Polycyclic Aromatic Hydrocarbons (PAHs) in Roasted Pork Meat and the Effect of Dried Fruits on PAH Content
Abstract
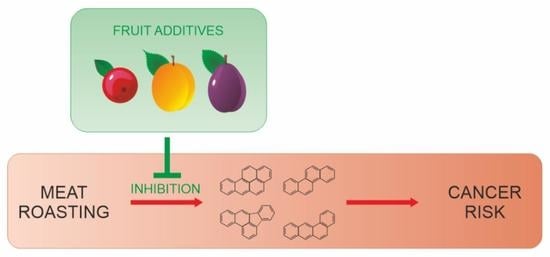
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Meat Dishes—Pork Loin Stuffed with Dried Fruits
2.3. Thermal Processing of Meat
2.4. Extraction of PAHs Fraction from Meat Samples
2.5. Determination of PAHs by HPLC and Fluorescence Detection (FLD)
2.6. Limits of Detection and Quantification, Calibration Plots, Repeatability and Reproducibility, and Recovery of PAHs from Meat Matrix
2.7. Identification of PAHs by Gas Chromatography—Mass Spectrometry/Mass Spectrometry (GC-MS/MS)
2.8. Statistical Analysis
2.9. Determination of Dry Mass and Water Containment in Fruits
3. Results and Discussion
3.1. PAHs in Meat Samples
3.2. Influence of Dried Fruits on PAHs
3.3. Identification of PAHs in Meat Samples Using GC-MS/MS Technique
3.4. Reduction in Exposure to PAHs through Consumption of Meat Dishes Prepared with Natural Additive
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Nigri, A.; Levantesi, S.; Piscopo, G. Causes-of-death specific estimates from synthetic health measure: A methodological framework. Soc. Indic. Res. 2022, 162, 887–908. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Anderson, R.N. The leading causes of death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Zhao, R.; Wang, D.; Ma, Y.; Li, A. Plant natural products: Promising resources for cancer chemoprevention. Molecules 2021, 26, 933. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Angua, K.M.; Rosner, B.A.; Barnett, J. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Red Meat and Processed Meat. In Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Publication: Lyon, France, 2018; p. 114. [Google Scholar]
- Kobets, T.; Smith, B.P.C.; Williams, G.M. Food-borne chemical carcinogens and the evidence for human cancer risk. Foods 2022, 11, 2828. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Ferreira, I. Domestic cooking of muscle foods: Impact on composition of nutrients and contaminants. Compr. Rev. Food Sci. Food Saf. 2018, 17, 255–509. [Google Scholar] [CrossRef] [Green Version]
- Agus, B.A.P.; Rajentran, K.; Selamat, J.; Lestari, S.D.; Umar, N.B.; Hussain, N. Determination of 16 EPA PAHs in food using gas and liquid chromatography. J Food Compos. Anal. 2023, 116, 105038. [Google Scholar] [CrossRef]
- Hamidi, E.N.; Hajeb, P.; Selamat, J.; Lee, S.Y.; Razis, A.F.A. Bioaccessibility of polycyclic aromatic hydrocarbons (PAHs) in grilled meat: The effects of meat doneness and fat content. Int. J. Environ. Res. Public Health 2022, 19, 736. [Google Scholar] [CrossRef]
- Molognoni, L.; Daguer, H.; Motta, G.E.; Merlo, T.C.; Lindner, J.D. Interactions of preservatives in meat processing: Formation of carcinogenic compounds, analytical methods, and inhibitory agents. Food Res. Int. 2019, 125, 108608. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]pyrene—Environmental occurrence, human exposure, and mechanisms of toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef]
- Wen, N.; Cai, K.; Li, Y.; Zhang, S.; Wang, Y.; Guo, J.; Chen, C.; Xu, B. Small molecular weight aldose (D-Glucose) and basic amino acids (L-lysine, L-arginine) increase the occurrence of PAHs in grilled pork sausages. Molecules 2018, 23, 3377. [Google Scholar]
- Lee, Y.N.; Lee, S.; Kim, J.S.; Patra, J.K.; Shin, H.S. Chemical analysis techniques and investigation of polycyclic aromatic hydrocarbons in fruit, vegetables and meats and their products. Food Chem. 2019, 277, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Polak-Śliwińska, M.; Paszczyk, B.; Śliwiński, M. Evaluation of polycyclic aromatic hydrocarbons in smoked cheeses made in Poland by HPLC Method. Molecules 2022, 27, 6909. [Google Scholar] [CrossRef]
- Roudbari, A.; Nazari, R.R.; Shariatifar, N.; Moazzen, M.; Abdolshahi, A.; Mirzamohammadi, S.; Madani-Tonekaboni, M.; Delvarianzadeh, M.; Arabameri, M. Concentration and health risk assessment of polycyclic aromatic hydrocarbons in commercial tea and coffee samples marketed in Iran. Environ. Sci. Pollut. Res. 2021, 28, 4827–4839. [Google Scholar] [CrossRef]
- Ji, J.; Jiang, M.; Zhang, Y.; Hou, J.; Sun, S. Polycyclic aromatic hydrocarbons contamination in edible oils: A review. Food Rev. Int. 2022. [Google Scholar] [CrossRef]
- Sakin, A.E.; Mert, C.; Tasdemir, Y. PAHs, PCBs and OCPs in olive oil during the fruit ripening period of olive fruits. Environ. Geochem. Health 2022. [Google Scholar] [CrossRef]
- Soceanu, A.; Dobrinas, S.; Popescu, V. Levels of polycyclic aromatic hydrocarbons in toasted bread. Polycycl. Aromat. Compd. 2022, 42, 6112–6123. [Google Scholar] [CrossRef]
- Kiani, A.; Ahmadloo, M.; Moazzen, M.; Shariatifar, N.; Shahsavari, S.; Arabameri, M.; Hasani, M.M.; Azari, A.; Abdel-Wahhab, M.A. Monitoring of polycyclic aromatic hydrocarbons and probabilistic health risk assessment in yogurt and butter in Iran. Food Sci. Nutr. 2021, 9, 2114–2128. [Google Scholar] [CrossRef]
- Zhu, B.T. Natural compounds as cancer chemopreventive and chemotherapeutic agents: Insights gained from mechanistic and pharmacologic studies. Anticancer Agents Med. Chem. 2012, 12, 1157–1158. [Google Scholar] [PubMed]
- Khizar, S.; Elkalla, E.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Magnetic nanoparticles: Multifunctional tool for cancer therapy. Expert Opin. Drug Deliv. 2023, 20, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Shankar, M.; Swetha, M.; Keerthana, C.K.; Rayginia, T.R.; Anto, R.J. Cancer chemoprevention: A strategic approach using phytochemicals. Front. Pharmacol. 2022, 12, 809308. [Google Scholar]
- Yousefi, M.; Shariatifar, N.; Ebrahimi, M.T.; Mortazavian, A.M.; Mohammadi, A.; Khorshidian, N.; Arab, M.; Hosseini, H. In vitro removal of polycyclic aromatic hydrocarbons by lactic acid bacteria. J. Appl. Microbiol. 2019, 126, 954–964. [Google Scholar] [CrossRef]
- The Commission of the European Communities. European Commission European Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; Di Domenico, A.; Cruz, M.L.; Fürst, P.; Fink-Gremmels, J.; Galli, C.; et al. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on polycyclic aromatic hydrocarbons in food. EFSA J. 2008, 724, 1–114. [Google Scholar]
- European Union. Commission Regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, L215, 4–8. [Google Scholar]
- Sharma, R.K.; Chan, K.G.; Hajaligol, M.R. Product compositions from pyrolysis of some aliphatic α-amino acids. J. Anal. Appl. Pyrolysis 2006, 75, 69–81. [Google Scholar] [CrossRef]
- Lai, Y.; Lee, Y.T.; Inbaraj, B.S.; Chen, B.H. Formation and inhibition of heterocyclic amines and polycyclic aromatic hydrocarbons in ground pork during marinating. Foods 2022, 11, 3080. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, T.; Simal-Gandar, J. Summarizing minimization of polycyclic aromatic hydrocarbons in thermally processed foods by different strategies. Food Control 2023, 146, 109514. [Google Scholar] [CrossRef]
- Cordeiro, T.; Viegas, M.; Silva, M.; Martins, Z.; Fernandes, I.; Ferreira, I.M.L.P.V.O.; Pinho, O.; Mateus, N.; Calhau, C. Inhibitory effect of vinegars on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. Meat Sci. 2020, 167, 108083. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.N.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.; Rogero, M.M.; Costa de Camargo, A.; Torres, E.A. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Duedahl-Olesen, L.; Ionas, A. Formation and mitigation of PAHs in barbecued meat—A review. Food Sci. Nutr. 2022, 62, 3553–3568. [Google Scholar] [CrossRef] [PubMed]
- de Moura Neves, T.; da Cunha, D.T.; de Rosso, V.V.; Álvares, S.M.D. Effects of seasoning on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in meats: A meta-analysis. Compr. Rev. Food Sci. Food Saf. 2021, 20, 526–541. [Google Scholar] [CrossRef]
- Hu, G.; Cai, K.; Li, Y.; Hui, T.; Wang, Z.; Chen, C.; Xu, B.; Zhang, D. Significant inhibition of garlic essential oil on benzo[a]pyrene formation in charcoal-grilled pork sausages relates to sulfide compounds. Food Res. Int. 2021, 141, 1101. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Wang, C.; Huang, S.; Lei, Y.; Huang, M.; Zhang, X. Inhibitory effect of coriander (Coriandrum sativum L.) extract marinades on the formation of polycyclic aromatic hydrocarbons in roasted duck wings. Food Sci. Hum. Wellness 2023, 12, 1128–1135. [Google Scholar] [CrossRef]
- Onopiuk, A.; Kołodziejczak, K.; Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Szpicer, A.; Stelmas, A. Influence of plant extract addition to marinades on polycyclic aromatic hydrocarbon formation in grilled pork meat. Molecules 2022, 27, 175. [Google Scholar] [CrossRef]
- Viegas, O.; Yebra-Pimentel, I.; Martínez-Carballo, E.; Simal-Gandara, J.; Ferreira, I.M.P.L.V.O. Effect of beer marinades on formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. J. Agric. Food Chem. 2014, 62, 2638–2643. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Y.; Wang, H.; Bai, Y.; Dai, C.; Li, C. Phenolic compounds in beer inhibit formation of polycyclic aromatic hydrocarbons from charcoal-grilled chicken wings. Food Chem. 2019, 294, 578–586. [Google Scholar] [CrossRef]
- Park, K.; Pyo, H.; Kim, W.; Sun, K. Effects of cooking methods and tea marinades on the formation of benzo[a]pyrene in grilled pork belly (Samgyeopsal). Meat Sci. 2017, 129, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Li, C.; Xu, X.; Zhou, G. Effects of phenolic acid marinades on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled chicken wings. J. Food Prot. 2019, 82, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Bulanda, S.; Janoszka, B. Consumption of thermally processed meat containing carcinogenic compounds (polycyclic aromatic hydrocarbons and heterocyclic aromatic amines) versus a risk of some cancers in humans and the possibility of reducing their formation by natural food additives—A literature review. Int. J. Environ. Res. Public Health 2022, 19, 4781. [Google Scholar] [PubMed]
- Lu, F.; Kuhnle, G.; Cheng, Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Control 2018, 92, 399–411. [Google Scholar] [CrossRef]
- Lai, Y.W.; Lee, Y.T.; Cao, H.; Zhang, H.L.; Chen, B.H. Extraction of heterocyclic amines and polycyclic aromatic hydrocarbons from pork jerky and the effect of flavoring on formation and inhibition. Food Chem. 2023, 402, 134290. [Google Scholar] [CrossRef]
- Cao, J.; Yang, L.; Yea, B.; Chai, Y.; Liu, L. Effect of apple polyphenol and three antioxidants on the formation of polycyclic aromatic hydrocarbon in barbecued pork. Polycycl. Aromat. Compd. 2022. [Google Scholar] [CrossRef]
- Janoszka, B. HPLC-fluorescence analysis of polycyclic aromatic hydrocarbons (PAHs) in pork meat and its gravy fried without additives and in the presence of onion and garlic. Food Chem. 2011, 126, 1344–1353. [Google Scholar] [CrossRef]
- Janoszka, B.; Warzecha, L.; Błaszczyk, U.; Bodzek, D. Organic compounds formed in thermally treated high-protein food, Part I: Polycyclic aromatic hydrocarbons. Acta Chromatogr. 2004, 14, 115–128. [Google Scholar]
- Rivera, L.; Curto, M.J.C.; Pais, P.; Galceran, M.T.; Puignou, L. Solid-phase extraction for the selective isolation of polycyclic aromatic hydrocarbons, azaarenes and heterocyclic aromatic amines in charcoal-grilled meat. J. Chromatogr. 1996, 731, 85–94. [Google Scholar] [CrossRef]
- Śnieżek, E.; Szumska, M.; Nowak, A.; Muzyka, R.; Janoszka, B. Application of high-performance liquid chromatography with fluorescence detection for non-polar heterocyclic aromatic amines and acridine derivatives determination in pork loin roasted in a roasting bag. Foods 2022, 27, 3385. [Google Scholar] [CrossRef]
- Konieczka, P.; Namieśnik, J. Evaluation and Quality Control of Analytical Measurement Results, 1st ed.; PWN: Warsaw, Poland, 2019; pp. 225–300. [Google Scholar]
- Karslıoğlu, B.; Kolsarıcı, N. The Effects of fat content and cooking procedures on the PAH content of beef doner kebabs. Polycycl. Aromat. Compd. 2022. [Google Scholar] [CrossRef]
- Worobiej, E. Oznaczanie Zawartości Wody w Produktach Spożywczych. In Wybrane Zagadnienia z Analizy Żywności; Wydawnictwo SGGW: Warszawa, Poland, 2009; 251p. [Google Scholar]
- Siddique, R.; Zahoor, A.F.; Ahmad, A.; Zahid, F.M.; Karrar, E. Impact of different cooking methods on polycyclic aromatic hydrocarbons in rabbit meat. Food Sci. Nutr. 2021, 9, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, A.; Jinap, S.; Abas, F.; Sakar, Z.I. Determination of polycyclic aromatic hydrocarbons in grilled meat. Food Control 2010, 21, 606–610. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Aaslyng, M.; Meinert, L.; Christensen, T.; Jensen, A.H.; Binderup, L.M. Polycyclic aromatic hydrocarbons (PAH) in Danish barbecued meat. Food Control 2015, 57, 169–176. [Google Scholar] [CrossRef]
- Aaslyng, A.D.; Duedahl-Olesen, L.; Jensen, K.; Meinert, L. Content of heterocyclic amines and polycyclic aromatic hydrocarbons in pork, beef and chicken barbecued at home by Danish consumers. Meat Sci. 2013, 93, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Samiee, S.; Fakhri, Y.; Sadighara, P.; Arabameri, M.; Rezaei, M.; Nabizadeh, R.; Shariatifar, N.; Khaneghah, A.M. The concentration of polycyclic aromatic hydrocarbons (PAHs) in the processed meat samples collected from Iran’s market: A probabilistic health risk assessment study. Environ. Sci. Pollut. Res. Int. 2020, 27, 21126–21139. [Google Scholar] [CrossRef]
- Min, S.; Patra, J.K.; Shin, H.S. Factors influencing inhibition of eight polycyclic aromatic hydrocarbons in heated meat model system. Food Chem. 2018, 239, 993–1000. [Google Scholar] [CrossRef]
- Gomes, A.; Santos, C.; Almeida, J.; Elias, M.; Roseiro, L.C. Effect of fat content, casing type and smoking procedures on PAHs contents of Portuguese traditional dry fermented sausages. Food Chem. Toxicol. 2013, 58, 369–374. [Google Scholar] [CrossRef]
- Lee, J.; Han, J.; Jung, M.; Lee, K.; Chung, M. Effects of thawing and frying methods on the formation of acrylamide and polycyclic aromatic hydrocarbons in chicken meat. Foods 2020, 9, 573. [Google Scholar] [CrossRef]
- Xin, L.; Hu, M.; Ma, X.; Wu, S.; Yoong, J.H.; Chen, S.; Tarmizi, A.H.A.; Zhang, G. Selection of 12 vegetable oils influences the prevalence of polycyclic aromatic hydrocarbons, fatty acids, tocol homologs and total polar components during deep frying. J. Food Compos. Anal. 2022, 114, 104840. [Google Scholar] [CrossRef]
- Büyükkurt, O.; Dinçer, E.A.; Çam, I.B.; Candal, C.; Erbas, M. The Influence of Cooking Methods and Some Marinades on Polycyclic Aromatic Hydrocarbon Formation in Beef Meat. Polycycl. Aromat. Compd. 2020, 40, 195–205. [Google Scholar] [CrossRef]
- Chen, B.H.; Lin, Y.S. Formation of polycyclic aromatic hydrocarbons during processing of duck meat. J. Agric. Food Chem. 1997, 45, 1394–1403. [Google Scholar] [CrossRef]
- Olatunji, O.S.; Fatoki, O.S.; Opeolu, B.O.; Ximba, B.J. Determination of polycyclic aromatic hydrocarbons [PAHs] in processed meat products using gas chromatography–flame ionization detector. Food Chem. 2014, 156, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Coroian, A.; Mireşan, V.; Coroian, C.O.; Răducu, C.; Cocan, D.; Andronie, L.; Naghiu, A.; Longodor, A.L.; Constantinescu, R.; Marchiş, Z. The level of polycyclic aromatic hydrocarbons (PAHs) from pork meat depending on the heat treatment applied. Rom. Biotechnol. Lett. 2020, 25, 1601–1606. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, S.Y.; Moon, J.S.; Kim, S.H.; Kang, D.H.; Yoon, H.J. Effects of grilling procedures on levels of polycyclic aromatic hydrocarbons in grilled meats. Food Chem. 2016, 199, 632–638. [Google Scholar] [CrossRef]
- Wang, X.; Nag, R.; Brunton, N.P.; Siddique, M.A.S.; Harrison, S.M.; Monahan, F.J.; Cummins, E. Human health risk assessment of bisphenol A (BPA) through meat products. Environ. Res. 2022, 213, 113734. [Google Scholar] [CrossRef]
- Savaş, A.; Oz, E.; Oz, F. Is oven bag really advantageous in terms of heterocyclic aromatic amines and bisphenol-A? Chicken meat perspective. Food Chem. 2021, 355, 129646. [Google Scholar] [CrossRef]
- Surma, M.; Sadowska-Rociek, A.; Cieślik, E. Assessment of thermal processing contaminant levels in dried and smoked fruits. Eur. Food Res. Technol. 2018, 244, 1533–1543. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Huang, X.; Tang, X.; Zhan, J.; Liu, S. The Effects of different natural plant extracts on the formation of polycyclic aromatic hydrocarbons (PAHs) in roast duck. Foods 2022, 11, 2104. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Y.; Wang, H.; Bai, Y.; Dai, C.; Li, C.; Xu, X.; Zhou, G. The influence of natural antioxidants on polycyclic aromatic hydrocarbon formation in charcoal-grilled chicken wings. Food Control 2019, 98, 34–41. [Google Scholar] [CrossRef]
- Kim, H.; Cho, J.; Kim, D.; Park, T.; Jin, S.; Hur, S.; Lee, S.; Jang, A. Effects of gochujang (Korean Red Pepper Paste) marinade on polycyclic aromatic hydrocarbon formation in charcoal-grilled pork belly. Food Sci. Anim. Resour. 2021, 41, 481–496. [Google Scholar] [CrossRef]
- Sadler, M.J.; Gibson, S.; Whelan, K.; Ha, M.A.; Lovegrove, J.; Higgs, J. Dried fruit and public health—What does the evidence tell us? Int. J. Food Sci. Nutr. 2019, 70, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Średnicka-Tober, D.; Kazimierczak, R.; Ponder, A.; Hallmann, E. Biologically active compounds in selected organic and conventionally produced dried fruits. Foods 2020, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Stacewicz-Sapuntzakis, M. Dried plums and their products: Composition and health effects-an updated review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1277–1302. [Google Scholar] [CrossRef] [PubMed]
- Dorofejeva, K.; Rakcejeva, T.; Skudra, L.; Dimins, F.; Kviesis, J. Changes in physically-chemical and microbiological parameters of Latvian wild cranberries during convective drying. Food Sci. 2010, 1, 132–137. [Google Scholar]
- Hartwig, N.; Ferreira, C.; Colazzo, C.; Kupski, L.; Badiale-Furlang, L. Dry fruit as source of fungal contaminants or functional compounds? Food Sci. Technol. 2020, 40, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Dorofejeva, K.; Rakcejevaa, T.; Galoburda, R.; Dukalska, L.; Kviesis, J. Vitamin C content in Latvian cranberries dried in convective and microwave vacuum driers. Proc. Food Sci. 2011, 1, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Wongmaneepratip, W.; Jom, K.; Vangnai, K. Inhibitory effects of dietary antioxidants on the formation of carcinogenic polycyclic aromatic hydrocarbons in grilled pork. Asian-Australas. J. Anim. Sci. 2019, 32, 1205–1210. [Google Scholar] [CrossRef] [Green Version]
- Śnieżek, E.; Szumska, M.; Janoszka, B. Ocena właściwości przeciwutleniających wybranych produktów roślinnych w aspekcie możliwości ich wykorzystania jako dodatków do żywności wysokobiałkowej poddawanej obróbce termicznej. ŻNTJ 2016, 23, 116–126. (In Polish) [Google Scholar]
- Bao, Y.; Zhu, Y.; Ren, X.; Zhang, Y.; Peng, Z.; Zhou, G. Formation and inhibition of lipid alkyl radicals in roasted meat. Foods 2020, 9, 572. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; He, Z.; Qin, F.; Chen, J. Effect of phenolic compounds from spices consumed in China on heterocyclic amine profiles in roast beef patties by UPLC-MS/MS and multivariate analysis. Meat Sci. 2016, 116, 50–57. [Google Scholar] [CrossRef]



| Name | Structure | Name | Structure |
|---|---|---|---|
| Benzo(a)pyrene BaP |  | Dibenzo(ah)anthracene DB(ah)A |  |
| Chrysene Chr |  | Benzo(k)fluoranthene BkF |  |
| Benzo(b)fluoranthene BbF |  | Benzo(ghi)perylene BghiP |  |
| Benzo(a)anthracene BaA |  |
| PAH | Abbreviation | Calibration Curve Concentration Range (ng/mL) | Regression Coefficients r | LOQ 1 (ng/mL) | LOD 2 (ng/g) | LOQ 1 (ng/g) |
|---|---|---|---|---|---|---|
| Benzo(a)anthracene | BaA | 0.5–200 | 0.9999 | 0.30 | 0.003 | 0.010 |
| Chrysene | Chr | 0.5–200 | 1.0 | 0.45 | 0.004 | 0.012 |
| Benzo(a)pyrene | BaP | 0.5–200 | 1.0 | 0.45 | 0.004 | 0.012 |
| Benzo(k)fluoranthene | BkF | 0.5–200 | 1.0 | 0.30 | 0.003 | 0.010 |
| Dibenzo(ah)anthracene | DiBahA | 0.5–200 | 1.0 | 0.45 | 0.004 | 0.012 |
| Benzo(ghi)perylene | BghiP | 0.5–200 | 1.0 | 0.45 | 0.004 | 0.012 |
| Benzo(b)fluoranthene | BbF | 1.0–200 | 1.0 | 0.6 | 0.006 | 0.020 |
| PAH | Recovery (%) and RSD 1 (%) for Spiking Level: | |
|---|---|---|
| 10 ng/g | 40 ng/g | |
| BaA | 87.2 (14.4) | 89.4 (14.9) |
| Chr | 88.6 (15.9) | 96.1 (8.9) |
| BbF | 83.9 (10.5) | 86.8 (11.7) |
| BaP | 61.2 (13.6) | 67.9 (12.7) |
| BkF | 73.5 (9.9) | 79,2 (11.3) |
| DiBahA | 72.1 (12.8) | 73.8 (7.8) |
| BghiP | 75.9 (8.1) | 75.3 (8.5) |
| PAH | Concentration 1 (ng/g) and Inhibition (%) in Pork Loin Samples | |||
|---|---|---|---|---|
| Without Additives (Control) | With Prunes | With Apricots | With Cranberries | |
| BaA | 1.42 ± 0.10 a | 0.83 ± 0.05 b (41.5%) | 0.92 ± 0.06 c (35.2%) | 0.57 ± 0.04 d (59.9%) |
| Chr | 2.01 ± 0.17 a | 1.14 ± 0.06 b,c (43.3%) | 1.22 ± 0.12 b (39.3) | 1.03 ± 0.06 c (48.8%) |
| BbF | 1.84 ± 0.22 a | 0.91 ± 0.13 b (50.5%) | 1.24 ± 0.12 c (32.6%) | 0.84 ± 0.08 b (54.4%) |
| BaP | 0.87 ± 0.07 a | 0.35 ± 0.03 b (59.8%) | 0.48 ± 0.07 c (44.8%) | n.q. 2 (100%) |
| BkF | 0.43 ± 0.06 a | 0.25 ± 0.04 b (41.9%) | 0.36 ± 0.04 c (16.3%) | 0.26 ± 0.01 b (39.5%) |
| DiBahA | 0.27 ± 0.04 a | n.q. 2 (100%) | 0.12 ± 0.02 b (55.6%) | 0.06 ± 0.004 c (77.8%) |
| BghiP | 0.54 ± 0.04 a | 0.34 ± 0.05 b (37.0%) | 0.50 ± 0.09 a (7.4%) | 0.35 ± 0.03 b (35.2%) |
| PAH4 3 | 6.14 | 3.23 (47.4%) | 3.86 (37.1%) | 2.44 (60.3%) |
| Total PAHs 4 | 7.38 | 3.82 (48.2%) | 4.84 (34.4%) | 3.11 (57.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulanda, S.; Janoszka, B. Polycyclic Aromatic Hydrocarbons (PAHs) in Roasted Pork Meat and the Effect of Dried Fruits on PAH Content. Int. J. Environ. Res. Public Health 2023, 20, 4922. https://doi.org/10.3390/ijerph20064922
Bulanda S, Janoszka B. Polycyclic Aromatic Hydrocarbons (PAHs) in Roasted Pork Meat and the Effect of Dried Fruits on PAH Content. International Journal of Environmental Research and Public Health. 2023; 20(6):4922. https://doi.org/10.3390/ijerph20064922
Chicago/Turabian StyleBulanda, Sylwia, and Beata Janoszka. 2023. "Polycyclic Aromatic Hydrocarbons (PAHs) in Roasted Pork Meat and the Effect of Dried Fruits on PAH Content" International Journal of Environmental Research and Public Health 20, no. 6: 4922. https://doi.org/10.3390/ijerph20064922
APA StyleBulanda, S., & Janoszka, B. (2023). Polycyclic Aromatic Hydrocarbons (PAHs) in Roasted Pork Meat and the Effect of Dried Fruits on PAH Content. International Journal of Environmental Research and Public Health, 20(6), 4922. https://doi.org/10.3390/ijerph20064922