Bioremediation of Wastewater Using Yeast Strains: An Assessment of Contaminant Removal Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Curve of Yeast Strains
2.2. Preparation of Synthetic Municipal Wastewater
2.3. Determination of Physico-Chemical Parameters of Synthetic Municipal Wastewater
2.4. Removal of Pollutants by Yeast Strains
2.5. Determination of the Biomass Amount
2.6. Determination of the Biosurfactant Influence on the Biomass Production Amount and the Heavy Metal Ion Removal Efficiency
2.7. Statistical Analysis
3. Results and Discussion
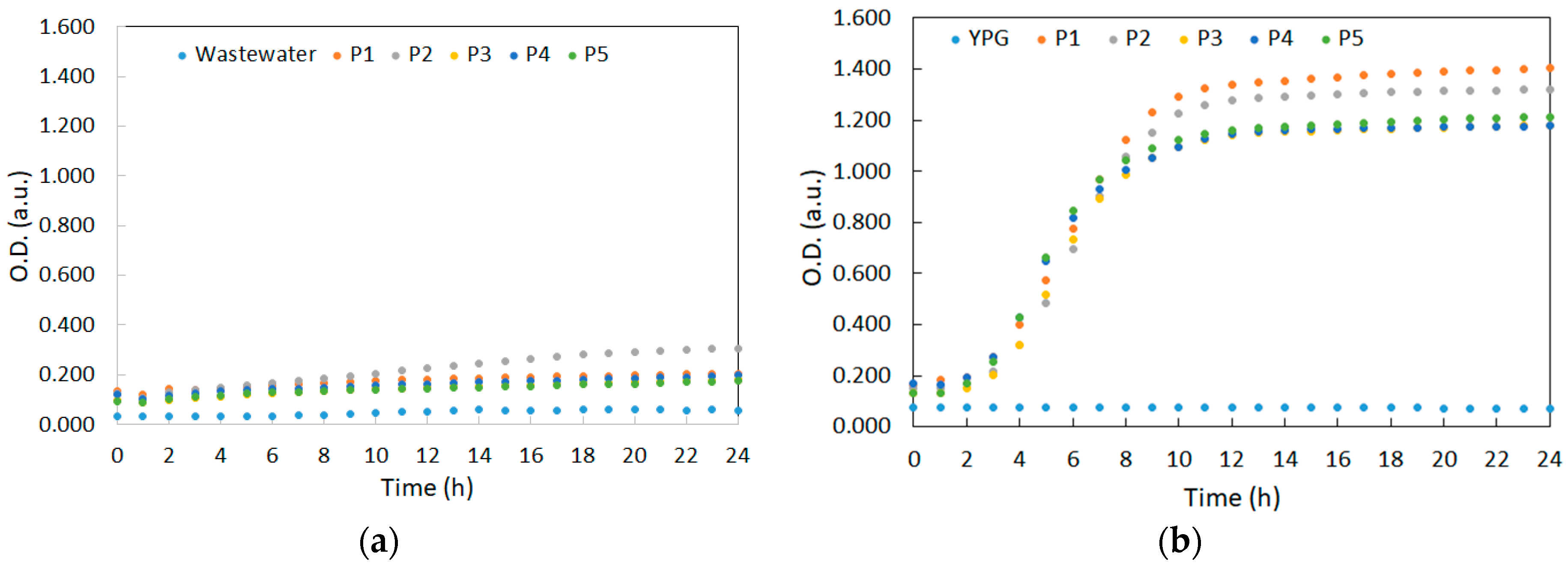
3.1. Yeast Strains’ Growth Kinetics in Synthetic Medium and in YPG Medium, under Controlled Conditions
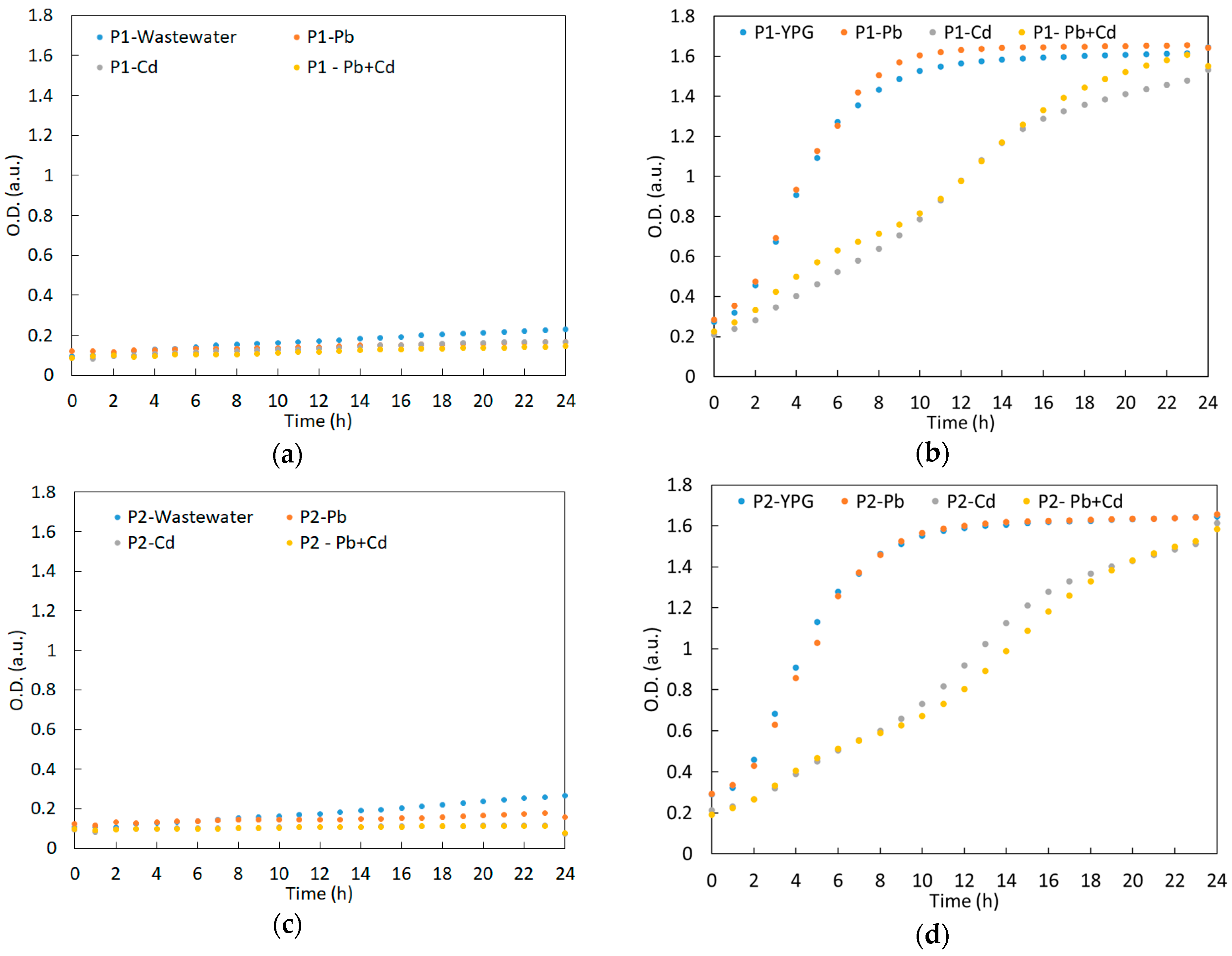
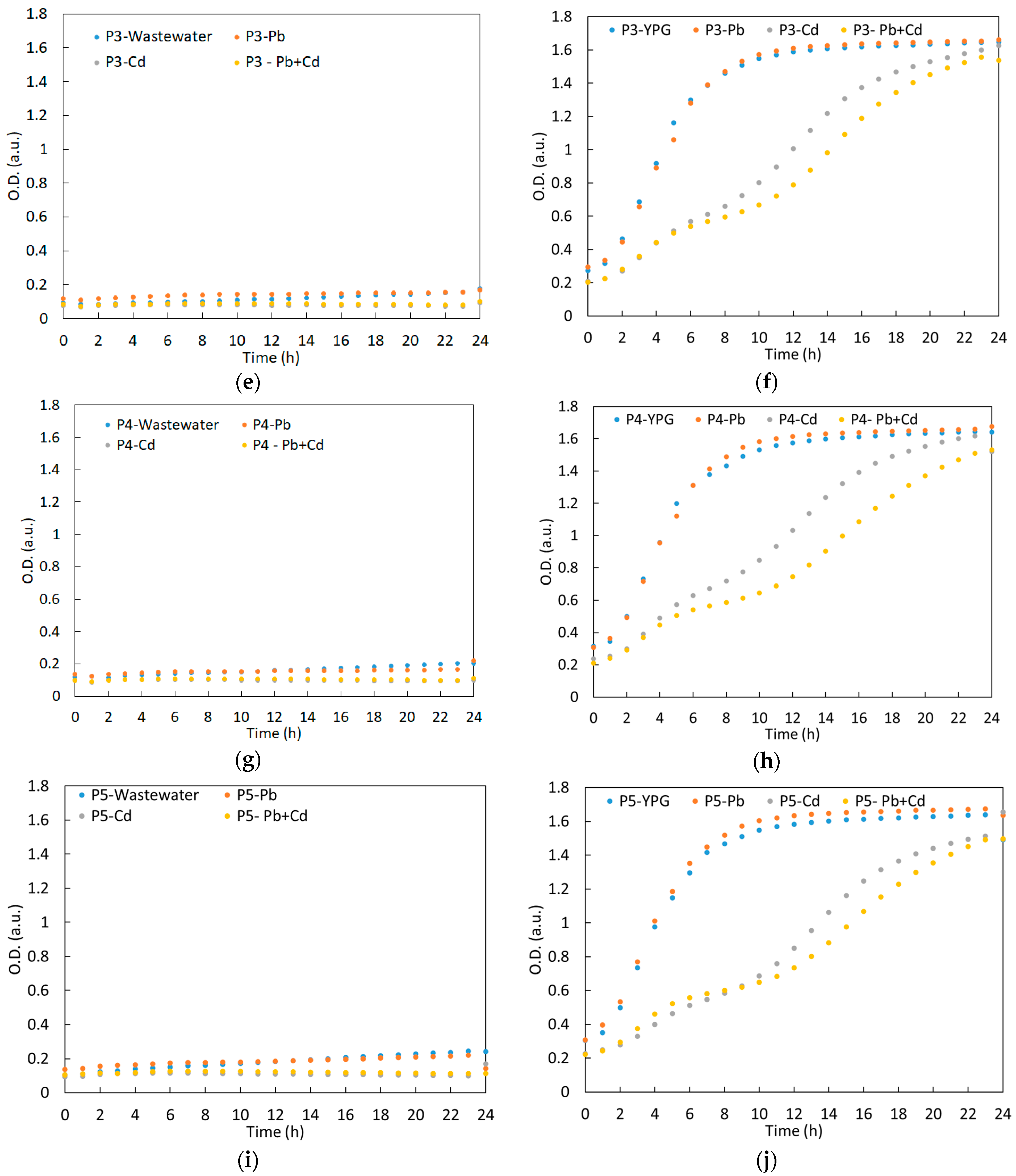
3.2. Growth Curve of the Yeast Strains in Synthetic Wastewater during the Experiment
3.3. Determination of the Physico-Chemical Parameters of the Inoculated Synthetic Wastewater during the Experiment
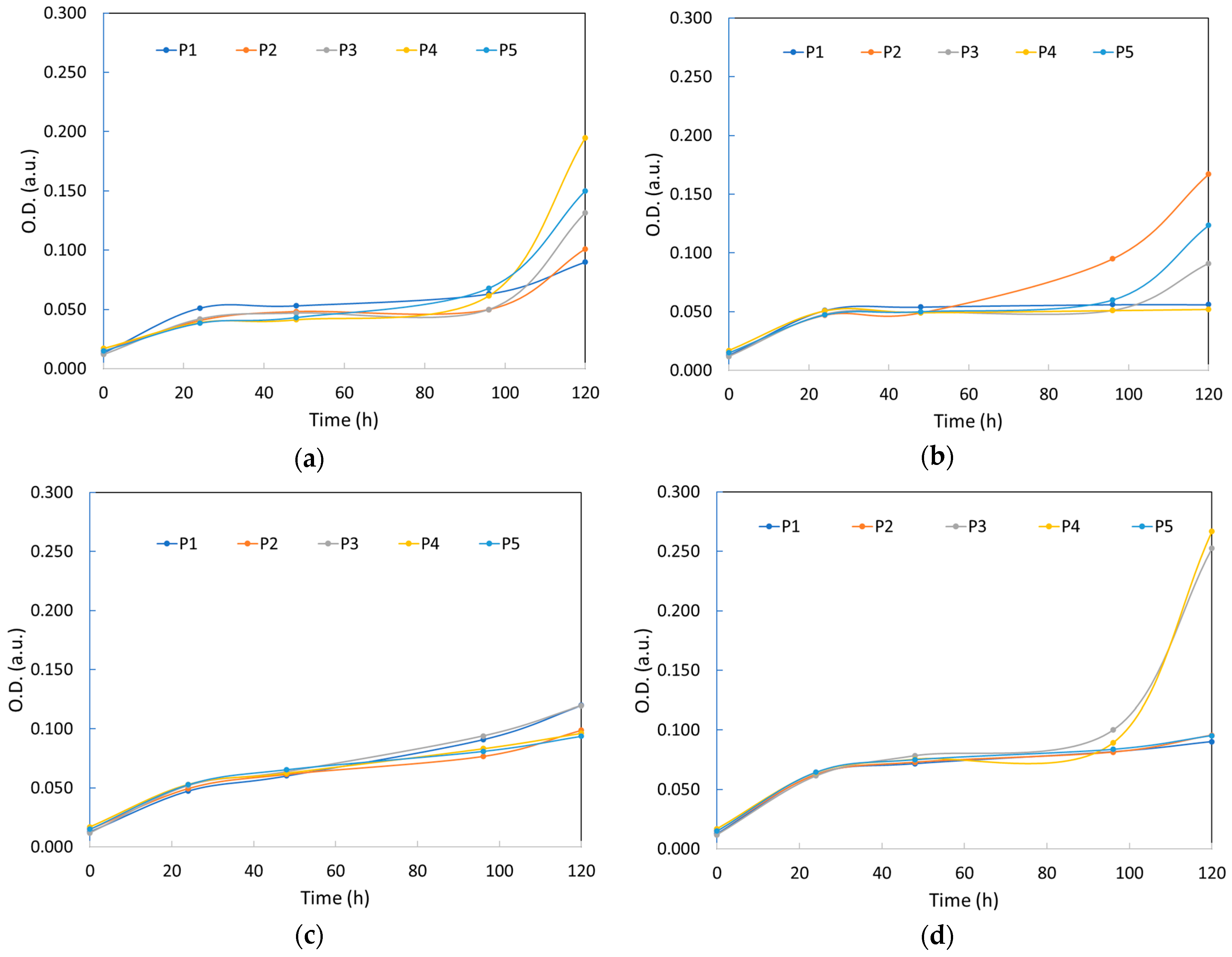
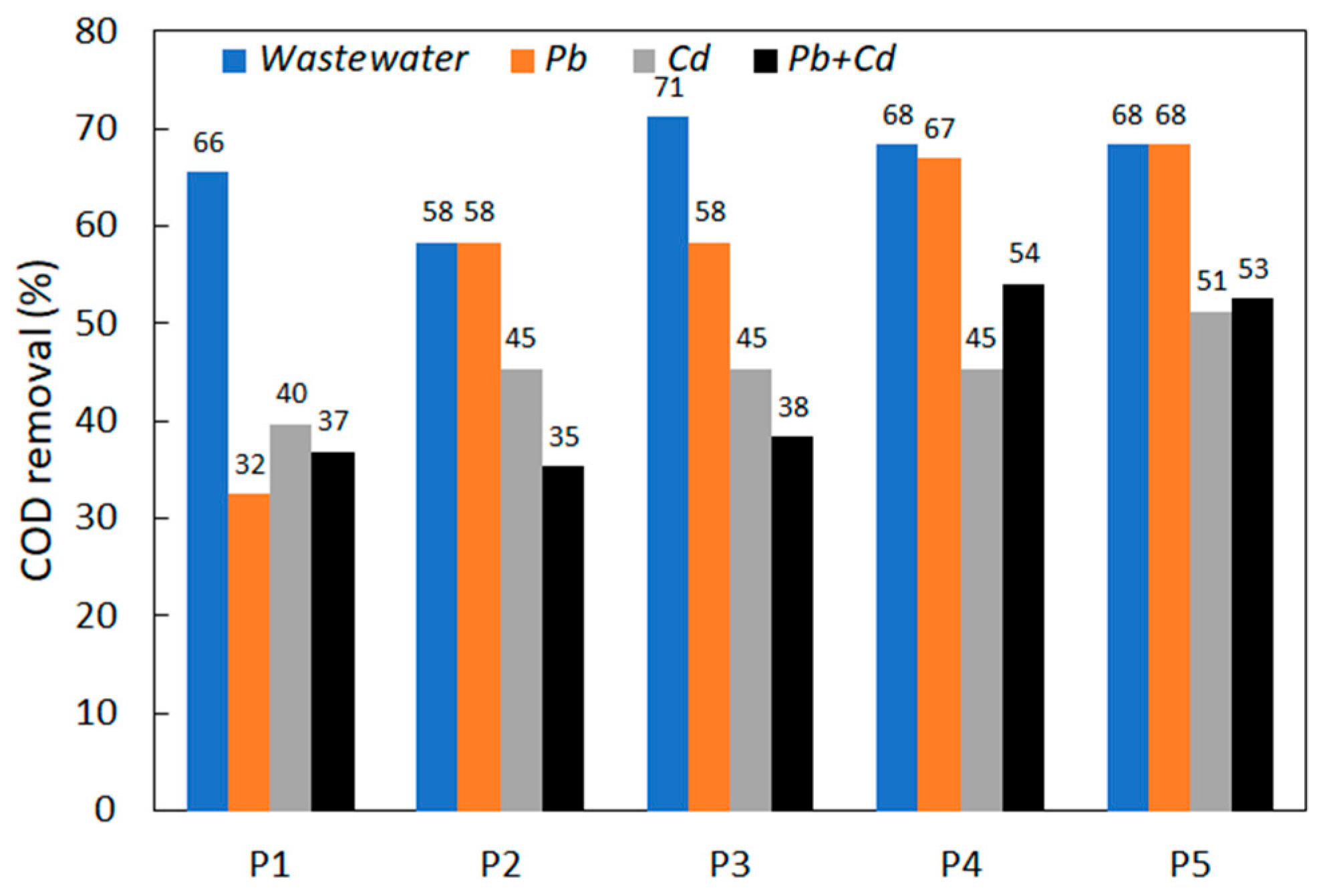
3.4. COD Removal and the Amount of Yeast Biomass
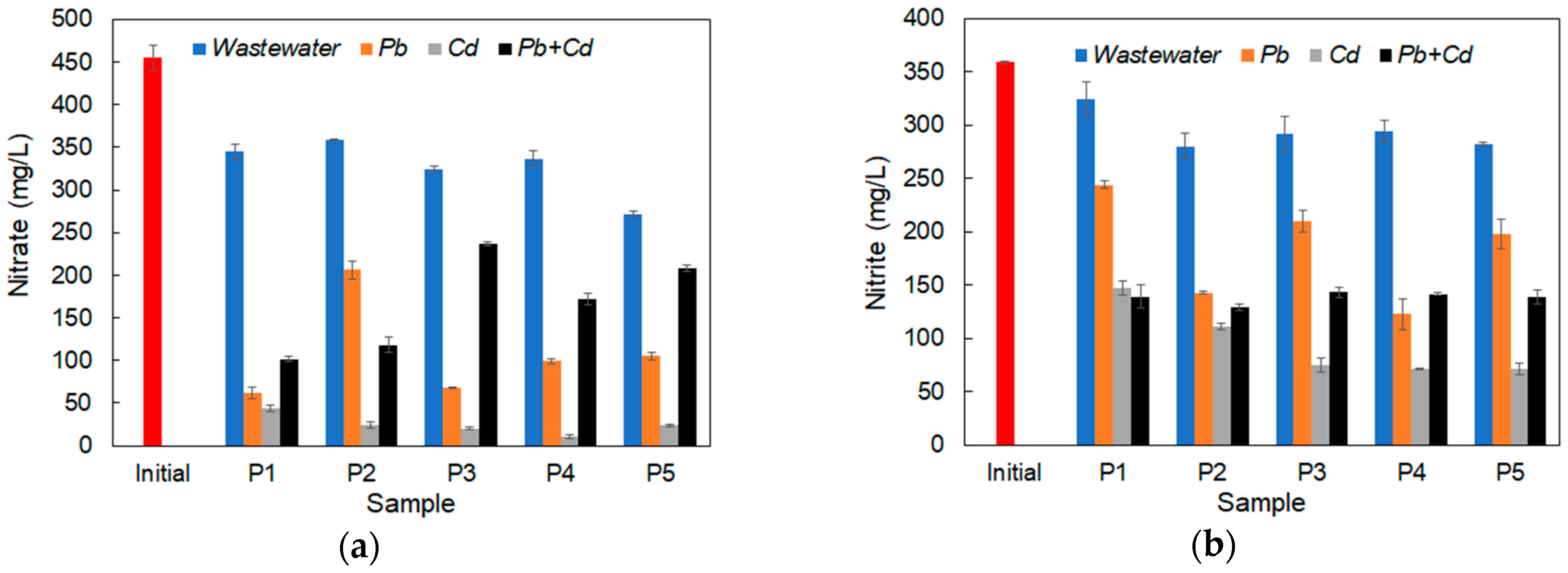
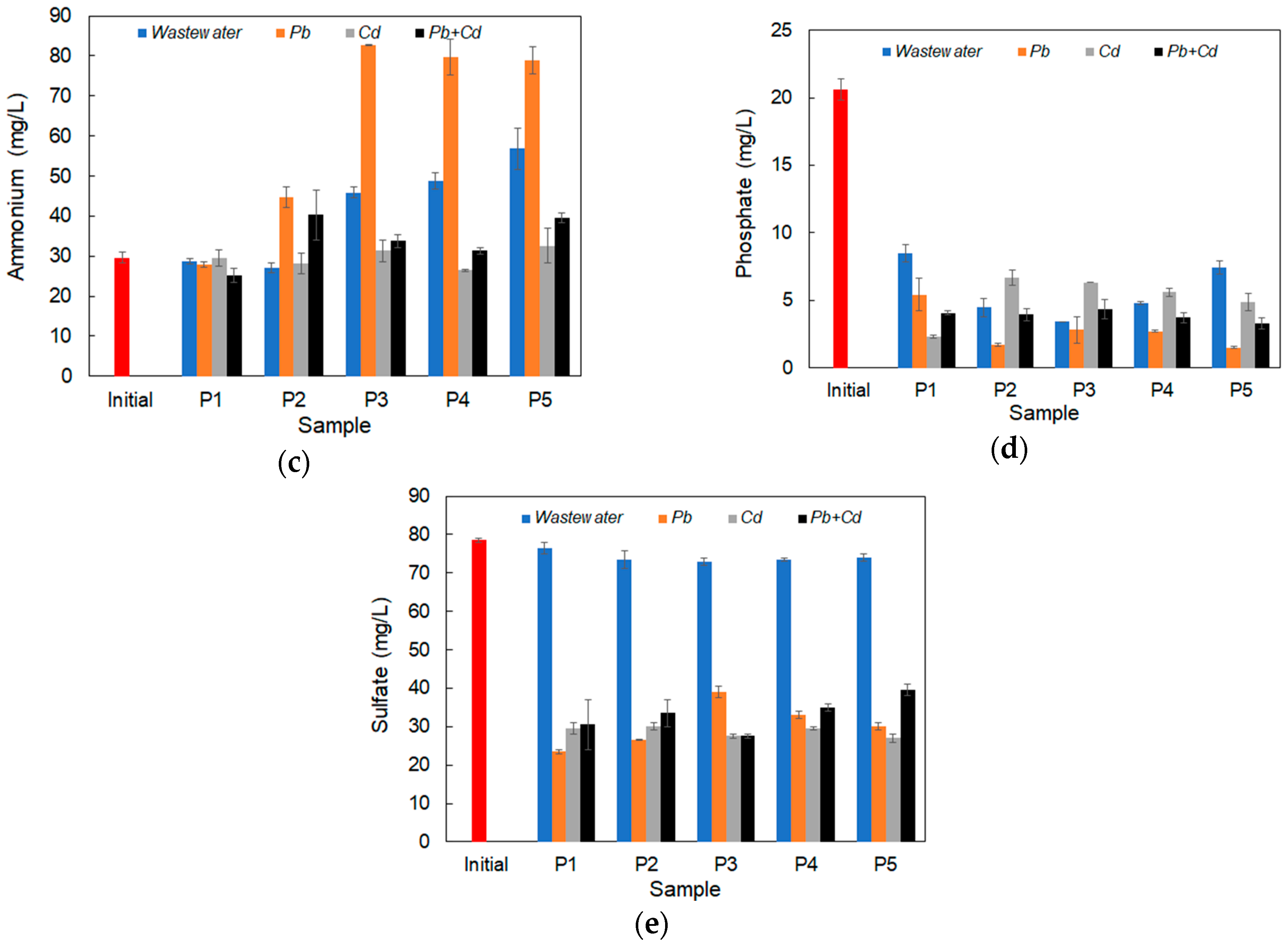
3.5. Nutrient Removal in Inoculated Synthetic Wastewater
3.6. Removal of Heavy Metal from Inoculated Wastewater
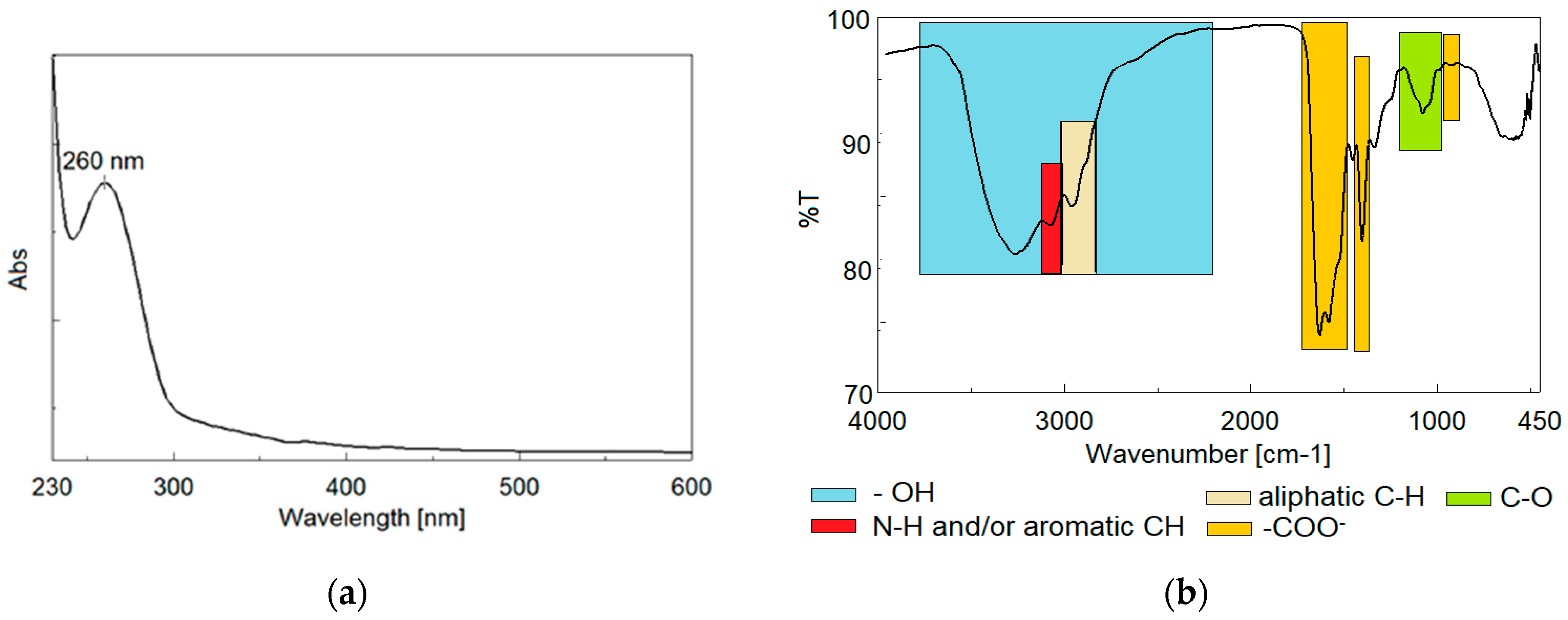
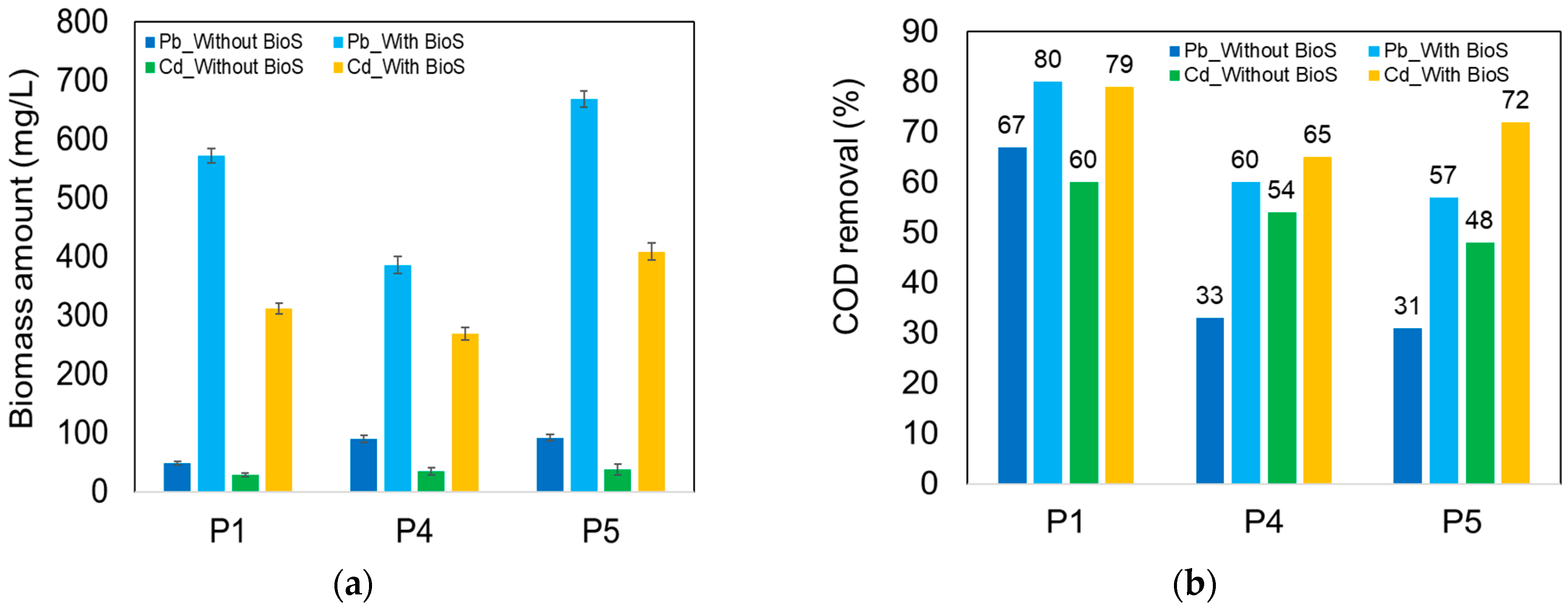
3.7. The Influence of Y. lipolytica Biosurfactant on the Reduction Capacity of Organic Compounds and Heavy Metals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Rao, D.G.; Senthilkumar, R.; Byrne, J.A.; Feroz, S. Wastewater Treatment: Advanced Processes and Technologies; Taylor & Francis: Abingdon, UK, 2012. [Google Scholar]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Yang, A.; Zhu, Q.; Sun, H.; Sun, P.; Yao, B.; Zang, Y.; Du, X.; Dong, L. Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water. Polymers 2022, 14, 1107. [Google Scholar] [CrossRef] [PubMed]
- Surgutskaia, N.S.; Martino, A.D.; Zednik, J.; Ozaltin, K.; Lovecká, L.; Bergerová, E.D.; Kimmer, D.; Svoboda, J.; Sedlarik, V. Efficient Cu2+, Pb2+ and Ni2+ ion removal from wastewater using electrospun DTPA-modified chitosan/polyethylene oxide nanofibers. Sep. Purif. Technol. 2020, 247, 116914. [Google Scholar] [CrossRef]
- Dong, L.; Shan, C.; Liu, Y.; Sun, H.; Yao, B.; Gong, G.; Jin, X.; Wang, S. Characterization and Mechanistic Study of Heavy Metal Adsorption by Facile Synthesized Magnetic Xanthate-Modified Chitosan/Polyacrylic Acid Hydrogels. Int. J. Environ. Res. Public Health 2022, 19, 11123. [Google Scholar] [CrossRef] [PubMed]
- Nicula, N.-O.; Lungulescu, E.-M.; Ieropoulos, I.A.; Rimbu, G.A.; Csutak, O. Nutrients Removal from Aquaculture Wastewater by Biofilter/Antibiotic-Resistant Bacteria Systems. Water 2022, 14, 607. [Google Scholar] [CrossRef]
- Nicula, N.-O.; Lungulescu, E.-M.; Rimbu, G.A.; Culcea, A.; Csutak, O. Nutrient and organic pollutants removal in synthetic wastewater by Pseudomonas aeruginosa and Chryseobacterium sp./biofilter systems. Environ. Monit. Assess. 2022, 194, 881. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Babel, S. Insights on microbial fuel cells for sustainable biological nitrogen removal from wastewater: A review. Environ. Res. 2022, 204, 112095. [Google Scholar] [CrossRef]
- Mu, R.; Jia, Y.; Ma, G.; Liu, L.; Hao, K.; Qi, F.; Shao, Y. Advances in the Use of Microalgal-Bacterial Consortia for Wastewater Treatment: Community Structures, Interactions, Economic Resource Reclamation and Study Techniques. Water Environ. Res. 2020, 93, 1217–1230. [Google Scholar] [CrossRef]
- Hamdan, A.M.; Abd-El-Mageed, H.; Ghanem, N. Biological treatment of hazardous heavy metals by Streptomyces rochei ANH for sustainable water management in agriculture. Sci. Rep. 2021, 11, 9314. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Hu, M. Application of yeast in the wastewater treatment. E3S Web Conf. 2018, 53, 04025. [Google Scholar] [CrossRef]
- Savastru, E.; Bulgariu, D.; Zamfir, C.-I.; Bulgariu, L. Application of Saccharomyces cerevisiae in the Biosorption of Co(II), Zn(II) and Cu(II) Ions from Aqueous Media. Water 2022, 14, 976. [Google Scholar] [CrossRef]
- Cherni, Y.; Botta, C.; Kasmi, M.; Franciosa, I.; Cocolin, L.; Chatti, A.; Trabelsi, I.; Elleuch, L. Mixed culture of Lactococcus lactis and Kluyveromyces marxianus isolated from kefir grains for pollutants load removal from Jebel Chakir leachate. Water Environ. Res. 2020, 92, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Vadkertiová, R.; Sláviková, E. Metal tolerance of yeasts isolated from water, soil and plant environments. J. Basic Microbiol. 2006, 46, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Zhou, J.; Luo, X. Uranium biosorption mechanism model of protonated Saccharomyces cerevisiae. J. Hazard. Mater. 2020, 385, 121588. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Reis, A.; Santos, J.A.L.; Lopes Da Silva, T. Concomitant wastewater treatment with lipid and carotenoid production by the oleaginous yeast Rhodosporidium toruloides grown on brewery effluent enriched with sugarcane molasses and urea. Process Biochem. 2020, 94, 1–14. [Google Scholar] [CrossRef]
- Chigusa, K.; Hasegawa, T.; Yamamoto, N.; Watanabe, Y. Treatment of wastewater from oil manufacturing plant by yeasts. Water Sci. Technol. 1996, 34, 51–58. [Google Scholar] [CrossRef]
- Massoud, R.; Hadiani, M.R.; Hamzehlou, P.; Khosravi-Darani, K. Bioremediation of heavy metals in food industry: Application of Saccharomyces cerevisiae. Electron. J. Biotechnol. 2019, 37, 56–60. [Google Scholar] [CrossRef]
- Al-Najar, J.A.; Lutfee, T.; Alwan, N.F. The action of yeast as an adsorbent in wastewater treatment: A Brief Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012054. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, C.; Gutierrez, T. Biosurfactants and Their Applications in the Oil and Gas Industry: Current State of Knowledge and Future Perspectives. Front. Bioeng. Biotech. 2021, 9, 6639. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.; Hatley, H. The role of surfactants in wastewater treatment: Impact, removal and future techniques: A critical review. Water Res. 2018, 147, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Malkapuram, S.T.; Sharma, V.; Gumfekar, S.P.; Sonawane, S.; Sonawane, S.; Boczkaj, G.; Seepana, M.M. A review on recent advances in the application of biosurfactants in wastewater treatment. Sustain. Energy Technol. Assess. 2021, 48, 101576. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Csutak, O.; Corbu, V.; Stoica, I.; Ionescu, R.; Vassu, T. Biotechnological Applications of Yarrowia lipolytica CMGB32. Agric. Agric. Sci. Procedia 2015, 6, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Corpuz, M.V.A.; Borea, L.; Senatore, V.; Castrogiovanni, F.; Buonerba, A.; Oliva, G.; Ballesteros, F.; Zarra, T.; Belgiorno, V.; Choo, K.-H.; et al. Wastewater treatment and fouling control in an electro algae-activated sludge membrane bioreactor. Sci. Total Environ. 2021, 786, 147475. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.; Elorza de Orellano, M.; Rezza, I.; Martinez, L.; Marchesvky, E.; Sanz de Tosetti, M. Removal of cadmium and lead from dilute aqueous solutions by Rhodotorula rubra. Bioresour. Technol. 2000, 72, 107–112. [Google Scholar] [CrossRef]
- ISO6060; ISO 6060-Determination of the Chemical Oxygen Demand. International Organization for Standardization: Geneva, Switzerland, 1989.
- Hanna. Hanna Instruments HI83300 Instruction Manual. Available online: https://www.haines.com.au/mwdownloads/download/link/id/222/ (accessed on 22 January 2022).
- Ortansa, C.; Ionela, S.; Elena, R.; Vassu, T.; Corbu, V. Antimicrobial and Antiadhesion Activity of Biosurfactants from Rhodotorula glutinis Grown on n-dodecane. Rev. Chim. Bucharest 2020, 71, 99–105. [Google Scholar] [CrossRef]
- Olivares-Marin, I.K.; Gonzalez-Hernandez, J.C.; Regalado-Gonzalez, C.; Madrigal-Perez, L.A. Saccharomyces cerevisiae Exponential Growth Kinetics in Batch Culture to Analyze Respiratory and Fermentative Metabolism. J. Vis. Exp. 2018, 139, e58192. [Google Scholar] [CrossRef] [Green Version]
- Van der Heggen, M.; Martins, S.; Flores, G.; Soares, E.V. Lead toxicity in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 88, 1355–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, E.V.; Hebbelinck, K.; Soares, H.M. Toxic effects caused by heavy metals in the yeast Saccharomyces cerevisiae: A comparative study. Can. J. Microbiol. 2003, 49, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Tewari, S.; Rai, J.P. Metals sorption from aqueous solutions by Kluyveromyces marxianus: Process optimization, equilibrium modeling and chemical characterization. Biotechnol. J. 2009, 4, 1471–1478. [Google Scholar] [CrossRef]
- Costa-Moreira, L.M.; Porto, B.A.A.; Haddad-Ribeiro, F.; Martins, F.S.; Menezes, M.A.B.C.; Rosa, C.A.; Neves, M.J. Membrane damage by lipid peroxidation retains the cadmium constraint and is not the primary cause of K+ extrusion in yeast. Ann. Microbiol. 2016, 66, 973–979. [Google Scholar] [CrossRef]
- Ozturk, M.; Metin, M.; Altay, V.; De Filippis, L.; Ünal, B.T.; Khursheed, A.; Gul, A.; Hasanuzzaman, M.; Nahar, K.; Kawano, T.; et al. Molecular Biology of Cadmium Toxicity in Saccharomyces cerevisiae. Biol. Trace Elem. Res. 2021, 199, 4832–4846. [Google Scholar] [CrossRef]
- Jacobson, T.; Priya, S.; Sharma, S.K.; Andersson, S.; Jakobsson, S.; Tanghe, R.; Ashouri, A.; Rauch, S.; Goloubinoff, P.; Christen, P.; et al. Cadmium Causes Misfolding and Aggregation of Cytosolic Proteins in Yeast. Mol. Cell Biol. 2017, 37, e00490-16. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, J. Response of Saccharomyces cerevisiae to lead ion stress. Appl. Microbiol. Biotechnol. 2007, 74, 683–687. [Google Scholar] [CrossRef]
- Hadiani, M.R.; Darani, K.K.; Rahimifard, N.; Younesi, H. Biosorption of low concentration levels of Lead (II) and Cadmium (II) from aqueous solution by Saccharomyces cerevisiae: Response surface methodology. Biocatal. Agric. Biotechnol. 2018, 15, 25–34. [Google Scholar] [CrossRef]
- Farhan, S.N.; Khadom, A.A. Biosorption of heavy metals from aqueous solutions by Saccharomyces Cerevisiae. Int. J. Ind. Chem. 2015, 6, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Pasternakiewicz, A. The growth of Saccharomyces cerevisiae yeast in cadmium enriched media. Acta Sci. Pol. Technol. Aliment. 2006, 5, 39–46. [Google Scholar]
- Breierová, E.; Vajcziková, I.; Sasinková, V.; Stratilová, E.; Fisera, I.; Gregor, T.; Sajbidor, J. Biosorption of cadmium ions by different yeast species. Z. Naturforsch. C. J. Biosci. 2002, 57, 634–639. [Google Scholar] [CrossRef]
- Cai, M.; Zhou, J.; Hao, T.; Du, K. Tolerance of phyllospheric Wickerhamomyces anomalus to BDE-3 and heavy metals. Environ. Sci. Pollut. Res. 2022, 29, 56555–56561. [Google Scholar] [CrossRef]
- Aibeche, C.; Selami, N.; Zitouni-Haouar, F.E.-H.; Oeunzar, K.; Addou, A.; Kaid-Harche, M.; Djabeur, A. Bioremediation potential and lead removal capacity of heavy metal-tolerant yeasts isolated from Dayet Oum Ghellaz Lake water (northwest of Algeria). Int. Microbiol. 2022, 25, 61–73. [Google Scholar] [CrossRef]
- Ilyas, S.; Rehman, A. Metal resistance and uptake by Trichosporon asahii and Pichia kudriavzevii isolated from industrial effluents. Arch. Environ. Prot. 2018, 44, 77–84. [Google Scholar] [CrossRef]
- Subhashini, S.S.; Velan, M.; Kaliappan, S. Biosorption of lead by Kluyveromyces marxianus immobilized in alginate beads. J Environ. Biol. 2013, 34, 831–835. [Google Scholar] [PubMed]
- Stahl, G.; Salem, S.N.; Chen, L.; Zhao, B.; Farabaugh, P.J. Translational accuracy during exponential, postdiauxic, and stationary growth phases in Saccharomyces cerevisiae. Eukaryot Cell 2004, 3, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbu, V.; Petrut, S.; Vassu, T.; Pelinescu, D.; Sarbu, I.; Rusu, E.; Csutak, O. Environmental stress responses in yeasts and lactic acid bacteria strains isolated from dairy traditional Romanian fermented products. Roman Biotechnol. Lett. 2021, 26, 2548–2559. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Sha, S.; Yin, H.; Zhang, H.; Wang, Y.; Zhao, B.; Song, F. Analysis of the tendency for the electronic conductivity to change during alcoholic fermentation. Sci. Rep. 2019, 9, 5512. [Google Scholar] [CrossRef] [Green Version]
- Narendranath, N.V.; Power, R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef] [Green Version]
- Recek, M.; Raspor, P. Yeast Growth Potential and COD Reduction in Waste Water from Ergot Alkaloid Production. Food Technol. Biotechol. 1999, 37, 159–163. [Google Scholar]
- OECD. Agriculture’s Impact on Aquaculture: Hypoxia and Eutrophication in Marine Waters; OECD: Paris, France, 2012. [Google Scholar]
- Zikánová, B.; Kuthan, M.; Řičicová, M.; Forstová, J.; Palková, Z. Amino acids control ammonia pulses in yeast colonies. Biochem. Biophys. Res. Commun. 2002, 294, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, S.K.; Özbaş, Z. Effects of Ammonium Sulphate Concentration on Growth and Glycerol Production Kinetics of Two Endogenic Wine Yeast Strains. Indian J. Biotechnol. 2008, 7, 89–93. [Google Scholar]
- Persson, B.L.; Lagerstedt, J.O.; Pratt, J.R.; Pattison-Granberg, J.; Lundh, K.; Shokrollahzadeh, S.; Lundh, F. Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr. Genet. 2003, 43, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Walls, L.E.; Velasquez-Orta, S.B.; Romero-Frasca, E.; Leary, P.; Yáñez Noguez, I.; Orta Ledesma, M.T. Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem. Eng. J. 2019, 151, 107319. [Google Scholar] [CrossRef]
- Kong, Y.; Xu, X.; Zhu, L.; Miao, L. Control of the Harmful Alga Microcystis aeruginosa and Absorption of Nitrogen and Phosphorus by Candida utilis. Appl. Biochem. Biotechnol. 2013, 169, 88–99. [Google Scholar] [CrossRef]
- Han, J.; Qiu, Q.; Gao, M.; Qiu, L.; Wang, Y.; Sun, S.; Song, D.; Ma, J. Phosphorus removal from municipal wastewater through a novel Trichosporon asahii BZ: Performance and mechanism. Chemosphere 2022, 298, 134329. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Gibney, P.; Cheng, L.; Liu, S.; Peck, G. Yeast assimilable nitrogen concentrations influence yeast gene expression and hydrogen sulfide production during cider fermentation. Front. Microbiol. 2020, 11, 1264. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Sagar Jena, P.; Pradhan, A.; Prakash Nanda, S.; Kishore Dash, A.; Naik, B. Biosorption of heavy metals from wastewater using Saccharomyces cerevisiae as a biosorbent: A mini review. Mater. Today Proc. 2022, 67, 1140–1146. [Google Scholar] [CrossRef]
- Martorell, M.M.; Fernández, P.M.; Fariña, J.I.; Figueroa, L.I.C. Cr(VI) reduction by cell-free extracts of Pichia jadinii and Pichia anomala isolated from textile-dye factory effluents. Int. Biodeterior. Biodegrad. 2012, 71, 80–85. [Google Scholar] [CrossRef]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. Yeast exopolysaccharides and their physiological functions. Folia Microbiol. 2021, 66, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Cheng, X.; Wang, L.; Hu, J.; Zhou, N.; Xia, J.; Xu, M.; Zhang, L.; Gao, H.; Ye, X.; et al. Remediation of Lead-Contaminated Water by Red Yeast and Different Types of Phosphate. Front. Bioeng. Biotechnol. 2022, 10, 775058. [Google Scholar] [CrossRef] [PubMed]
- Massoud, R.; Khosravi-Darani, K.; Sharifan, A.; Asadi, G.H. Lead bioremoval from milk by Saccharomyces cerevisiae. Biocatal. Agric. Biotechnol. 2019, 22, 101437. [Google Scholar] [CrossRef]
- Franzetti, A.; Gandolfi, I.; Fracchia, L.; Van Hamme, J.; Gkorezis, P.; Marchant, R.; Banat, I.M. Biosurfactant use in heavy metal removal from industrial effluents and contaminated sites. Biosurfactants Prod. Util. Process. Technol. Econ. 2014, 159, 361. [Google Scholar]
- Van Oss, C.J. Specifically impermeable precipitate membranes formed through double diffusion in gels: Behavior with complex forming and with simple systems. J. Colloid Interface Sci. 1968, 27, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; de Luna, J.M.; de Campos Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. 2014, 17, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Hazra, C.; Samanta, T.; Mahalingam, V. A resonance energy transfer approach for the selective detection of aromatic amino acids. J. Mater. Chem. C 2014, 2, 10157–10163. [Google Scholar] [CrossRef]
- Magthalin, C.J.; Varadharajan, A.; Swarnalatha, S.; Sekaran, G. Utilization of Chicken Tallow for the Production of Cationic Biosurfactant and Thereof for Decontamination of Cr(III) Containing Soil. Procedia Environ. Sci. 2016, 35, 895–913. [Google Scholar] [CrossRef]
- Saito, K.; Xu, T.; Ishikita, H. Correlation between C=O Stretching Vibrational Frequency and pK(a) Shift of Carboxylic Acids. J Phys. Chem. B 2022, 126, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Lungulescu, E.M.; Lingvay, I.; Ungureanu, L.C.; Rus, T.; Bors, A.M. Thermooxidative Behavior of Some Paint Materials in Natural Ester Based Electro-insulating Fluid. Mater. Plast. 2018, 55, 201–206. [Google Scholar] [CrossRef]
- Yilmaz, F.; Ergene, A.; Yalçin, E.; Tan, S. Production and characterization of biosurfactants produced by microorganisms isolated from milk factory wastewaters. Environ. Technol. 2009, 30, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Radha, P.; Suhazsini, P.; Prabhu, K.; Jayakumar, A.; Kandasamy, R. Chicken Tallow, a Renewable Source for the Production of Biosurfactant by Yarrowia lipolytica MTCC9520, and its Application in Silver Nanoparticle Synthesis. J. Surfactants Deterg. 2020, 23, 119–135. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; da Silva, J.M.; Lehocky, M.; Barros-Timmons, A.M.V.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A.P. Production and characterization of a bioemulsifier from Yarrowia lipolytica. Process Biochem. 2006, 41, 1894–1898. [Google Scholar] [CrossRef]
- Cirigliano, M.C.; Carman, G.M. Purification and Characterization of Liposan, a Bioemulsifier from Candida lipolytica. Appl. Environ. Microbiol. 1985, 50, 846–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, F.P.; Menezes, A.J.d.; Tonello, P.S.; Dos Santos, A.C.A.; Duarte, I.C.S. Characterization of biosurfactant from yeast using residual soybean oil under acidic conditions and their use in metal removal processes. Fems. Microbiol. Lett. 2018, 365, fny098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Lin, Z.Q.; Pang, S.M.; Zhang, Y.M.; Bhatt, P.; Chen, S.H. Biosurfactant is a powerful tool for the bioremediation of heavy metals from contaminated soils. J. Hazard. Mater. 2021, 418, 126253. [Google Scholar] [CrossRef] [PubMed]
| Time (h) | Synthetic Wastewater | |||||
|---|---|---|---|---|---|---|
| Parameters | P1 | P2 | P3 | P4 | P5 | |
| 0 | Conductivity (µS·cm−1) | 1767 | 1767 | 1746 | 1767 | 1754 |
| pH | 7.74 | 7.77 | 7.74 | 7.73 | 7.74 | |
| 24 | Conductivity (µS·cm−1) | 1750 | 1754 | 1744 | 1751 | 1751 |
| pH | 6.34 | 6.54 | 6.52 | 6.53 | 6.53 | |
| 48 | Conductivity (µS·cm−1) | 1797 | 1745 | 1756 | 1762 | 1758 |
| pH | 6.44 | 6.45 | 6.41 | 6.44 | 6.45 | |
| 96 | Conductivity (µS·cm−1) | 1797 | 1773 | 1775 | 1784 | 1790 |
| pH | 7.00 | 6.42 | 6.84 | 7.18 | 7.14 | |
| 120 | Conductivity (µS·cm−1) | 1798 | 1722 | 1777 | 1784 | 1789 |
| pH | 7.47 | 6.52 | 7.2 | 7.35 | 7.46 | |
| Synthetic wastewater + Pb2+ | ||||||
| 0 | Conductivity (µS·cm−1) | 1770 | 1776 | 1766 | 1767 | 1768 |
| pH | 7.53 | 7.51 | 7.5 | 7.5 | 7.49 | |
| 24 | Conductivity (µS·cm−1) | 1754 | 1763 | 1761 | 1753 | 1762 |
| pH | 6.28 | 6.52 | 6.54 | 6.43 | 6.49 | |
| 48 | Conductivity (µS·cm−1) | 1765 | 1762 | 1771 | 1767 | 1774 |
| pH | 6.35 | 6.47 | 6.47 | 6.47 | 6.52 | |
| 96 | Conductivity (µS·cm−1) | 1776 | 1762 | 1792 | 1798 | 1799 |
| pH | 6.42 | 6.71 | 6.22 | 7.02 | 6.64 | |
| 120 | Conductivity (µS·cm−1) | 1781 | 1778 | 1801 | 1810 | 1788 |
| pH | 6.46 | 7.19 | 6.35 | 7.36 | 6.84 | |
| Synthetic wastewater + Cd2+ | ||||||
| 0 | Conductivity (µS·cm−1) | 1780 | 1776 | 1781 | 1778 | 1770 |
| pH | 7.7 | 7.71 | 7.70 | 7.71 | 7.72 | |
| 24 | Conductivity (µS·cm−1) | 1774 | 1780 | 1781 | 1782 | 1767 |
| pH | 7.43 | 7.43 | 7.44 | 7.45 | 7.48 | |
| 48 | Conductivity (µS·cm−1) | 1774 | 1782 | 1781 | 1772 | 1759 |
| pH | 7.48 | 7.43 | 7.45 | 7.50 | 7.53 | |
| 96 | Conductivity (µS·cm−1) | 1773 | 1790 | 1785 | 1743 | 1747 |
| pH | 7.64 | 7.68 | 7.70 | 7.68 | 7.72 | |
| 120 | Conductivity (µS·cm−1) | 1781 | 1756 | 1760 | 1772 | 1743 |
| pH | 7.65 | 7.67 | 7.62 | 7.63 | 7.69 | |
| Synthetic wastewater + Pb2+ + Cd2+ | ||||||
| 0 | Conductivity (µS·cm−1) | 1704 | 1775 | 1724 | 1749 | 1747 |
| pH | 7.91 | 7.67 | 7.56 | 7.49 | 7.47 | |
| 24 | Conductivity (µS·cm−1) | 1781 | 1777 | 1774 | 1776 | 1770 |
| pH | 7.33 | 7.39 | 7.35 | 7.34 | 7.34 | |
| 48 | Conductivity (µS·cm−1) | 1782 | 1779 | 1787 | 1793 | 1785 |
| pH | 7.58 | 7.56 | 7.45 | 7.41 | 7.47 | |
| 96 | Conductivity (µS·cm−1) | 1797 | 1777 | 1816 | 1826 | 1806 |
| pH | 7.71 | 7.67 | 7.55 | 7.59 | 7.49 | |
| 120 | Conductivity (µS·cm−1) | 1772 | 1784 | 1802 | 1742 | 1730 |
| pH | 7.59 | 7.50 | 7.46 | 7.51 | 7.44 | |
| Yeast Strain | Biomass Amount (mg/L) | |||
|---|---|---|---|---|
| Synthetic Wastewater | Synthetic Wastewater/Pb2+ | Synthetic Wastewater/Cd2+ | Synthetic Wastewater/Pb2+ + Cd2+ | |
| P1 | 61.00 ± 5.20 | 49.25 ± 1.30 | 28.38 ± 0.55 | 32.25 ± 0.70 |
| P2 | 80.38 ± 5.25 | 88.25 ± 1.40 | 29.75 ± 0.70 | 34.38 ± 0.85 |
| P3 | 96.75 ± 0.80 | 71.63 ± 7.85 | 30.13 ± 0.25 | 34.50 ± 1.50 |
| P4 | 91.00 ± 2.60 | 90.00 ± 2.00 | 35.13 ± 0.05 | 34.50 ± 0.70 |
| P5 | 97.88 ± 2.85 | 92.38 ± 3.75 | 37.88 ± 1.15 | 39.63 ± 0.30 |
| Time (h) | Removal Efficiency (%) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | ||||||||||||||||
| Pb | Cd | Pb/Cd | Pb | Cd | Pb/Cd | Pb | Cd | Pb/Cd | Pb | Cd | Pb/Cd | Pb | Cd | Pb/Cd | ||||||
| Pb | Cd | Pb | Cd | Pb | Cd | Pb | Cd | Pb | Cd | |||||||||||
| 24 | 70 | 10 | 65 | 23 | 51 | 2 | 70 | 27 | 45 | 2 | 81 | 26 | 66 | 0 | 85 | 26 | 68 | 27 | 84 | 3 |
| 48 | 78 | 10 | 81 | 30 | 63 | 3 | 74 | 32 | 58 | 12 | 85 | 32 | 68 | 3 | 87 | 32 | 75 | 32 | 85 | 11 |
| 96 | 92 | 10 | 82 | 30 | 85 | 10 | 83 | 33 | 81 | 24 | 85 | 32 | 88 | 23 | 87 | 37 | 85 | 34 | 89 | 26 |
| 120 | 96 | 15 | 92 | 39 | 86 | 20 | 85 | 33 | 89 | 26 | 94 | 33 | 90 | 30 | 95 | 40 | 90 | 39 | 95 | 32 |
| Time (h) | Removal Efficiency (%) | |||||
|---|---|---|---|---|---|---|
| P1 | P4 | P5 | ||||
| Pb | Cd | Pb | Cd | Pb | Cd | |
| 24 | 72 | 14 | 70 | 12 | 69 | 16 |
| 48 | 82 | 26 | 79 | 22 | 84 | 26 |
| 96 | 93 | 43 | 94 | 40 | 88 | 37 |
| 120 | 97 | 56 | 99 | 51 | 98 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicula, N.-O.; Lungulescu, E.-M.; Rîmbu, G.A.; Marinescu, V.; Corbu, V.M.; Csutak, O. Bioremediation of Wastewater Using Yeast Strains: An Assessment of Contaminant Removal Efficiency. Int. J. Environ. Res. Public Health 2023, 20, 4795. https://doi.org/10.3390/ijerph20064795
Nicula N-O, Lungulescu E-M, Rîmbu GA, Marinescu V, Corbu VM, Csutak O. Bioremediation of Wastewater Using Yeast Strains: An Assessment of Contaminant Removal Efficiency. International Journal of Environmental Research and Public Health. 2023; 20(6):4795. https://doi.org/10.3390/ijerph20064795
Chicago/Turabian StyleNicula, Nicoleta-Oana, Eduard-Marius Lungulescu, Gimi A. Rîmbu, Virgil Marinescu, Viorica Maria Corbu, and Ortansa Csutak. 2023. "Bioremediation of Wastewater Using Yeast Strains: An Assessment of Contaminant Removal Efficiency" International Journal of Environmental Research and Public Health 20, no. 6: 4795. https://doi.org/10.3390/ijerph20064795








