Collaborative Purification of Tert-Butanol and N2O over Fe/Co-Zeolite Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalytic Performance Tests
2.3. Catalyst Characterization
3. Results and Discussion
3.1. Characterization
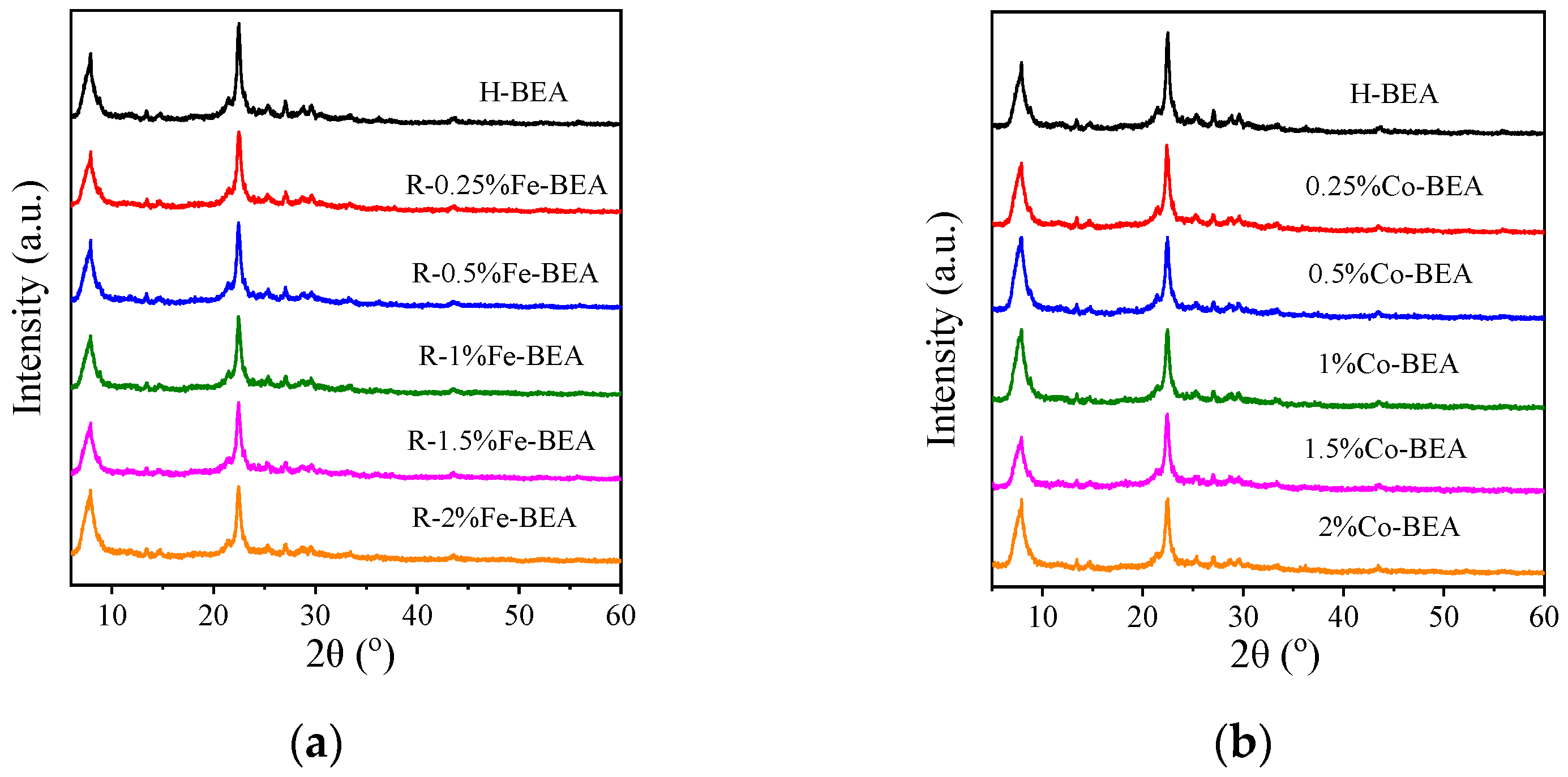
3.1.1. XRD
3.1.2. N2 Adsorption/Desorption
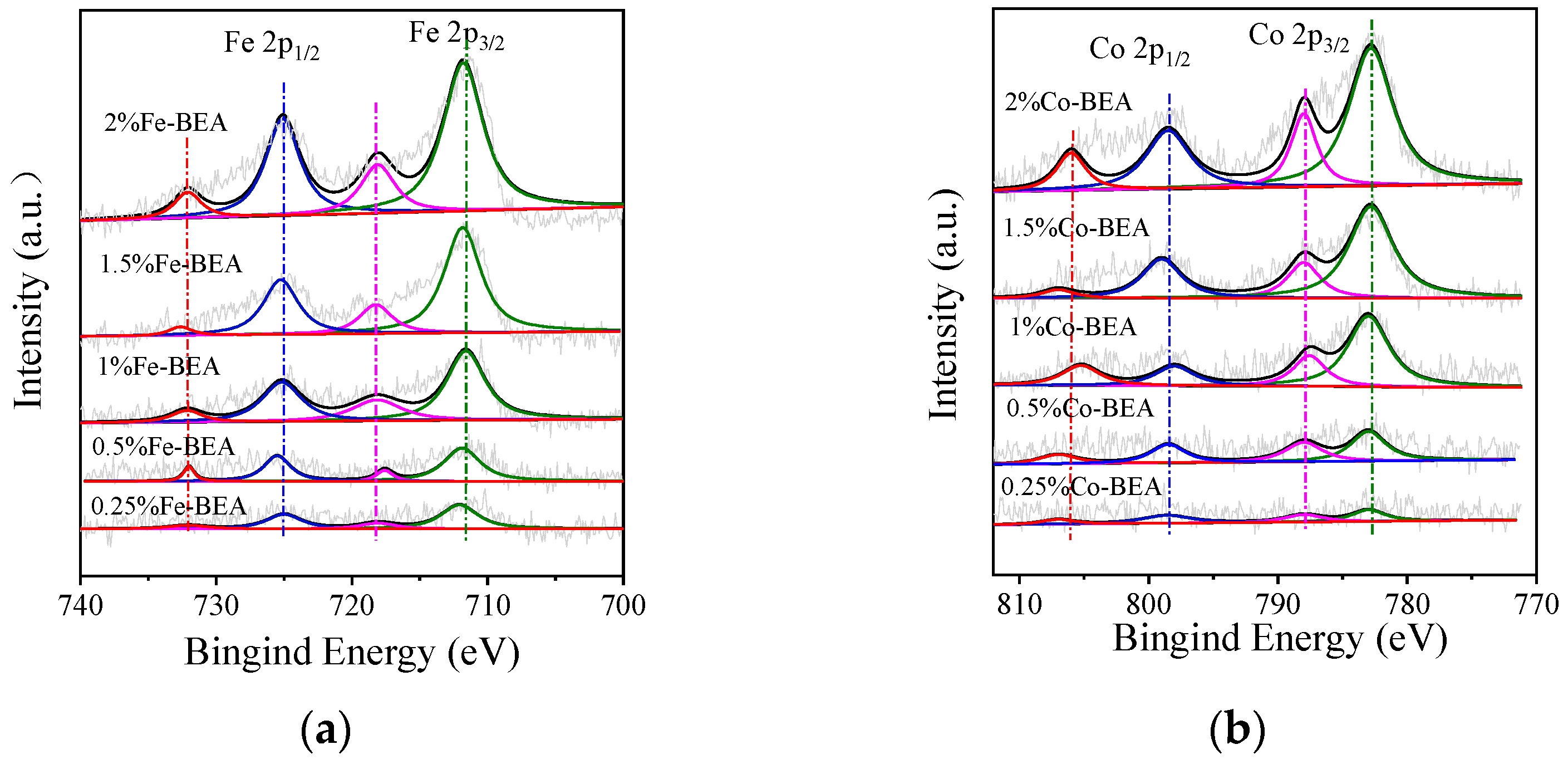
3.1.3. XPS
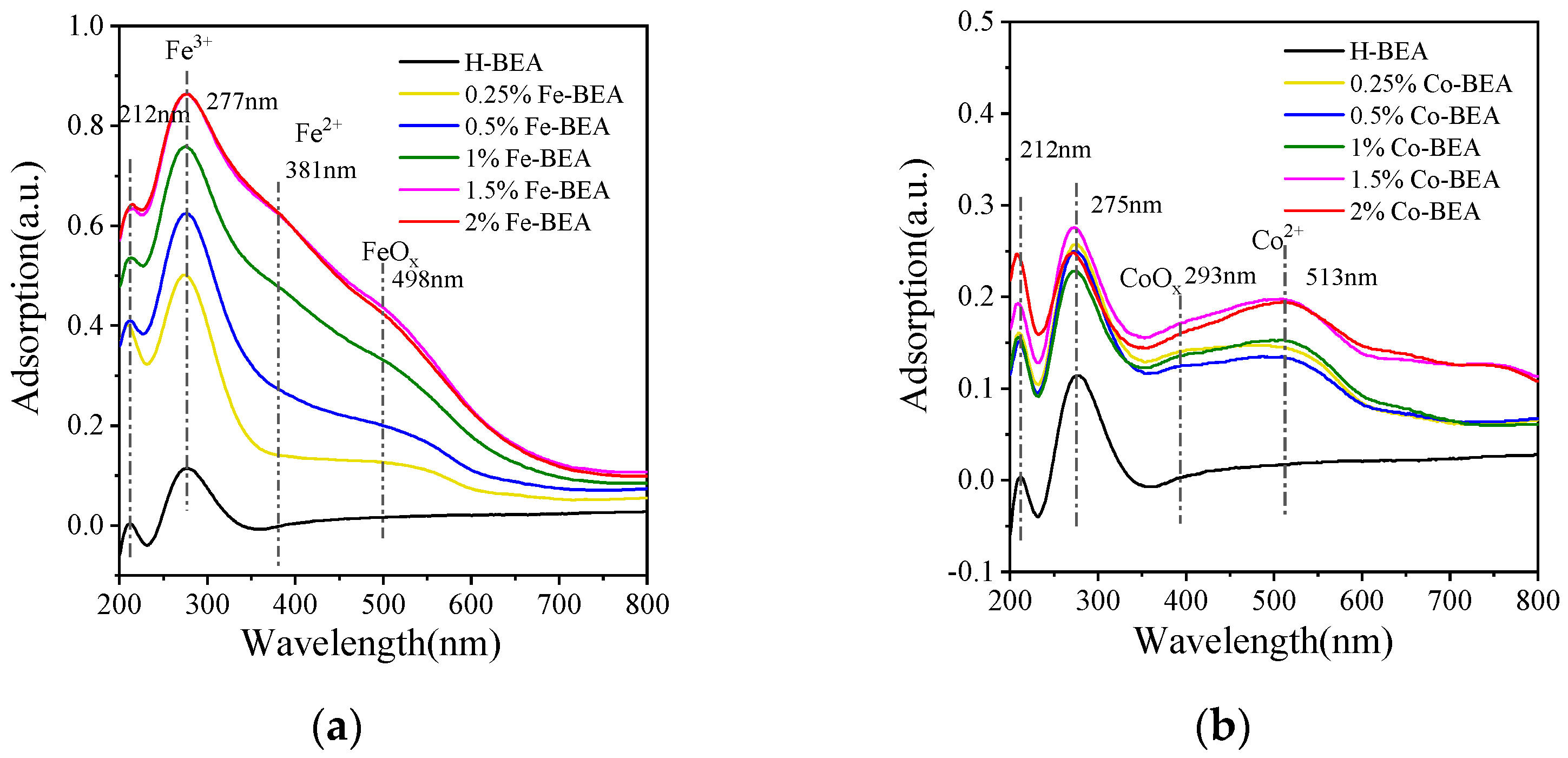
3.1.4. UV−Vis
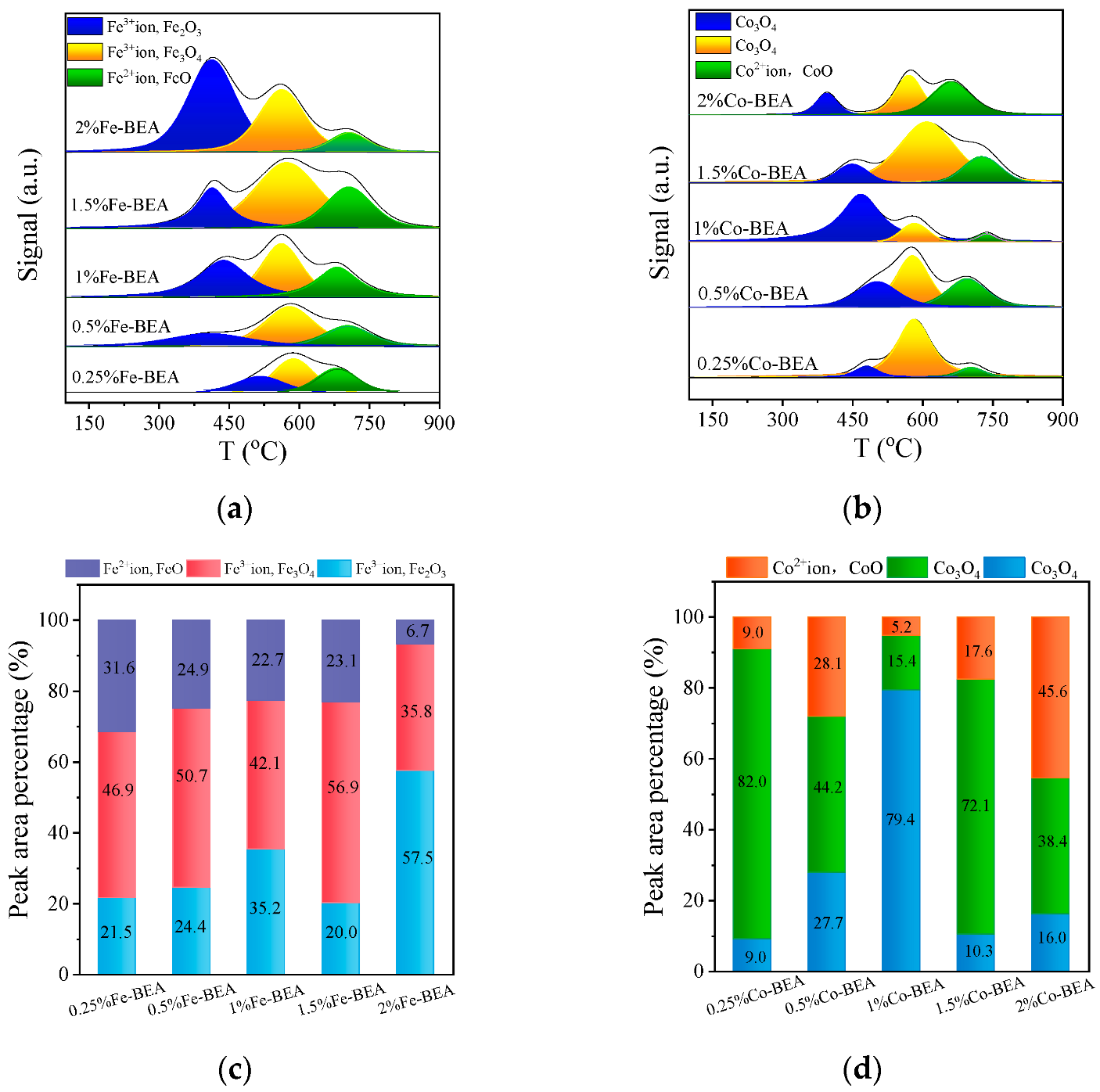
3.1.5. Temperature−Programmed Reduction by Hydrogen
3.2. Activity Measurement
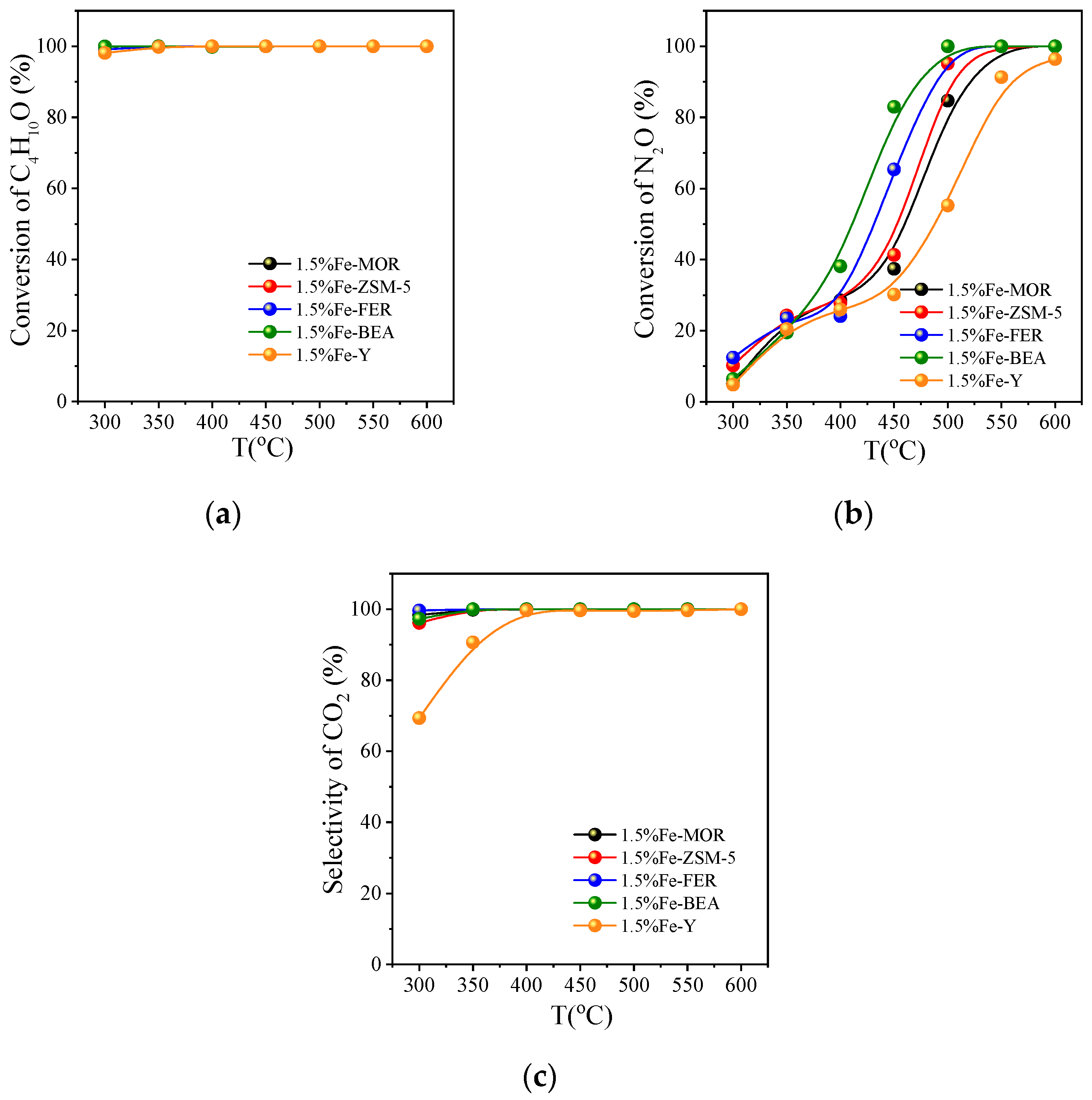
3.2.1. Activity Comparison on Different Fe Zeolite Catalysts
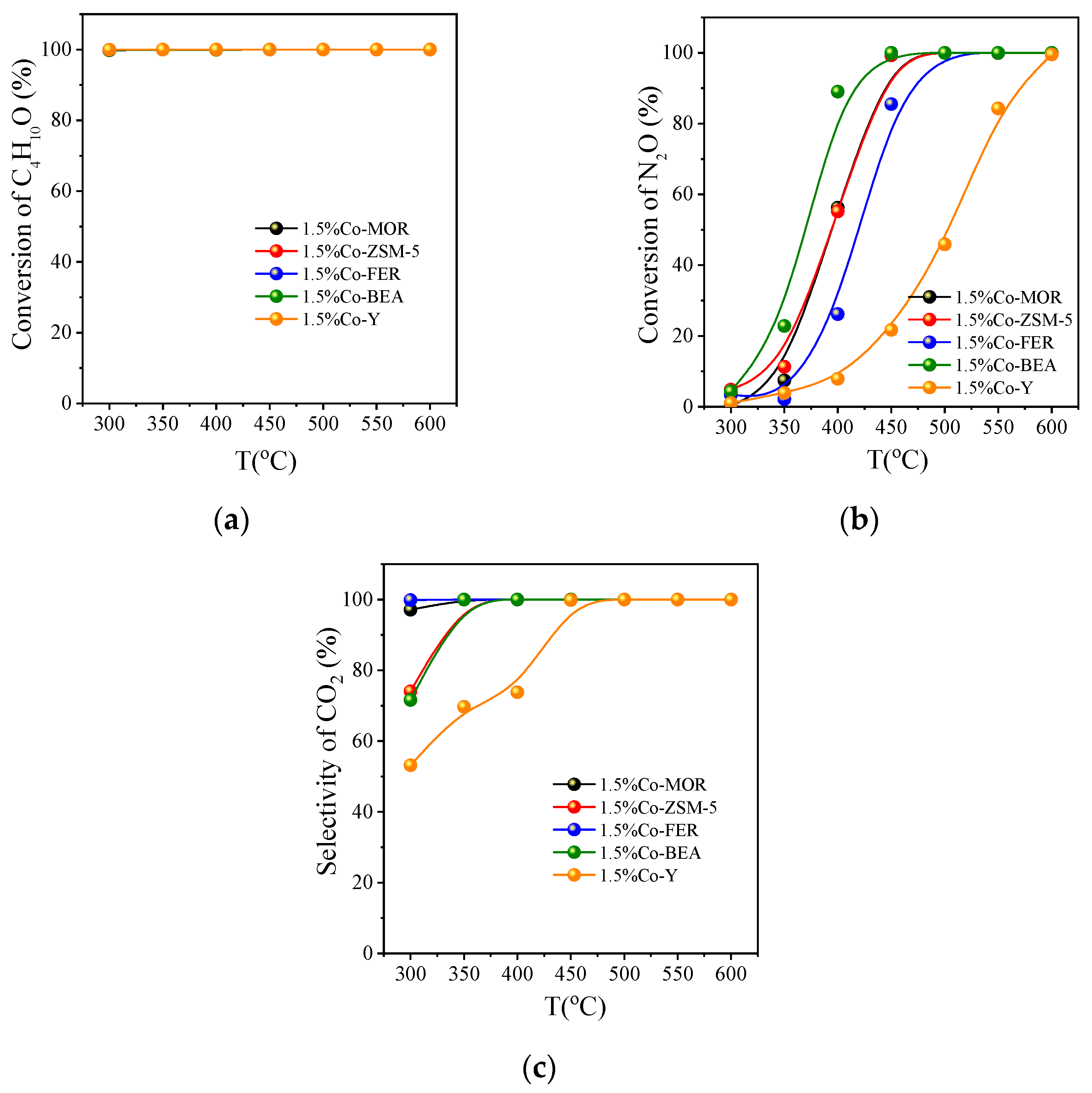
3.2.2. Activity Comparison of Different Co Zeolite Catalysts
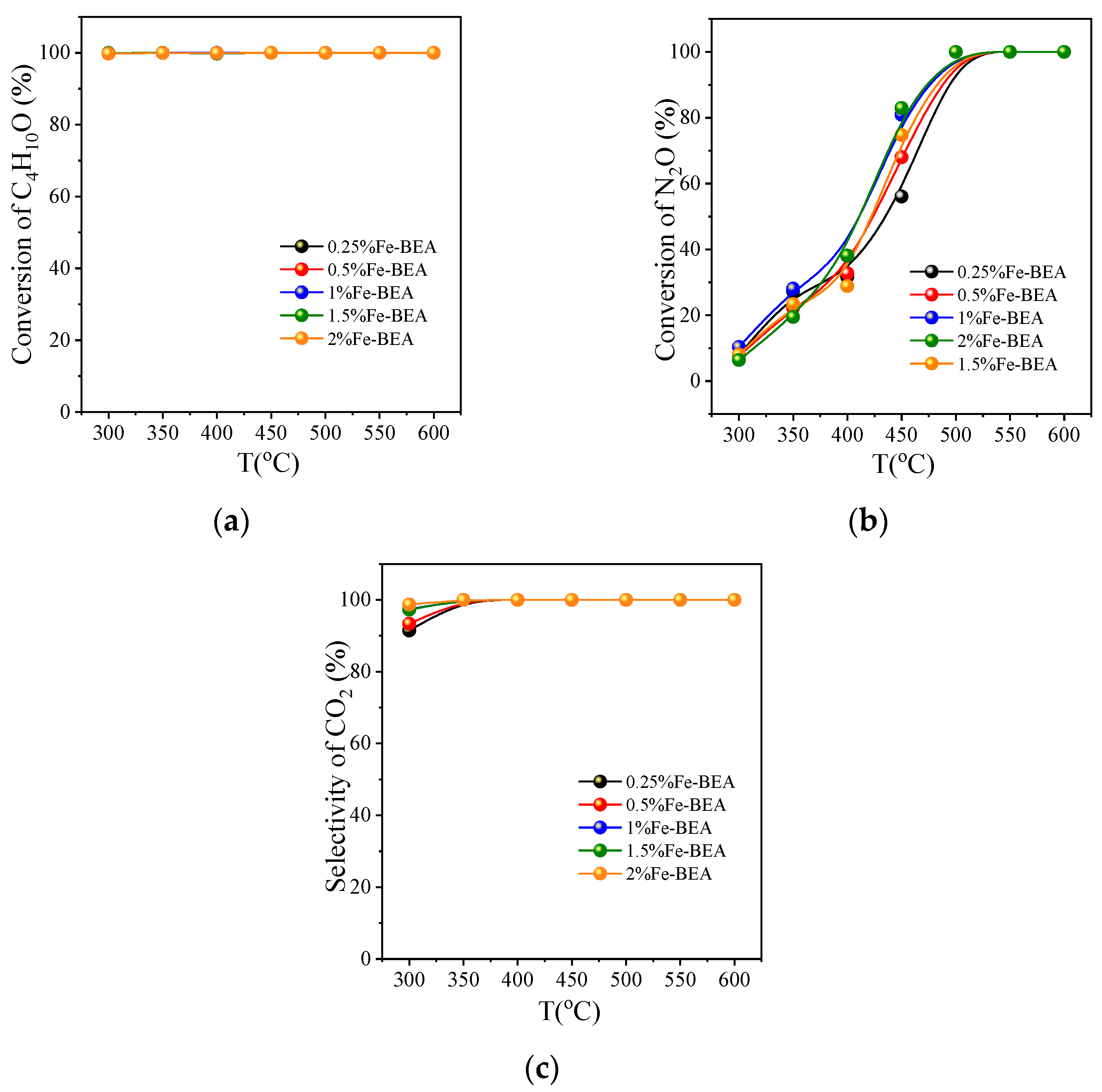
3.2.3. Activity Comparison of Fe-BEA with Different Concentrations
3.2.4. Activity Comparison of Co-BEA with Different Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Dang, X.; Li, S.; Zhang, J.; Zhang, Q.; Cao, L. A comparison of in- and post-plasma catalysis for toluene abatement through continuous and sequential processes in dielectric barrier discharge reactors. J. Clean. Prod. 2020, 276, 124251. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.; Hossain, M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, Q.; Qin, Z.; Li, Q.; Feng, X.; Song, Z.; Cai, Z.; Liu, Y.; Chen, X.; Chen, D.; et al. Reversing Titanium Oligomer Formation towards High-Efficiency and Green Synthesis of Titanium-Containing Molecular Sieves. Angew Chem. Int. Ed. Eng. 2021, 60, 3443–3448. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, M.; Feng, X.; Zhao, S.; Zhou, X.; Li, S.; Zha, M.; Meng, F.; Chen, X.; Liu, Y.; et al. PO(4) (3-) Coordinated Robust Single-Atom Platinum Catalyst for Selective Polyol Oxidation. Angew Chem. Int. Ed. Engl. 2022, 61, e202116059. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qin, H.; Feng, X.; Jin, X.; Liang, W.; Sheng, N.; Zhu, C.; Wang, H.; Yin, B.; Liu, Y.; et al. Synergistic Pt/MgO/SBA-15 nanocatalysts for glycerol oxidation in base-free medium: Catalyst design and mechanistic study. J. Catal. 2019, 370, 434–446. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Walter, E.; Kukkadapu, R.; Guo, Y.; Lu, G.; Weber, R.; Wang, Y.; Peden, C.; Gao, F. Catalytic N2O decomposition and reduction by NH3 over Fe/Beta and Fe/SSZ-13 catalysts. J. Catal. 2018, 358, 199–210. [Google Scholar] [CrossRef]
- Sazama, P.; Sathu, N.; Tabor, E.; Wichterlová, B.; Sklenák, Š.; Sobalík, Z. Structure and critical function of Fe and acid sites in Fe-ZSM-5 in propane oxidative dehydrogenation with N2O and N2O decomposition. J. Catal. 2013, 299, 188–203. [Google Scholar] [CrossRef]
- Kaucky, D.; Sobalik, Z.; Schwarze, M.; Vondrova, A.; Wichterlova, B. Effect of FeH-zeolite structure and Al-Lewis sites on N2O decomposition and NO/NO2-assisted reaction. J. Catal. 2006, 238, 293–300. [Google Scholar] [CrossRef]
- Yan, H.; Li, S.; Feng, X.; Lu, J.; Zheng, X.; Li, R.; Zhou, X.; Chen, X.; Liu, Y.; Chen, D.; et al. Rational screening of metal catalysts for selective oxidation of glycerol to glyceric acid from microkinetic analysis. AIChE J. 2022, 69, e17868. [Google Scholar] [CrossRef]
- Szegedi, Á.; Popova, M.; Minchev, C. Catalytic activity of Co/MCM-41 and Co/SBA-15 materials in toluene oxidation. J. Mater. Sci. 2009, 44, 6710–6716. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Divakar, D.; Aranzabal, A.; González-Velasco, J.; González-Marcos, J. Catalytic oxidation of trichloroethylene over Fe-ZSM-5: Influence of the preparation method on the iron species and the catalytic behavior. Appl. Catal. B Environ. 2016, 180, 210–218. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, J.; Yang, P.; Zuo, S. Preparation of MnCo/MCM-41 catalysts with high performance for chlorobenzene combustion. Chin. J. Catal. 2018, 39, 1994–2001. [Google Scholar] [CrossRef]
- Zhao, W.; Ruan, S.; Qian, S.; Feng, B.; Ao, C.; Wang, L.; Liu, F.; Zhang, L. Abatement of n-Butane by Catalytic Combustion over Co-ZSM-5 Catalysts. Energy Fuels 2020, 34, 12880–12890. [Google Scholar] [CrossRef]
- Dubkov, K.A.; Panov, G.; Parmon, V. Nitrous oxide as a selective oxidant for ketonization of C=C double bonds in organic compounds. Russ. Chem. Rev. 2017, 86, 510–529. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Zhuang, J.; Xiao, Q.; Zhong, Y.; Zhu, W. Direct oxidation of benzene to phenol by N2O over meso-Fe-ZSM-5 catalysts obtained via alkaline post-treatment. Catal. Sci. Technol. 2011, 1, 1250–1255. [Google Scholar] [CrossRef]
- Mi, G.; Li, J.; Zhang, J.; Chen, B. Reaction kinetics of phenol synthesis through one-step oxidation of benzene with N2O over Fe-ZSM-5 zeolite. Korean J. Chem. Eng. 2010, 27, 1700–1706. [Google Scholar] [CrossRef]
- Richards, N.; Nowicka, E.; Carter, J.; Morgan, D.; Dummer, N.; Golunski, S.; Hutchings, G. Investigating the Influence of Fe Speciation on N2O Decomposition Over Fe–ZSM-5 Catalysts. Top. Catal. 2018, 61, 1983–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wang, M.; Li, Z. Fe-Based Metal–Organic Frameworks for Highly Selective Photocatalytic Benzene Hydroxylation to Phenol. ACS Catal. 2015, 5, 6852–6857. [Google Scholar] [CrossRef]
- Xin, H. Novel Carbon-Templating Procedure for Synthesis of Mesoporous Fe/ZSM-5 with Enhanced Performance in Hydroxylation of Benzene with Nitrous Oxide. Energy Environ. Focus 2014, 3, 297–308. [Google Scholar] [CrossRef]
- Suzuki, E.; Nakashiro, K.; Ono, Y. Hydroxylation of Benzene with Dinitrogen Monoxide over H-ZSM-5 Zeolite. Chem. Lett. 2006, 6, 953–956. [Google Scholar] [CrossRef]
- Navarro, R.; Lopez-Pedrajas, S.; Luna, D.; Marinas, J.; Bautista, F. Direct hydroxylation of benzene to phenol by nitrous oxide on amorphous aluminium-iron binary phosphates. Appl. Catal. A Gen. 2014, 474, 272–279. [Google Scholar] [CrossRef]
- Li, L.; Meng, Q.; Wen, J.; Wang, J.; Tu, G.; Xu, C.; Zhang, F.; Zhong, Y.; Zhu, W.; Xiao, Q. Improved performance of hierarchical Fe-ZSM-5 in the direct oxidation of benzene to phenol by N2O. Microporous Mesoporous Mater. 2016, 227, 252–257. [Google Scholar] [CrossRef]
- Ouyang, C.; Li, Y.; Li, J. The ZSM-5-Catalyzed Oxidation of Benzene to Phenol with N2O: Effect of Lewis Acid Sites. Catalysts 2019, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lei, Z.; Zhang, R.; Chen, B. Adsorption of NO and N2O on Cu-BEA zeolite. J. Mol. Struct. THEOCHEM 2010, 957, 41–46. [Google Scholar] [CrossRef]
- Ates, A.; Hardacre, C.; Goguet, A. Oxidative dehydrogenation of propane with N2O over Fe-ZSM-5 and Fe–SiO2: Influence of the iron species and acid sites. Appl. Catal. A Gen. 2012, 441–442, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Su, D.; Wang, Z.; Ma, C.; Wang, M. Catalytic reduction of nitrogen oxide by carbon monoxide, methane and hydrogen over transition metals supported on BEA zeolites. Int. J. Hydrogen Energy 2018, 43, 21969–21981. [Google Scholar] [CrossRef]
- Qi, X.; Kong, D.; Yuan, X.; Xu, Z.; Wang, Y.; Zheng, J.; Xie, Z. Studies on the crystallization process of BEA/MOR co-crystalline zeolite. J. Mater. Sci. 2008, 43, 5626–5633. [Google Scholar] [CrossRef]
- Lin, Q.; Feng, X.; Zhang, H.; Lin, C.; Liu, S.; Xu, H.; Chen, Y. Hydrothermal deactivation over CuFe/BEA for NH3-SCR. J. Ind. Eng. Chem. 2018, 65, 40–50. [Google Scholar] [CrossRef]
- Zhang, R.; Hedjazi, K.; Chen, B.; Li, Y.; Lei, Z.; Liu, N. M(Fe, Co)-BEA washcoated honeycomb cordierite for N2O direct decomposition. Catal. Today 2016, 273, 273–285. [Google Scholar] [CrossRef]
- Shwan, S.; Jansson, J.; Olsson, L.; Skoglundh, M. Effect of post-synthesis hydrogen-treatment on the nature of iron species in Fe-BEA as NH3-SCR catalyst. Catal. Sci. Technol. 2014, 4, 2932–2937. [Google Scholar] [CrossRef]
- Essid, S.; Ayari, F.; Bulánek, R.; Vaculík, J.; Mhamdi, M.; Delahay, G.; Ghorbel, A. Improvement of the conventional preparation methods in Co/BEA zeolites: Characterization and ethane ammoxidation. Solid State Sci. 2019, 93, 13–23. [Google Scholar] [CrossRef]
- Shwan, S.; Jansson, J.; Olsson, L.; Skoglundh, M. Chemical deactivation of Fe-BEA as NH3-SCR catalyst—Effect of phosphorous. Appl. Catal. B: Environ. 2014, 147, 111–123. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.; Wang, Z.; Ma, C.; Qin, Y. Investigation on Fe-Co binary metal oxides supported on activated semi-coke for NO reduction by CO. Appl. Catal. B: Environ. 2017, 201, 636–651. [Google Scholar] [CrossRef]
- Jabłońska, M.; Delahay, G.; Kruczała, K.; Błachowski, A.; Tarach, K.; Brylewska, K.; Petitto, C.; Góra-Marek, K. Standard and Fast Selective Catalytic Reduction of NO with NH3 on Zeolites Fe-BEA. J. Phys. Chem. C 2016, 120, 16831–16842. [Google Scholar] [CrossRef]
- Baran, R.; Krafft, J.-M.; Onfroy, T.; Grzybek, T.; Dzwigaj, S. Influence of the nature and environment of cobalt on the catalytic activity of Co-BEA zeolites in selective catalytic reduction of NO with ammonia. Microporous Mesoporous Mater. 2016, 225, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Zheng, D.; Yu, G.; Xu, R.; Dai, C.; Liu, N.; Wang, N.; Chen, B. N2O Catalytic Decomposition and NH3-SCR Coupling Reactions over Fe-SSZ-13 Catalyst: Mechanisms and Interactions Unraveling via Experiments and DFT Calculations. ACS Catal. 2022, 13, 934–947. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Dai, C.; Xu, R.; Yu, G.; Wang, N.; Chen, B. H2O in situ induced active site structure dynamics for efficient methane direct oxidation to methanol over Fe-BEA zeolite. J. Catal. 2022, 414, 302–312. [Google Scholar] [CrossRef]
- Essid, S.; Ayari, F.; Bulánek, R.; Vaculík, J.; Mhamdi, M.; Delahay, G.; Ghorbel, A. Over– and low–exchanged Co/BEA catalysts: General characterization and catalytic behaviour in ethane ammoxidation. Catal. Today 2018, 304, 103–111. [Google Scholar] [CrossRef]


| Samples | Surface Area m2/g | Micropore Volume cm3/g | Average Pore Diameter nm |
|---|---|---|---|
| H-BEA | 562.0 | 0.18 | 15.0 |
| 0.25% Fe-BEA | 490.1 | 0.14 | 14.0 |
| 0.5% Fe-BEA | 463.7 | 0.15 | 15.8 |
| 1% Fe-BEA | 449.8 | 0.13 | 14.7 |
| 1.5% Fe-BEA | 414.7 | 0.11 | 13.2 |
| 2% Fe-BEA | 421.1 | 0.15 | 15.2 |
| 0.25% Co-BEA | 544.31 | 0.17 | 16.70 |
| 0.5% Co-BEA | 561.71 | 0.17 | 14.85 |
| 1% Co-BEA | 471.31 | 0.14 | 15.80 |
| 1.5% Co-BEA | 488.65 | 0.14 | 15.79 |
| 2% Co-BEA | 483.66 | 0.13 | 14.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Liu, N.; Dai, C.; Xu, R.; Wang, N.; Yu, G.; Chen, B. Collaborative Purification of Tert-Butanol and N2O over Fe/Co-Zeolite Catalysts. Int. J. Environ. Res. Public Health 2023, 20, 4902. https://doi.org/10.3390/ijerph20064902
Wu R, Liu N, Dai C, Xu R, Wang N, Yu G, Chen B. Collaborative Purification of Tert-Butanol and N2O over Fe/Co-Zeolite Catalysts. International Journal of Environmental Research and Public Health. 2023; 20(6):4902. https://doi.org/10.3390/ijerph20064902
Chicago/Turabian StyleWu, Ruiqi, Ning Liu, Chengna Dai, Ruinian Xu, Ning Wang, Gangqiang Yu, and Biaohua Chen. 2023. "Collaborative Purification of Tert-Butanol and N2O over Fe/Co-Zeolite Catalysts" International Journal of Environmental Research and Public Health 20, no. 6: 4902. https://doi.org/10.3390/ijerph20064902
APA StyleWu, R., Liu, N., Dai, C., Xu, R., Wang, N., Yu, G., & Chen, B. (2023). Collaborative Purification of Tert-Butanol and N2O over Fe/Co-Zeolite Catalysts. International Journal of Environmental Research and Public Health, 20(6), 4902. https://doi.org/10.3390/ijerph20064902










