Effect of Humus on the Solidification and Stabilization of Heavy Metal Contaminated River Sediment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
- (1)
- Test sediment: the test sediment was taken from a river channel in Chongming District, Shanghai. The sediments with high organic matter and low organic matter were collected in two places for the subsequent experiment to adjust the content of the organic matter. The sampling depth was 0~20 cm. The physical and chemical properties of the sediment are shown in Table 1. The additional amount of heavy metal salts Cd (NO3) 2⋯4H2O, Cu (NO3) 2⋯3H2O, Pb (NO3) 2 is based on the Soil Environmental Quality Standard for Soil Pollution Risk Control of Agricultural Land (GB15618-2018), simulating heavy metal contaminated sediment. Composite heavy metal contaminated sediment was prepared after even stirring and aging for 1 month, with the moisture content kept at about 30%. The heavy metal content before and after sediment pollution is shown in Table 2.
- (2)
- Curing agent: Shanghai Conch PO.42.5 ordinary Portland cement was selected.
- (3)
- Commercial HA/FA was purchased from Shanghai Sinopharm Chemical Reagents Co., Ltd.
2.2. Experimental Method
2.3. Testing Method
2.4. Statistical Analysis
3. Results and Discussion
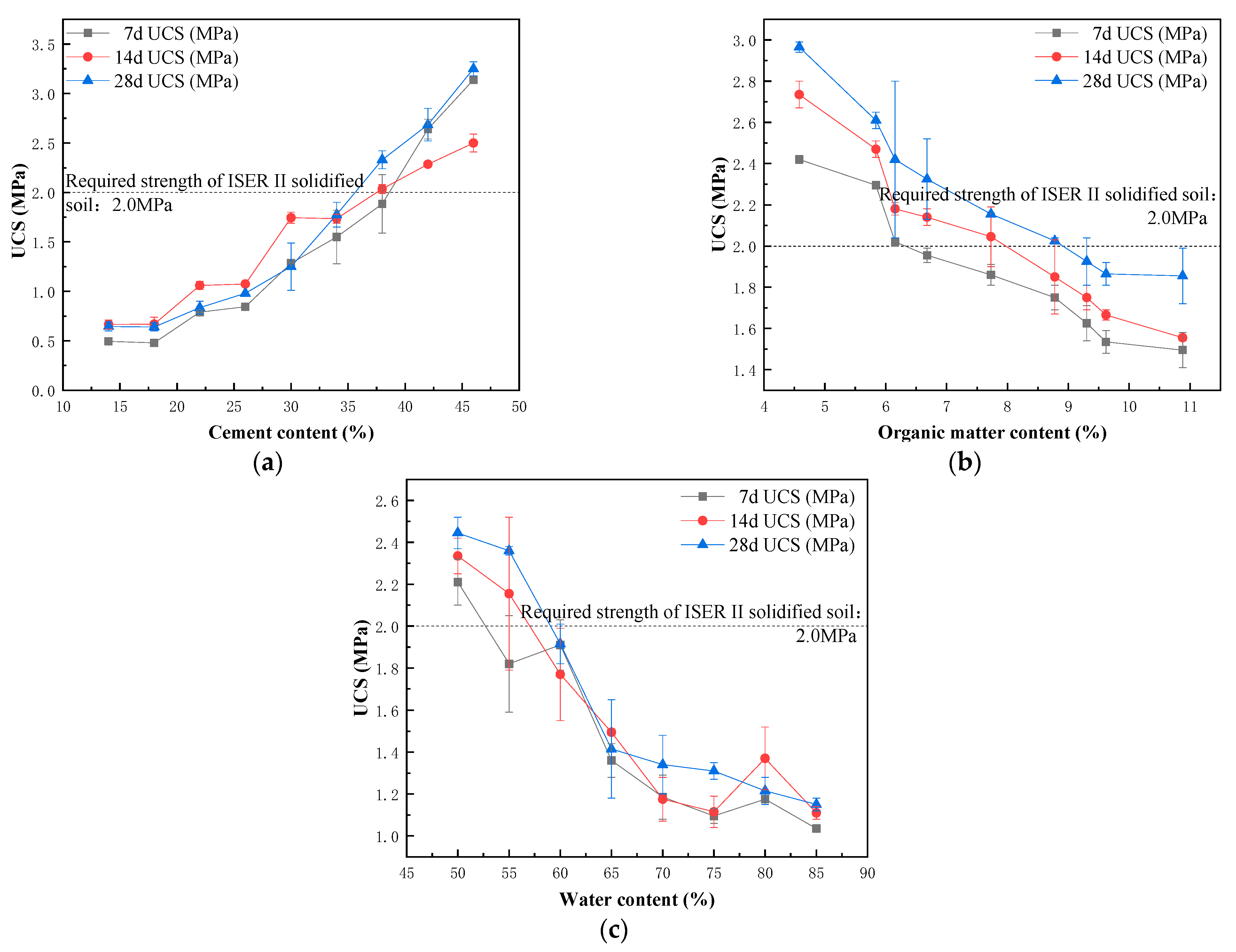
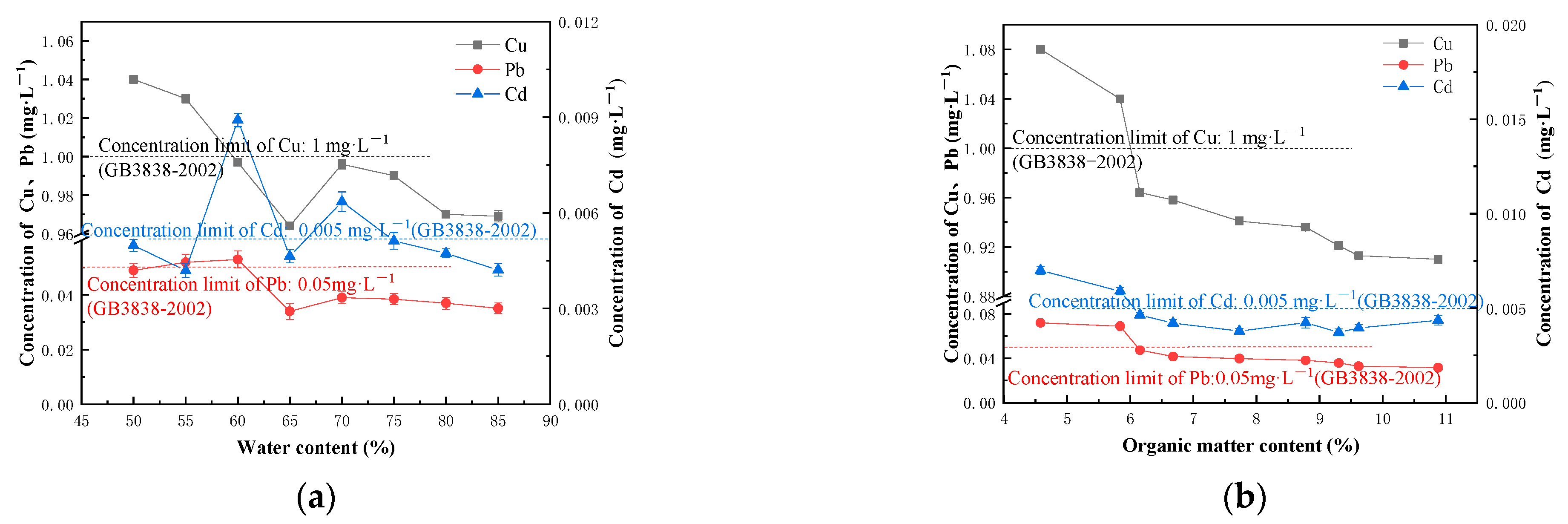
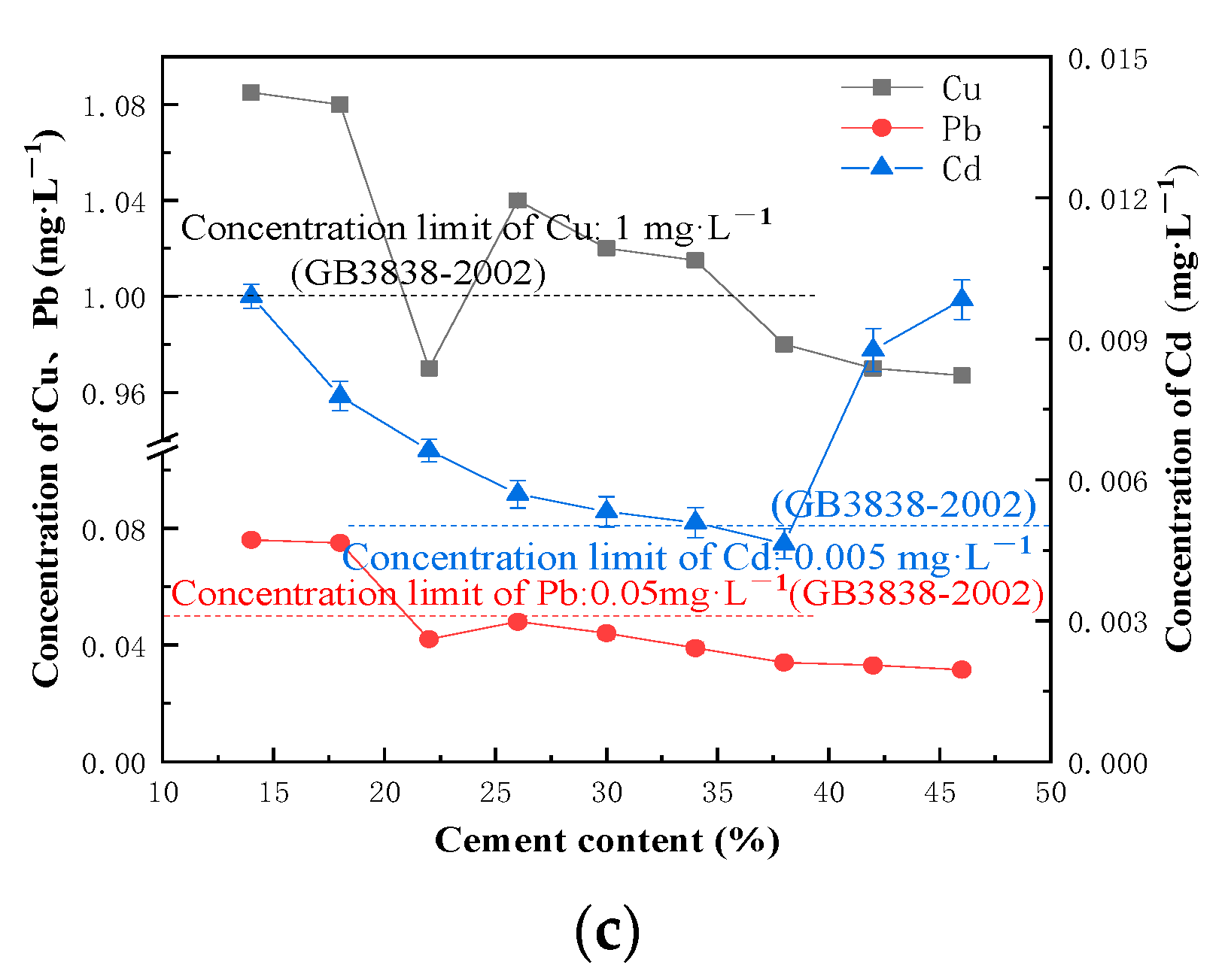
3.1. Factors Influencing the Cement Solidification and Stabilization of Heavy Metal Polluted Sediment in River Course
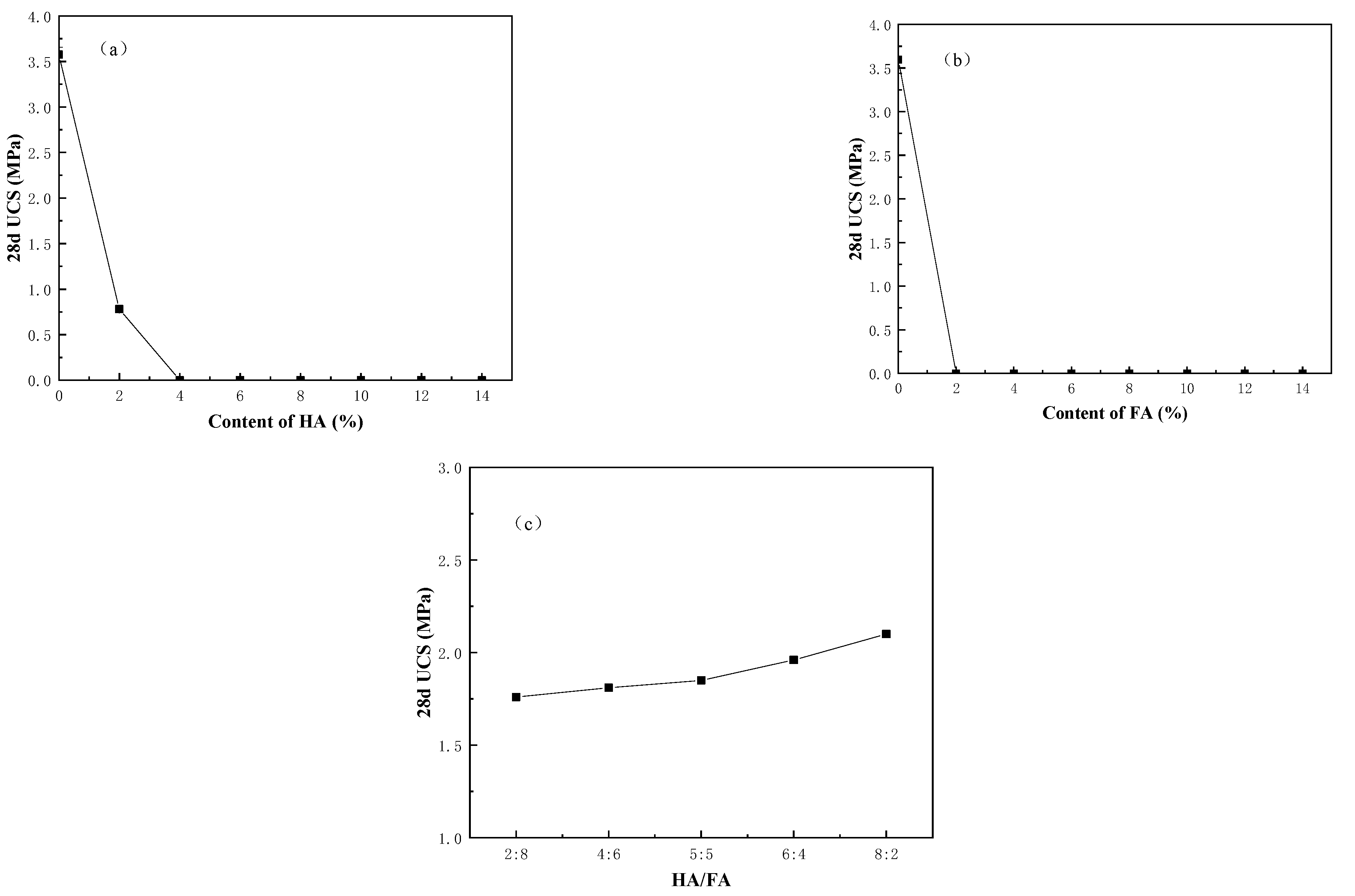
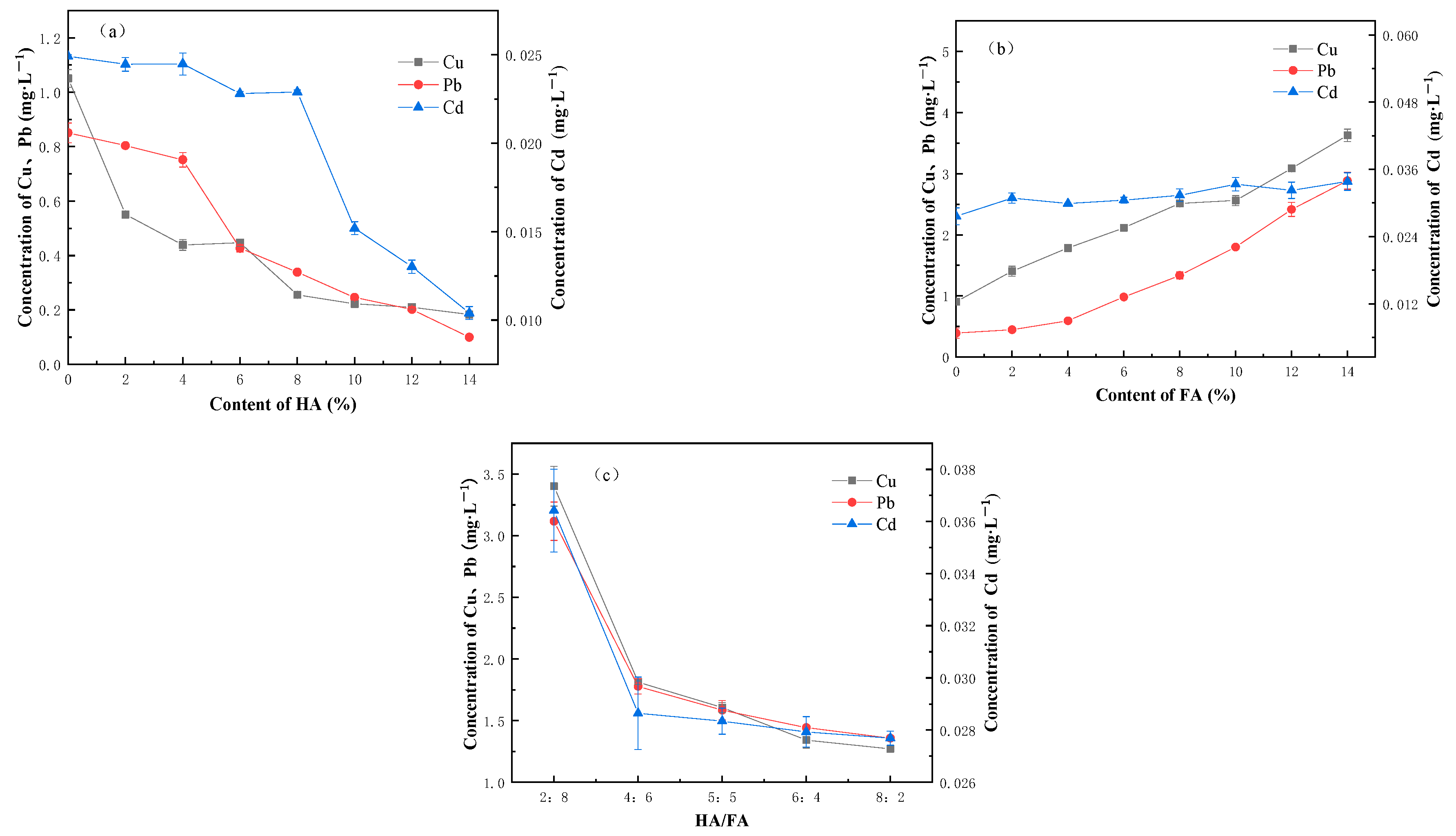
3.2. Effect of Humus on the Solidification and Stabilization of Heavy Metal Contaminated River Sediment
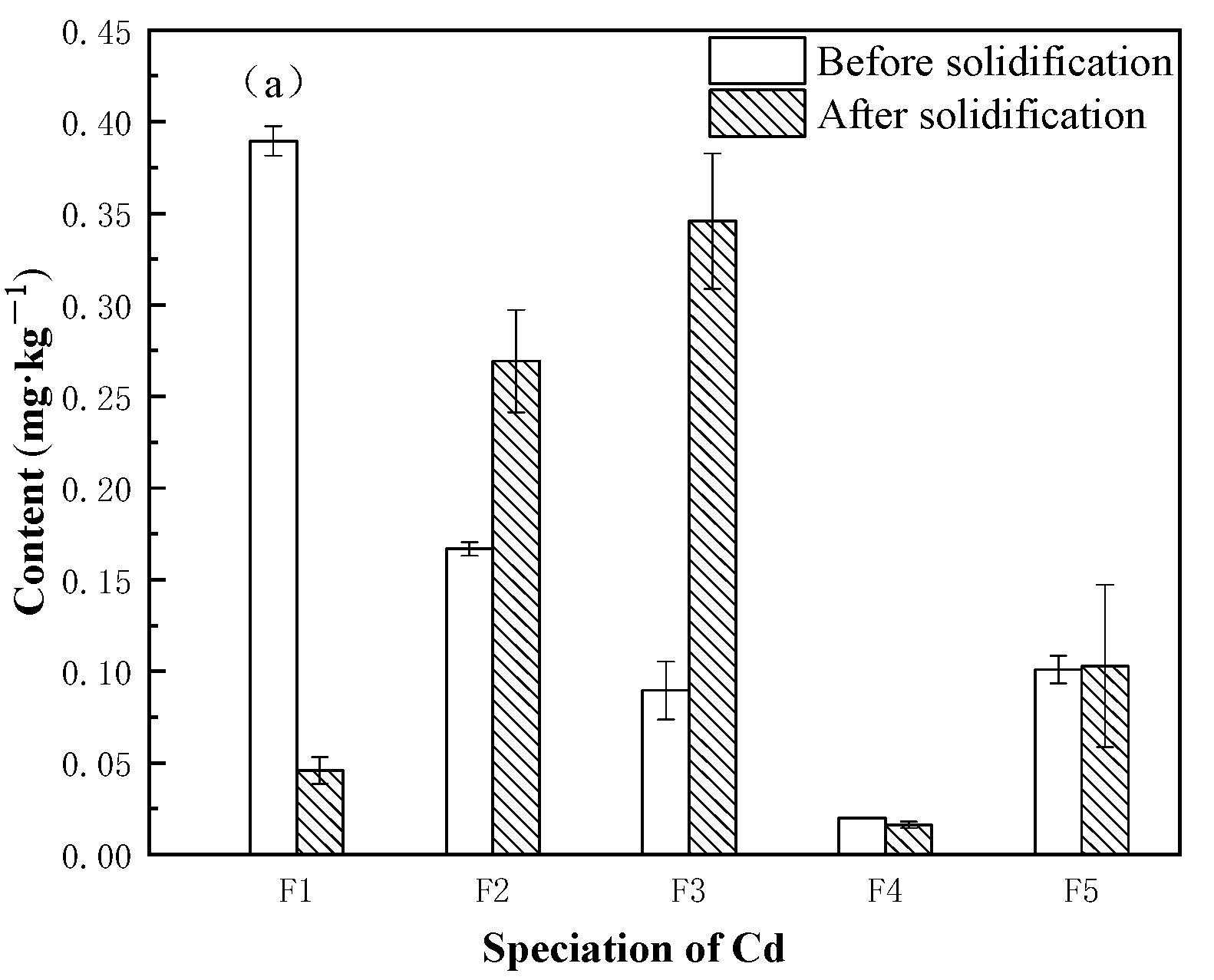
3.3. Speciation Changes of Heavy Metals in Sediment before and after Solidification
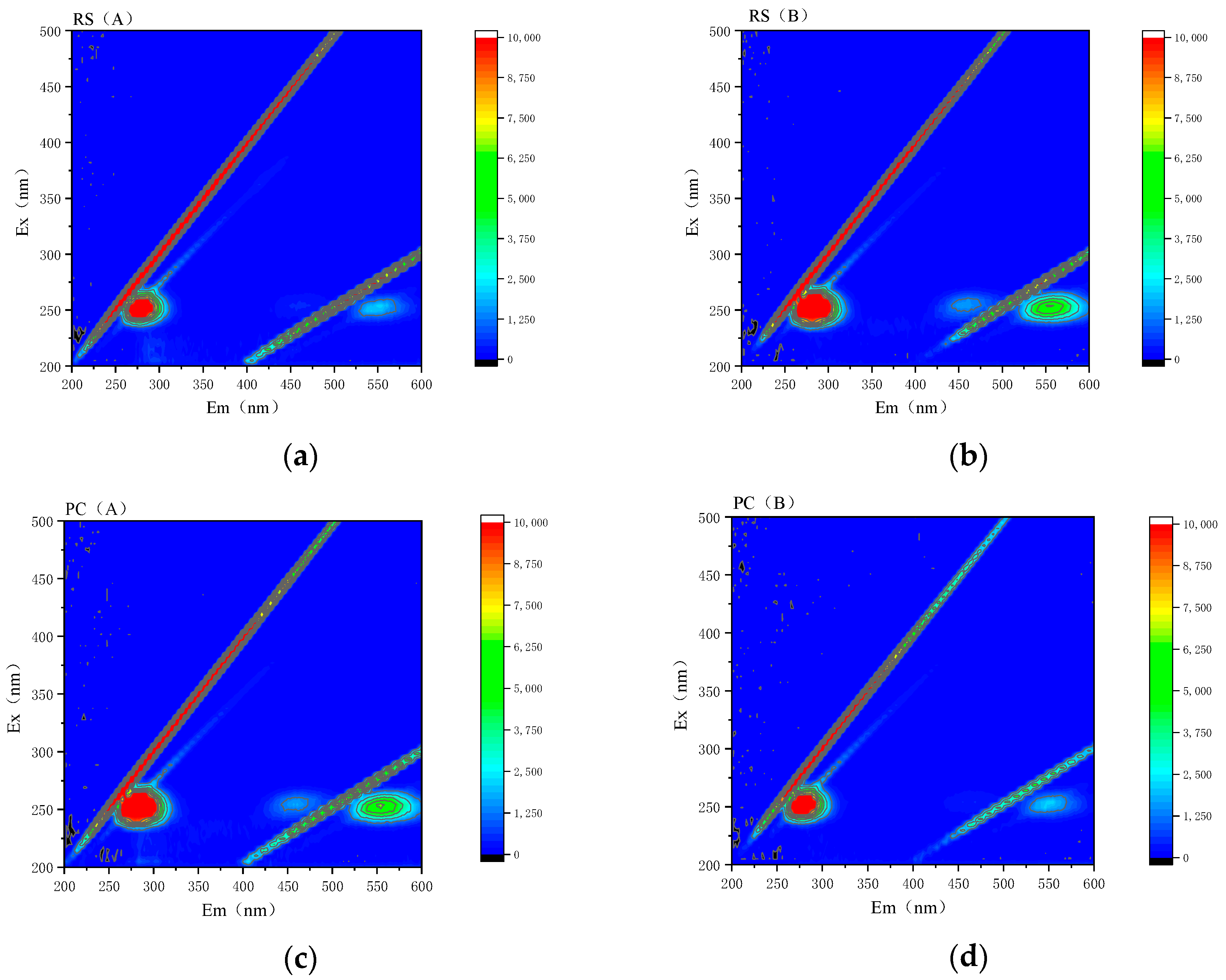
3.4. Characterization of Organic Matter in Sediment before and after Solidification
4. Conclusions
- (1)
- With the increase in the cement content and curing age, the UCS of the solidified block increases, and the leaching concentration of heavy metals decreases. Organic matter has an inhibitory effect on the hydration reaction of the solidified block. When the content of the organic matter in the sediment increases within the limit value, the UCS of the solidified block shows a linear downward trend, the leaching concentration of Cu and Pb decreases, and the leaching concentration of Cd does not change significantly. Water content is negatively correlated with the UCS and the heavy metal leaching concentration of the solidified block. When the water content exceeds 60%, the decline of UCS slows down.
- (2)
- The weakening effect of commercial humic acid/fulvic acid on the strength of cured samples is more obvious than that of natural organic matter, no matter if it is mixed acid or a single humic acid/fulvic acid, both of which have consistent inhibiting and delaying effects on the hydration reaction of cement. Among them, fulvic acid has a stronger weakening effect on the strength of cured samples than humic acid. Humic acid shows a stabilizing effect on heavy metals, while fulvic acid demonstrates an activating effect on heavy metals. With the increase in the humification index (DI), the leaching concentration of heavy metals decreases and their stability improves.
- (3)
- The main speciation of heavy metals in raw sediment includes unstable F1, F2, and F3, with high mobility and high biological toxicity. The key to the stabilization of heavy metals is the treatment of unstable heavy metals. Solidification converts unstable heavy metals into stable heavy metals through adsorption, encapsulation, and co-precipitation, effectively reducing the environmental risk of the sediment.
- (4)
- The fluorescent groups in the sediment before and after curing are essentially the same, and no new fluorescent groups are generated. The fluorescence characteristic index of the sediment samples before and after solidification is 1.96~4.61, indicating that the humus extracted from the samples mainly comes from microbial metabolism, mainly from autogenous sources. After solidification, the content of organic matter in the sediment decreases and the consumption of fulvic acid is more significant than that of humic acid.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pejman, A.; Bidhendi, G.N.; Ardestani, M.; Saeedi, M.; Baghvand, A. A new index for assessing heavy metals contamination in sediments: A case study. Ecol. Indic. 2015, 58, 365–373. [Google Scholar] [CrossRef]
- Gu, Z.J.; Chen, Y.P.; Feng, J.P.; Cao, G.; Li, F.P.; Mao, L.C.; Tao, H. Bottleneck of development and sustainable utilization approaches of urban river sediments disposal based on solidification and stabilizationtechnology. Water Purif. Technol. 2017, 36, 22–29. [Google Scholar]
- Kononova, M.M. Soil Organic Matter, Its Nature, Its Role in Soil Formation and in Soil Fertility; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Xiao, Y.C. The Study on Grouping and Characteristics of Soil Humi; Jilin Agricultural University: Jilin, China, 2004. [Google Scholar]
- Zhang, S.B. The Infection on the Humic Acid in the Progress of Solidifying Soft Soil by Cement Soil; Jilin University: Jilin, China, 2007. [Google Scholar]
- Yang, C.B.; Chu, C.F. Effect of organic matter content on strength of cement-stabilized soil and its countermeasures. Shanxi Archit. 2005, 2, 97–98. [Google Scholar]
- Shao, Y.F. Study on Humus-Containing Soil Stabilization; Zhejiang University: Zhejiang, China, 2006. [Google Scholar]
- Ashworth, D.J.; Alloway, B.J. Influence of dissolved organic matter on the solubility of heavy metals in sewage-sludge-amended soils. Commun. Soil Sci. Plant Anal. 2008, 39, 538–550. [Google Scholar] [CrossRef]
- Cui, S.P.; Lan, M.Z.; Zhang, J.; Wang, C. Effect and incorporation mechanism of heavy metal elements in hazardors Industrial wastes during clinker formation. J. Chin. Ceram. Soc. 2004, 32, 1264–1270. [Google Scholar]
- Li, S.C. Remediation and Evaluation of Different Plants on Typical Heavy Metal Contaminated Sediment; Tianjin University: Tianjin, China, 2014. [Google Scholar]
- Yang, K.; Tao, H.; Li, F.; Gu, Z. lnfuences of mixed chlorde salt on cement solidification and river sediment stabilization. Water Purif. Technol. 2018, 37, 116–121. [Google Scholar]
- Wan, Q. Distribution Characteristics and Risk Assessment of Heavy Metals and Organophosphorus Flame Retardants in Electronic Waste Dismantling Workshop; Shanghai Second Polytechnic University: Shanghai, China, 2021. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Seguential chemical extraction procedure for the speciation of particulate trace metals. Anal Chem. 1979, 51, 844–850. [Google Scholar] [CrossRef]
- GB/T 50123-1999; Standard for Geotechnical Testing Method. China Planning Press: Beijing, China, 1999.
- HJ/T 300-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Acetic Acid Buffer Solution Method. China Environmental Science Press: Beijing, China, 2007.
- GB 3838-2002; Environmental Quality Standards for Surface Water. China Environmental Science Press: Beijing, China, 2002.
- DG/TJ 08-2331-2020; Technical Standard for Construction of In-Situ Ecological Revetment Using Dredged Mud. Shanghai Municipal Commission of Housing and Urban-Rural Development: Shanghai, China, 2020.
- Liang, C.S.; Dang, Z.; Liu, C.Q. Structure and adsorption behavior of humic acid. Chin. J. Anal. Chem. 2006, 34, 288–292. [Google Scholar]
- Liang, C.S.; Dang, Z.; Liu, C.Q. Structure characterization of soil humic acids and adsorption equilibria on phenanthrene. Chin. J. Anal. Chem. 2006, 34, 288–292. [Google Scholar]
- Lam, L.; Wong, Y.L.; Poon, C.S. Degree of hydration and gel/space ratio of high-volume fly ash/cement systems. Cem. Conerete Res. 2000, 30, 747–756. [Google Scholar] [CrossRef]
- Saride, S.; Puppala, A.J.; Chikyala, S.R. Swell-shrink and strength behaviors of lime and cement stabilized expansive organic clays. Appl. Clay Sci. 2013, 85, 39–45. [Google Scholar] [CrossRef]
- Lin, G.L. On the Reaction of Humic Acids on Some Heavy Metal Ions and the Affecting Factors; Southwest Agricultural University: Chongqing, China, 2002. [Google Scholar]
- Wong, J.W.C.; Li, K.L.; Zhou, L.X.; Selvam, A. The sorption of Cdand Zn by different soils in the presence of dissolved organic matter from sludge. Geoderma 2007, 137, 310–317. [Google Scholar] [CrossRef]
- Lamy, I.; Bourgeois, S.; Bermond, A. Soil Cadmiummobility as a consequence of sewage sludge disposal. J. Environ. Qual. 1993, 22, 931–937. [Google Scholar] [CrossRef]
- Pan, Y.Z. Stabilization/Solidification Characteristicsand Mechanism of Organic Claycontaminated by Lead When Using Cement; Zhejiang University: Zhejiang, China, 2019. [Google Scholar]
- Zhang, W.; Li, S.Q.; Fu, J.B. An improved method of solidifying heavy metals in river-lake sediment. J. Yangtze River Sci. Res. Inst. 2021, 38, 15–120. [Google Scholar]
- Ma, C.; Chen, B.; Chen, L. Effect of organic matter on strength development of self compacting earth-based construction stabilized with cement-based composites. Constr. Build. Mater. 2016, 123, 414–423. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhao, B.; Ma, L.T.; Li, L.; Deng, Y.; Xu, Z. Humification process and microbial driving mechanism of composting. Biotechnol. Bull. 2022, 38, 22–28. [Google Scholar]
- Ke, R. Release Pattern and Erosion Effect on Solidified Sludgecontaining Humic Acid; China Three Gorges University: Yichang, China, 2019. [Google Scholar]
- Tremblay, H.; Duchesne, J.; Locat, J.; Leroueil, S. Influence of the nature of organic compounds on fine soil stabilization with cement. Can. Geotech. J. 2002, 39, 535–546. [Google Scholar] [CrossRef]
- Long, L.J. Characteristics of Humic Acid from Sewage Sludge and Adsorption of Heavy Metals after Modification; Chongqing University: Chongqing, China, 2018. [Google Scholar]
- Xiao, D.D. Effects of Humic Acid on Heavy Metal Forms and Antioxidant Enzymes Activities in Soil Contaminated by Lead and Cadmium; Shandong Agricultural University: Tai’an, China, 2017. [Google Scholar]
- Ji, Y.; Shang, C.C.; Feng, Y.; Li, Z.; Zhu, Y.C. Behavior of cement clinker solidified heavy Metal Cu2+ and Zn2+ and hydration leaching. J. Mater. Sci. Eng. 2022, 40, 62–69. [Google Scholar]
- Lin, C.F.; Lin, T.T.; Huang, T.H. Laching processes of the dicalcium and copper oxide solidification/stabilization system. Toxicol. Environ. Chem. 1994, 44, 89–90. [Google Scholar] [CrossRef]
- Xiang, C.Y. Solidification Behavior of Heavy Metal Ions in Portland Cement Hydration Products; Wuhan University of Technology: Wuhan, China, 2015. [Google Scholar]
- Fang, S.R.; Xu, Y.; Wei, X.Y.; Lu, J.L. Speciation distribution and correlation of heavy metals in sediments of typical urban polluted water bodies. Ecol. Environ. Sci. 2009, 18, 2066–2070. [Google Scholar]
- Larid, D.A.; Martens, D.A.; Kingery, W.L. Nature of clay-humic complexes is an agricultural soil: 1. Chemical, biochemical and spectroscopic analyses. Soil Sci. Soc. Am. J. 2001, 65, 1413–1418. [Google Scholar] [CrossRef]
- Li, H.J. Study on Extraction and Characterization of HA and FA; Huazhong University of Science and Technology: Wuhan, China, 2012. [Google Scholar]
- Du, S.L.; Li, Q.; Ding, T.T. Fluorescence spectra analysis of DOM in water of Shaying River basin. Environ. Chem. 2019, 11, 4016. [Google Scholar]
- Cory, R.M.; McKnight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef] [PubMed]
- Ponomareva, B.B. Formation of Humus and Soil; Agricultural Press: Beijing, China, 1987. [Google Scholar]

| Sediment | Classification | Organic Matter Content (%) | Plasticity Index | C (%) | N (%) | H (%) | S (%) |
|---|---|---|---|---|---|---|---|
| High organic sediment | Organic soil | 10.88 | 14.53 | 16.16 | 1.55 | 2.37 | 1.12 |
| Low organic sediment | Low liquid limit silt | 4.58 | 14.62 | 2.88 | 0.20 | 0.67 | 0.22 |
| Heavy Metal | Cd | Cr | Ni | Pb | Zn | Cu |
|---|---|---|---|---|---|---|
| High organic sediment | 0.27 | 83.06 | 41.02 | 32.29 | 91.61 | 27.60 |
| High organic sediment (after pollution) | 0.95 | 83.06 | 41.02 | 170.22 | 91.61 | 151.72 |
| Low organic sediment | 0.28 | 49.53 | 31.44 | 17.04 | 62.80 | 28.27 |
| Low organic sediment (after pollution) | 0.99 | 49.53 | 31.44 | 197.98 | 62.80 | 149.86 |
| Background value | 0.14 | 70.2 | 29.9 | 25.5 | 86.1 | 27.2 |
| GB15618filter value | 0.3 | 200 | 100 | 120 | 250 | 100 |
| Group | Humic Acid Content (mg/g) | Fulvic Acid Content (mg/g) |
|---|---|---|
| RS | 1.89 | 1.83 |
| PC | 1.74 | 1.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Tao, H.; Yang, Y.; Che, Q.; Tang, Q.; Gu, Y. Effect of Humus on the Solidification and Stabilization of Heavy Metal Contaminated River Sediment. Int. J. Environ. Res. Public Health 2023, 20, 4882. https://doi.org/10.3390/ijerph20064882
Gao H, Tao H, Yang Y, Che Q, Tang Q, Gu Y. Effect of Humus on the Solidification and Stabilization of Heavy Metal Contaminated River Sediment. International Journal of Environmental Research and Public Health. 2023; 20(6):4882. https://doi.org/10.3390/ijerph20064882
Chicago/Turabian StyleGao, Huimin, Hong Tao, Yang Yang, Qingyang Che, Qinyi Tang, and Yong Gu. 2023. "Effect of Humus on the Solidification and Stabilization of Heavy Metal Contaminated River Sediment" International Journal of Environmental Research and Public Health 20, no. 6: 4882. https://doi.org/10.3390/ijerph20064882
APA StyleGao, H., Tao, H., Yang, Y., Che, Q., Tang, Q., & Gu, Y. (2023). Effect of Humus on the Solidification and Stabilization of Heavy Metal Contaminated River Sediment. International Journal of Environmental Research and Public Health, 20(6), 4882. https://doi.org/10.3390/ijerph20064882






