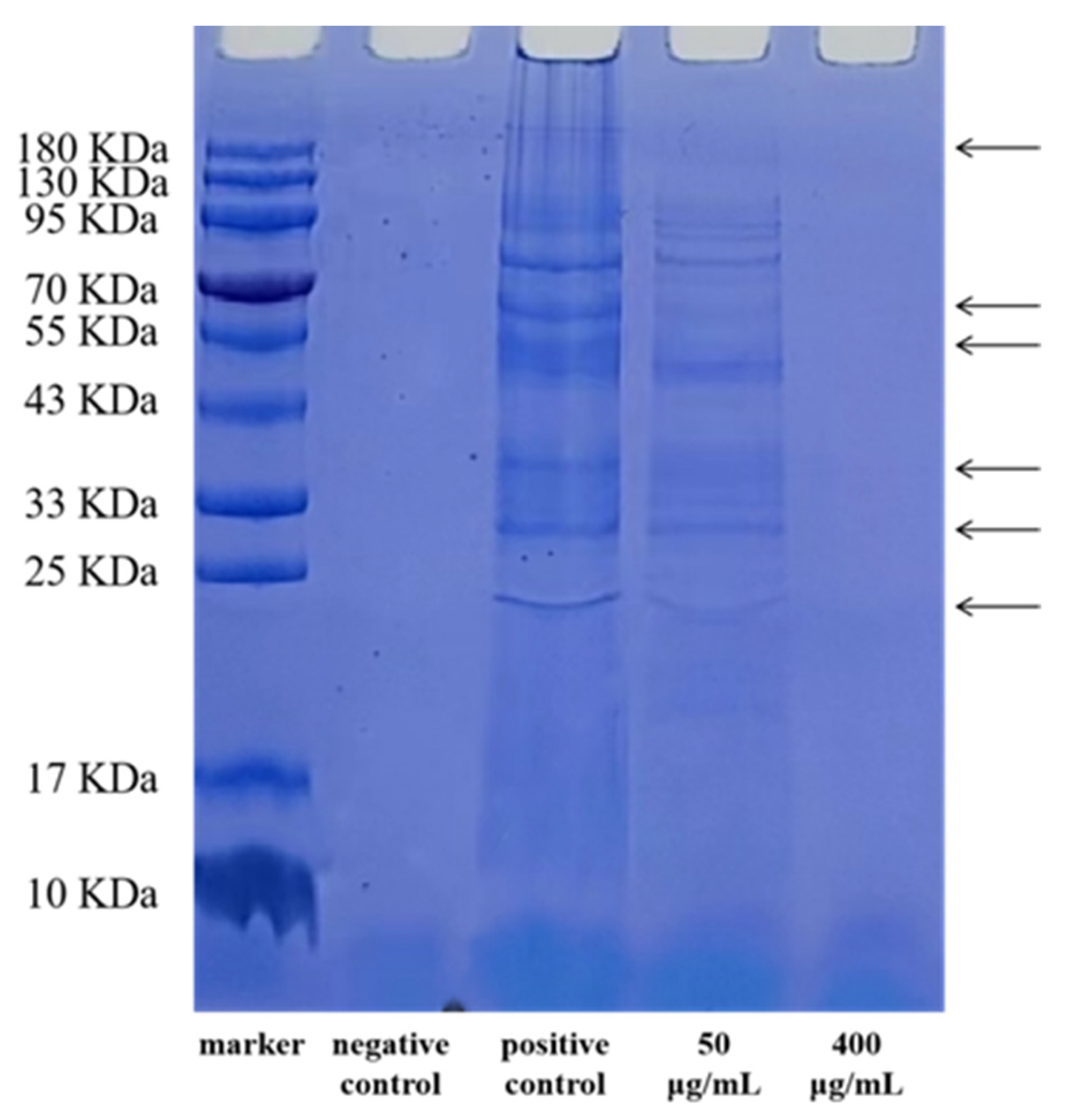
2.2.2. SDS-PAGE of Bacterial Proteins
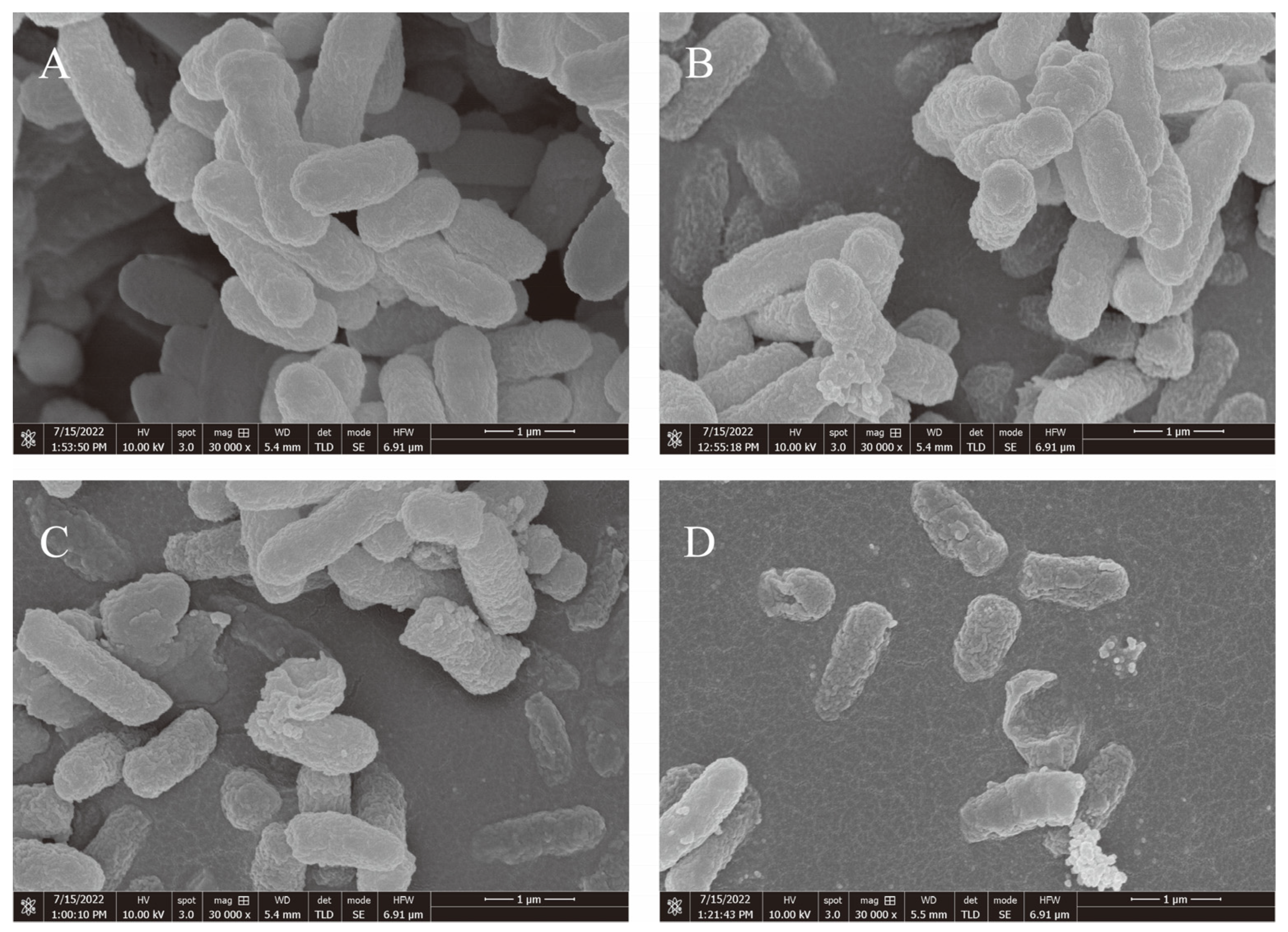
2.2.3. Analysis Using a Field Emission Scanning Electron Microscope (FESEM)
2.3. Detection of EGCG’s Impact on the Development of Biofilms
2.3.1. Quantitative Evaluation of S. flexneri Crystal Violet Biofilm Development
2.3.2. Determination of Viable Bacteria Inside and Outside S. flexneri Biofilm
2.3.3. RNA Extraction and Quantitative RT-PCR Evaluation
2.4. Antibacterial Mechanism
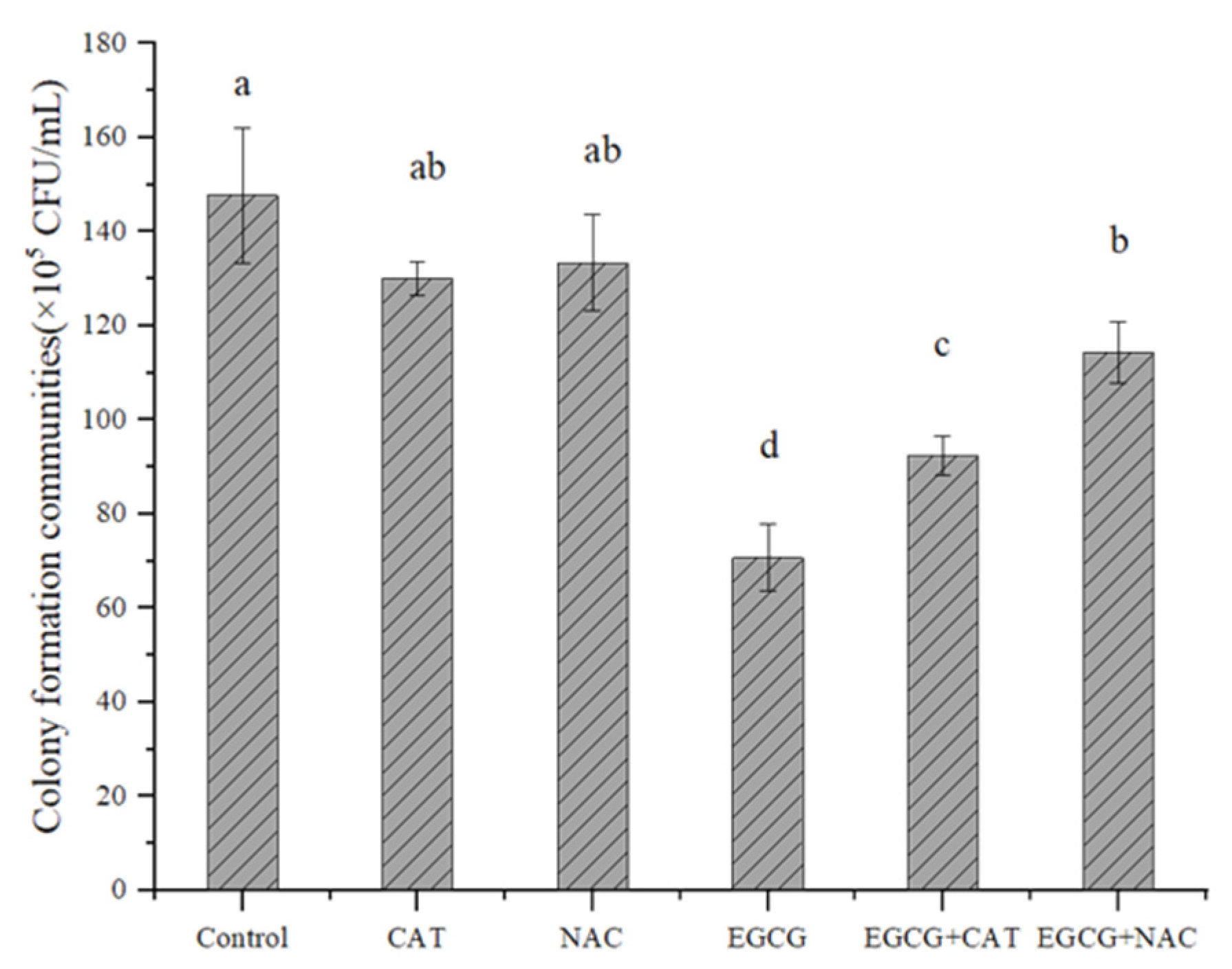
2.4.1. Determination of the Effect of Antioxidants on EGCG
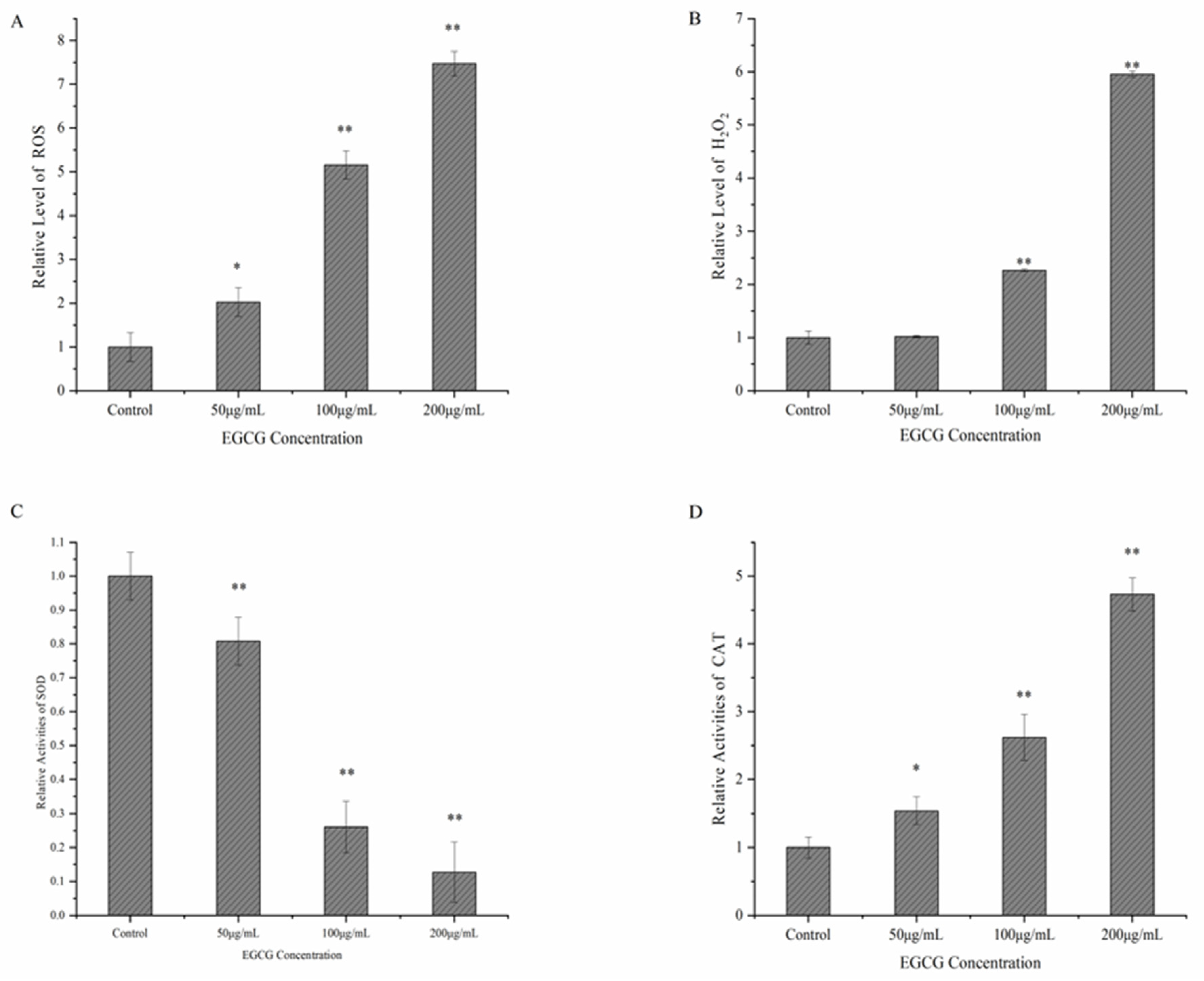
2.4.2. Determination of ROS, H2O2, SOD, and CAT
2.5. Analytical Statistics
3. Results
3.1. The Antibacterial Activity of EGCG against a Strain of S. flexneri
3.2. EGCG Inhibits the Formation of Biofilms
3.3. Possible Mechanism of EGCG against S. flexneri
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Koestler, B.J.; Ward, C.M.; Payne, S.M. Shigella Pathogenesis Modeling with Tissue Culture Assays. Curr. Protoc. Microbiol. 2018, 50, e57. [Google Scholar] [CrossRef]
- Li, Y.; Cao, B.; Liu, B.; Liu, D.; Gao, Q.; Peng, X.; Wu, J.; Bastin, D.A.; Feng, L.; Wang, L. Molecular detection of all 34 distinct O-antigen forms of Shigella. J. Med. Microbiol. 2009, 58, 69–81. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Yang, C.; Liang, B.; Ma, Q.; Yi, S.; Li, H.; Liu, H.; Li, P.; Wu, Z.; et al. Prevalence and antimicrobial resistance of Shigella flexneri serotype 2 variant in China. Front. Microbiol. 2015, 6, 435. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Boerma, T.; Fat, D.M. Global and regional causes of death. Br. Med. Bull. 2009, 92, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella Isolates From the Global Enteric Multicenter Study Inform Vaccine Development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Köhler, H.; Rodrigues, S.P.; McCormick, B.A. Shigella flexneri Interactions with the Basolateral Membrane Domain of Polarized Model Intestinal Epithelium: Role of Lipopolysaccharide in Cell Invasion and in Activation of the Mitogen-Activated Protein Kinase ERK. Infect. Immun. 2002, 70, 1150–1158. [Google Scholar] [CrossRef]
- Luo, W.; Dai, W.; Zhang, X.; Zheng, L.; Zhao, J.; Xie, X.; Xu, Y. Effects of Shigella flexneri exposure on development of Xenopus Tropicals embryo and its immune response. J. Hazard. Mater. 2022, 427, 128153. [Google Scholar] [CrossRef]
- Koestler, B.J.; Ward, C.M.; Fisher, C.R.; Rajan, A.; Maresso, A.W.; Payne, S.M. Human Intestinal Enteroids as a Model System of Shigella Pathogenesis. Infect. Immun. 2019, 87, e00733-18. [Google Scholar] [CrossRef]
- Uyanik, M.H.; Yazgi, H.; Ayyildiz, A. Survival of salmonella typhi and shigella flexneri in different water samples and at different temperatures. Turk. J. Med. Sci. 2008, 4, 307–310. [Google Scholar]
- Tang, F.; Cheng, Y.; Bao, C.; Hu, J.; Liu, W.; Liang, Q.; Wu, Y.; Norris, J.; Peng, Z.; Yu, R.; et al. Spatio-Temporal Trends and Risk Factors for Shigella from 2001 to 2011 in Jiangsu Province, People’s Republic of China. PLoS ONE 2014, 9, e83487. [Google Scholar] [CrossRef] [PubMed]
- Garedew, L.; Hagos, Z.; Zegeye, B.; Addis, Z. The detection and antimicrobial susceptibility profile of Shigella isolates from meat and swab samples at butchers’ shops in Gondar town, Northwest Ethiopia. J. Infect. Public Health 2015, 9, 348–355. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T. Molecular characterization of multidrug-resistant Shigella spp. of food origin. Int. J. Food Microbiol. 2015, 194, 78–82. [Google Scholar] [CrossRef]
- Philpott, D.J.; Edgeworth, J.D.; Sansonetti, P.J. The pathogenesis of Shigella flexneri infection: Lessons from in vitro and in vivo studies. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 575–586. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New Weapons to Fight Old Enemies: Novel Strategies for the (Bio)control of Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.; Zhang, Y.; Shan, Y.; Peng, Z.; Gu, B.; Yang, H. Au Nanoclusters Ameliorate Shigella Infectious Colitis by Inducing Oxidative Stress. Int. J. Nanomed. 2021, 16, 4545–4557. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Zhang, Y.; Ye, Y.; Gao, Y.; Li, J. In vitro and in vivo activity of ciprofloxacin/fosfomycin combination therapy against ciprofloxacin-resistant Shigella flexneri isolates. Infect. Drug Resist. 2019, 12, 1619–1628. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014, 20, 1–16. [Google Scholar]
- Bai, X.; Li, X.; Liu, X.; Xing, Z.; Su, R.; Wang, Y.; Xia, X.; Shi, C. Antibacterial Effect of Eugenol on Shigella flexneri and Its Mechanism. Foods 2022, 11, 2565. [Google Scholar] [CrossRef] [PubMed]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Du, W.; Zhou, M.; Liu, Z.; Chen, Y.; Li, R. Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes. Food Control 2017, 85, 119–126. [Google Scholar] [CrossRef]
- Senanayake, S.N. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Bansal, S.; Choudhary, S.; Sharma, M.; Kumar, S.S.; Lohan, S.; Bhardwaj, V.; Syan, N.; Jyoti, S. Tea: A native source of antimicrobial agents. Food Res. Int. 2013, 53, 568–584. [Google Scholar] [CrossRef]
- Yoda, Y.; Hu, Z.-Q.; Shimamura, T.; Zhao, W.-H. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Oh, Y.; Lim, J.; Youn, M.; Lee, I.; Pak, H.; Park, W.; Jo, W.; Park, S. AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol. 2012, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Sun, Z.; Wang, T.; Yang, M.; Liu, M.; Zhang, J.; Li, Y. Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb. Pathog. 2019, 139, 103924. [Google Scholar] [CrossRef]
- Liu, M.; Wu, X.; Li, J.; Liu, L.; Zhang, R.; Shao, D.; Du, X. The specific anti-biofilm effect of gallic acid on Staphylococcus aureus by regulating the expression of the ica operon. Food Control 2017, 73, 613–618. [Google Scholar] [CrossRef]
- Puzari, M.; Sharma, M.; Chetia, P. Emergence of antibiotic resistant Shigella species: A matter of concern. J. Infect. Public Health 2018, 11, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Kahsay, A.G.; Muthupandian, S. A review on Sero diversity and antimicrobial resistance patterns of Shigella species in Africa, Asia and South America, 2001–2014. BMC Res. Notes 2016, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Updated Knowledge about Polyphenols: Functions, Bioavailability, Metabolism, and Health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef]
- Asahi, Y.; Noiri, Y.; Miura, J.; Maezono, H.; Yamaguchi, M.; Yamamoto, R.; Azakami, H.; Hayashi, M.; Ebisu, S. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J. Appl. Microbiol. 2014, 116, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, X.D.; Wu, C.D. The Tea Catechin Epigallocatechin Gallate Suppresses Cariogenic Virulence Factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef]
- China, R.; Mukherjee, S.; Sen, S.; Bose, S.; Datta, S.; Koley, H.; Ghosh, S.; Dhar, P. Antimicrobial activity of Sesbania grandiflora flower polyphenol extracts on some pathogenic bacteria and growth stimulatory effect on the probiotic organism Lactobacillus acidophilus. Microbiol. Res. 2012, 167, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Rene, K.; Hortense, G.K.; Pascal, W.; Alexis, M.N.J.; Vidal, P.E.; Archange, F.T.M.; Christine, F.M. Activity of aqueous ethanol extract of Euphorbia prostrata ait on Shigella dysenteriae type 1-induced diarrhea in rats. Indian J. Pharmacol. 2007, 39, 240. [Google Scholar] [CrossRef]
- Babaei, S.; Bajelani, F.; Mansourizaveleh, O.; Abbasi, A.; Oubari, F. A study of the bactericidal effect of copper oxide nanoparticles on shigella sonnei and salmonella typhimurium. J. Babol Univ. Med. Sci. 2017, 19, 76–81. [Google Scholar] [CrossRef]
- Kitichalermkiat, A.; Kurahachi, M.; Nonaka, A.; Nakayama, M.; Shimatani, K.; Shigemune, N.; Tsugukuni, T.; Hitomi, J.; Sato, J.; Sonoda, T.; et al. Effects of Epigallocatechin Gallate on Viability and Cellular Proteins of Staphylococcus aureus. Food Sci. Technol. Res. 2019, 25, 277–285. [Google Scholar] [CrossRef]
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of Hydrogen Peroxide in Bactericidal Action of Catechin. Biol. Pharm. Bull. 2004, 27, 277–281. [Google Scholar] [CrossRef]
- Lee, P.; Tan, K.S. Effects of Epigallocatechin gallate against Enterococcus faecalis biofilm and virulence. Arch. Oral Biol. 2015, 60, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lu, J.; Duan, H.; Yang, J.; Tang, C. Biofilm inhibition and mode of action of epigallocatechin gallate against Vibrio mimicus. Food Control 2020, 113, 107148. [Google Scholar] [CrossRef]
- Jeon, M.-J.; Ha, J.-W. Bactericidal and synergistic effects of X-ray irradiation and gallic acid against foodborne pathogens on lettuce. Food Microbiol. 2020, 92, 103584. [Google Scholar] [CrossRef]
- Kang, J.; Li, Q.; Liu, L.; Jin, W.; Wang, J.; Sun, Y. The specific effect of gallic acid on Escherichia coli biofilm formation by regulating pgaABCD genes expression. Appl. Microbiol. Biotechnol. 2018, 102, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Su, T.-Y.; Wang, M.-Y.; Yang, S.-F.; Mar, K.; Hung, S.-L. Inhibitory effects of tea catechin epigallocatechin-3-gallate against biofilms formed from Streptococcus mutans and a probiotic lactobacillus strain. Arch. Oral Biol. 2018, 94, 69–77. [Google Scholar] [CrossRef]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef]
- Feng, C.; Wang, T.; Wang, C.; Chen, X.; Guo, Z.; Chen, Z. Disinfection Effects and Operating Conditions of Tea Polyphenols Combined with Ozone. Ozone: Sci. Eng. 2020, 42, 551–557. [Google Scholar] [CrossRef]
- Feng, C.M.; Xie, H.; Wang, X.T.; Yang, T.; Huang, H. Study and exploration of drinking water disinfection using tea polyphenols. Environ. Sci. Technol. 2016, 39, 63–67. [Google Scholar] [CrossRef]
- Feng, C.-M.; Yu, H.-Y.; Wang, T.; Li, J.; Sun, L.-H.; Tao, X.-C. Effect of ozone–tea polyphenols as a drinking water disinfection process on antibiotic resistance genes. J. Water Supply Res. Technol. 2022, 71, 507–517. [Google Scholar] [CrossRef]
- Zhu, N.; Feng, C.; Li, Y.; Xu, Z.; Fu, L.; Wang, Z. Research progress on antibacterial properties of tea polyphenols and its use as drinking water disinfectants. Appl. Chem. Ind. 2022, 51, 567–573. [Google Scholar] [CrossRef]

| Gene | Nucleotide Sequence of Primers (5′-3′) | Amplicon Size (bp) |
|---|---|---|
| mdoH | 5-TACCATCCGCCGTTACATTC-3 | 130 |
| 5-ATCCTGACCAACCATATCCATAG-3 | ||
| 16S rRNA | 5-GGGACCCGCACAAGCGGTGG-3 | 191 |
| 5-GGGTTGCGCTCGTTGCGGGA-3 |
| Reagent Groups | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| EGCG | - | 200 μg/mL | - | - | 200 μg/mL | 200 μg/mL |
| CAT | - | - | 5 μg/mL | - | 5 μg/mL | - |
| NAC | - | - | - | 16.2 μg/mL | - | 16.2 μg/mL |
| Strain | EGCG Concentration (μg/mL) | |||||
|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 400 | 800 | |
| S. flexneri | + | + | + | + | - | - |
| Initial Concentration of EGCG (μg/mL) | OD595 of EGCG | OD595 of Bacterial Solution after Inhibition | OD595 Difference (OD595 of Bacterial Solution after Inhibition—OD595 of EGCG) |
|---|---|---|---|
| 0 | 0.069 | 0.688 | 0.619 |
| 50 | 0.158 | 0.564 | 0.406 |
| 100 | 0.168 | 0.497 | 0.329 |
| 200 | 0.204 | 0.449 | 0.245 |
| 400 | 0.331 | 0.398 | 0.067 |
| 800 | 0.586 | 0.837 | 0.251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Y.; Ma, R.; Sun, W.; Ji, Z. Antibacterial Activity of Epigallocatechin Gallate (EGCG) against Shigella flexneri. Int. J. Environ. Res. Public Health 2023, 20, 4676. https://doi.org/10.3390/ijerph20064676
Zhang Y, Zhang Y, Ma R, Sun W, Ji Z. Antibacterial Activity of Epigallocatechin Gallate (EGCG) against Shigella flexneri. International Journal of Environmental Research and Public Health. 2023; 20(6):4676. https://doi.org/10.3390/ijerph20064676
Chicago/Turabian StyleZhang, Yini, Yeyue Zhang, Ruiqing Ma, Wanting Sun, and Zheng Ji. 2023. "Antibacterial Activity of Epigallocatechin Gallate (EGCG) against Shigella flexneri" International Journal of Environmental Research and Public Health 20, no. 6: 4676. https://doi.org/10.3390/ijerph20064676
APA StyleZhang, Y., Zhang, Y., Ma, R., Sun, W., & Ji, Z. (2023). Antibacterial Activity of Epigallocatechin Gallate (EGCG) against Shigella flexneri. International Journal of Environmental Research and Public Health, 20(6), 4676. https://doi.org/10.3390/ijerph20064676






