Ecological Implications in a Human-Impacted Lake—A Case Study of Cyanobacterial Blooms in a Recreationally Used Water Body
Abstract
:1. Introduction
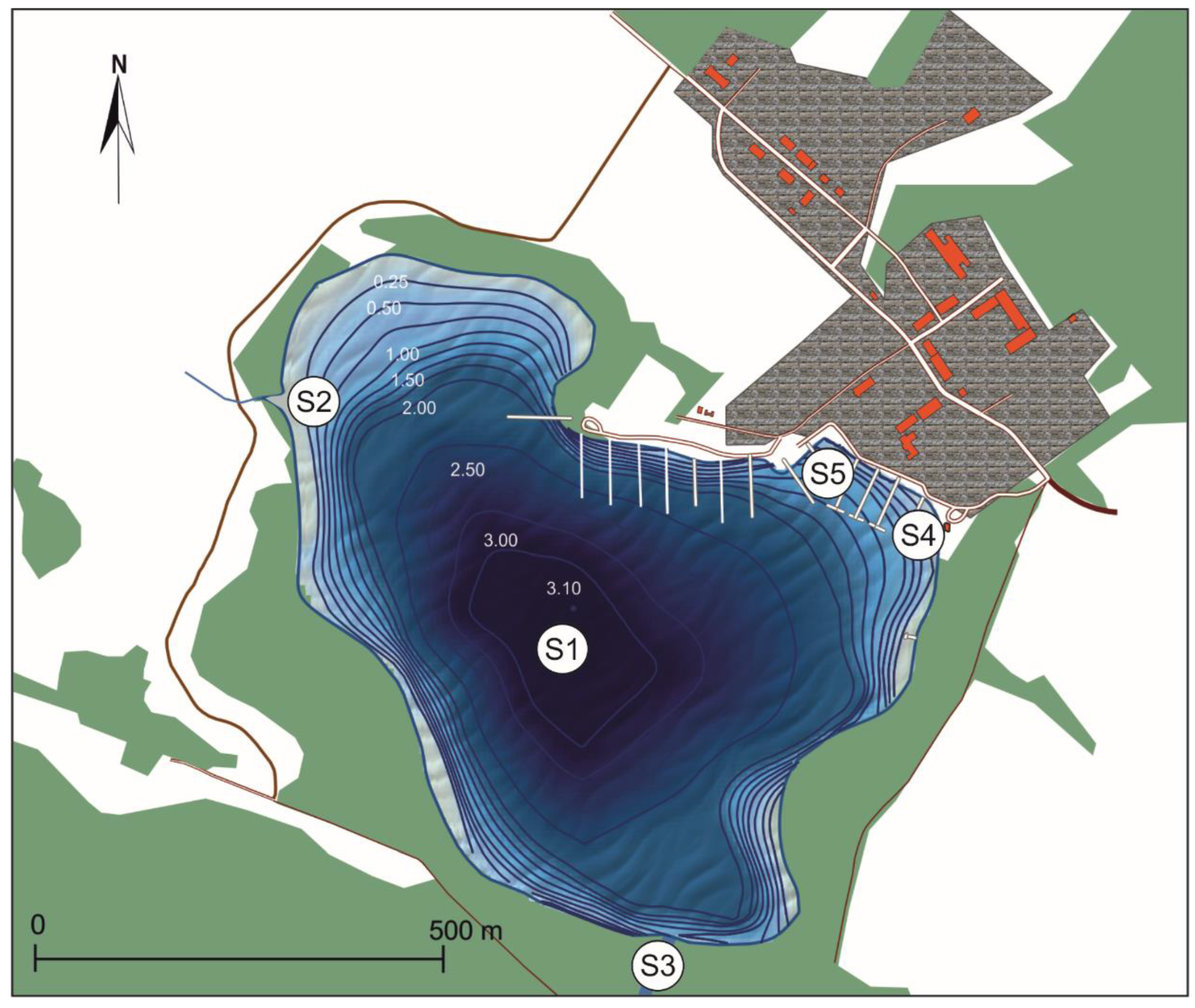
2. Materials and Methods
3. Results
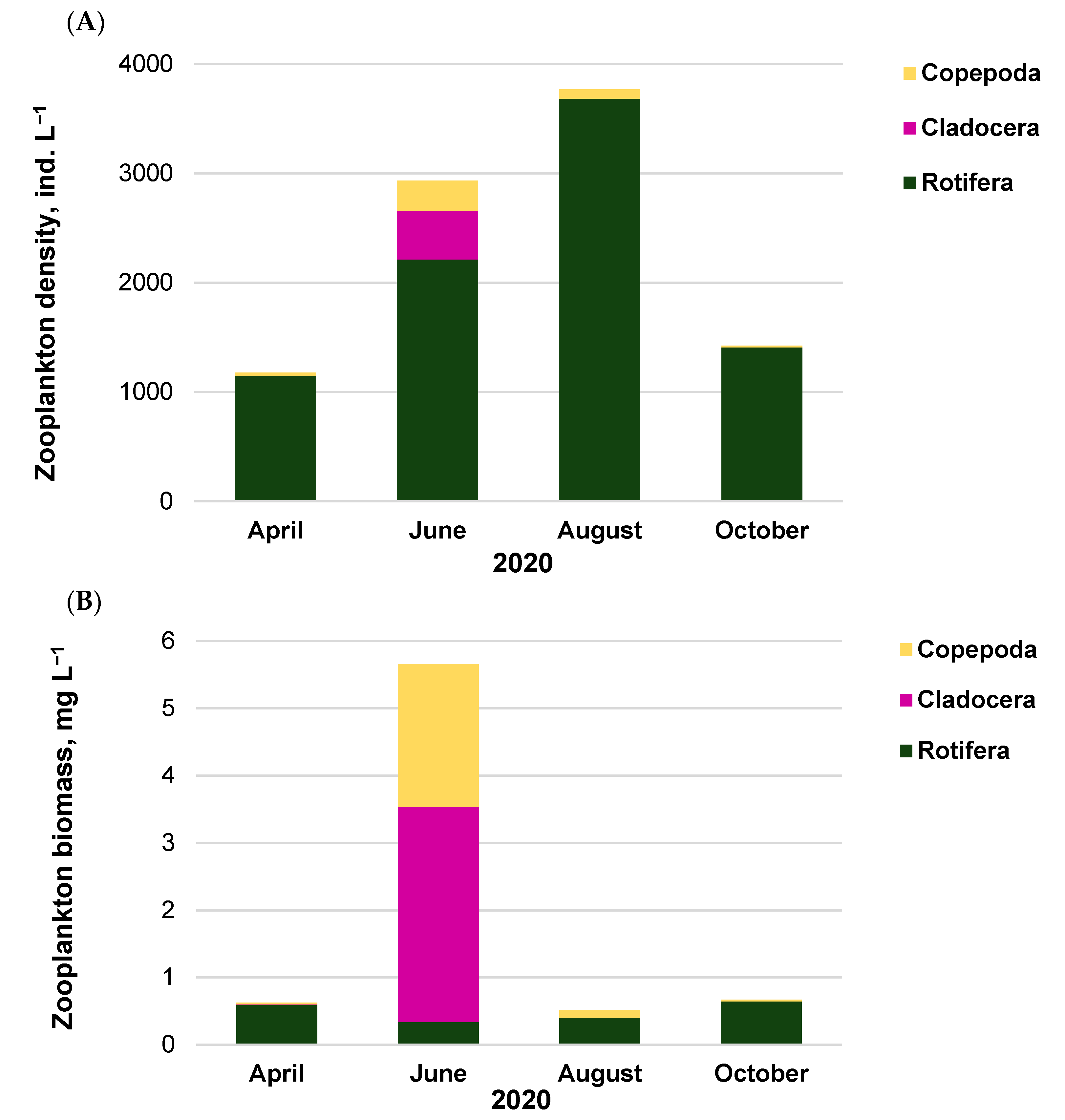
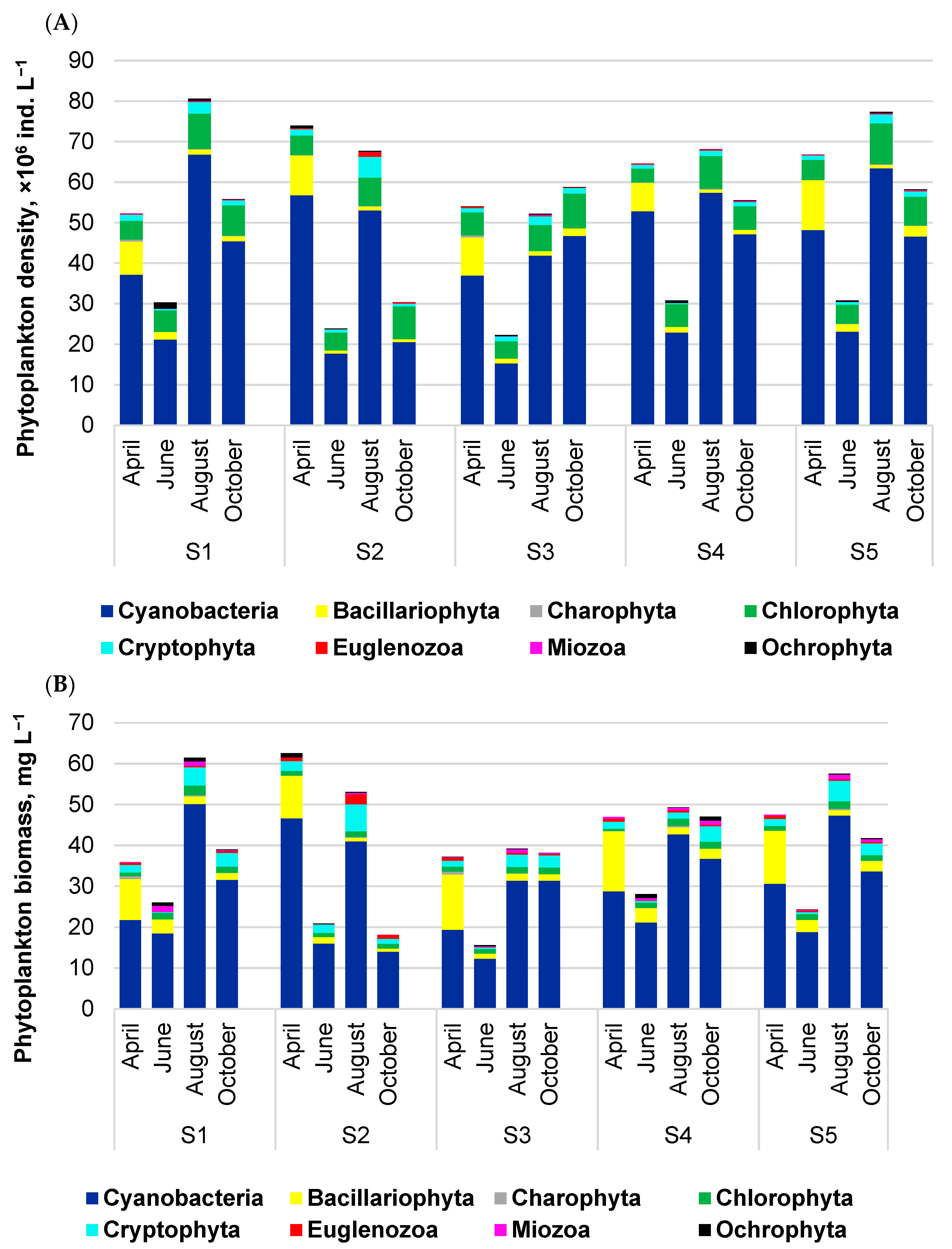
3.1. Phytoplankton and Zooplankton Assemblages within Routine Monitoring System
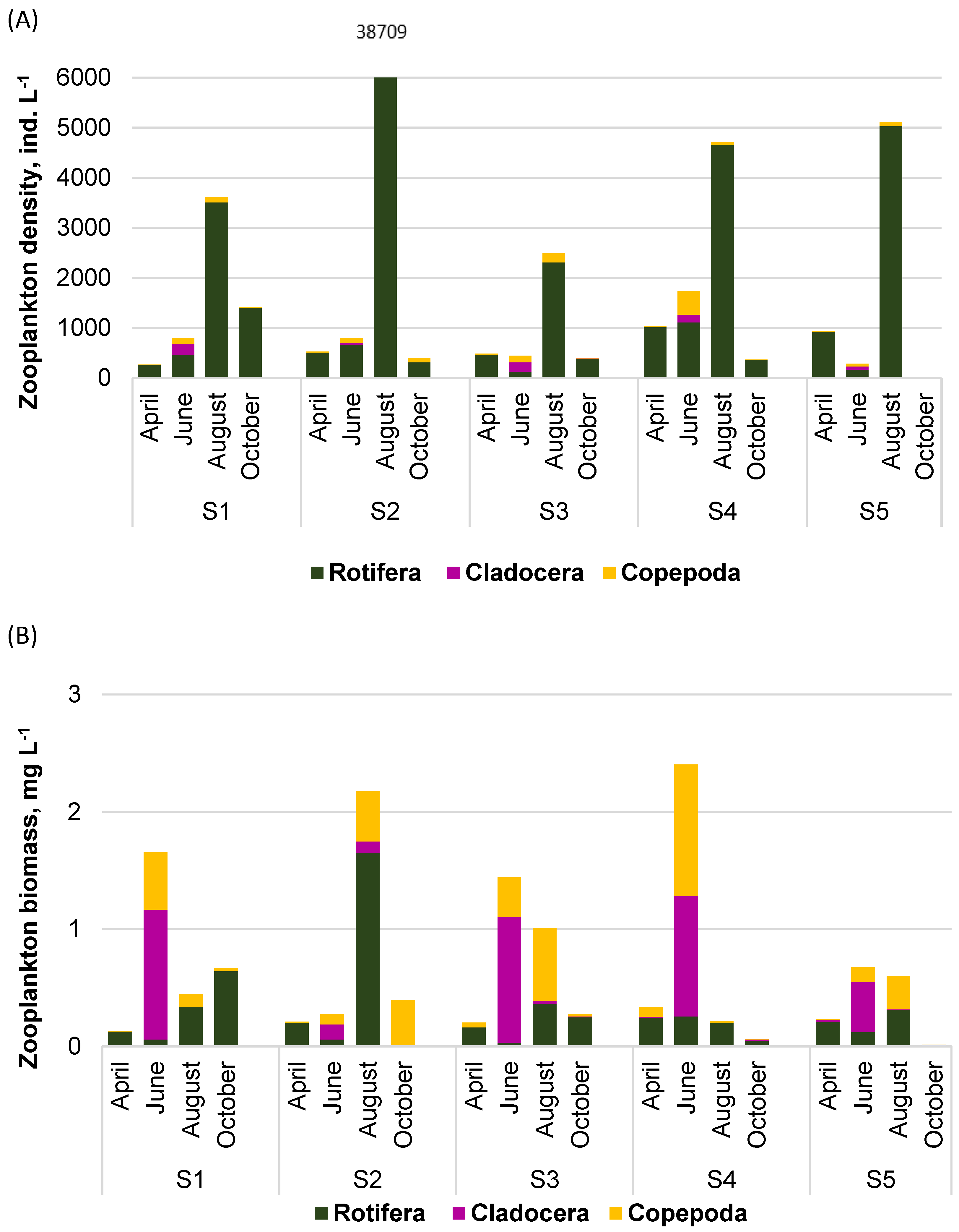
3.2. Spatial Changes in Phytoplankton and Zooplankton Assemblages
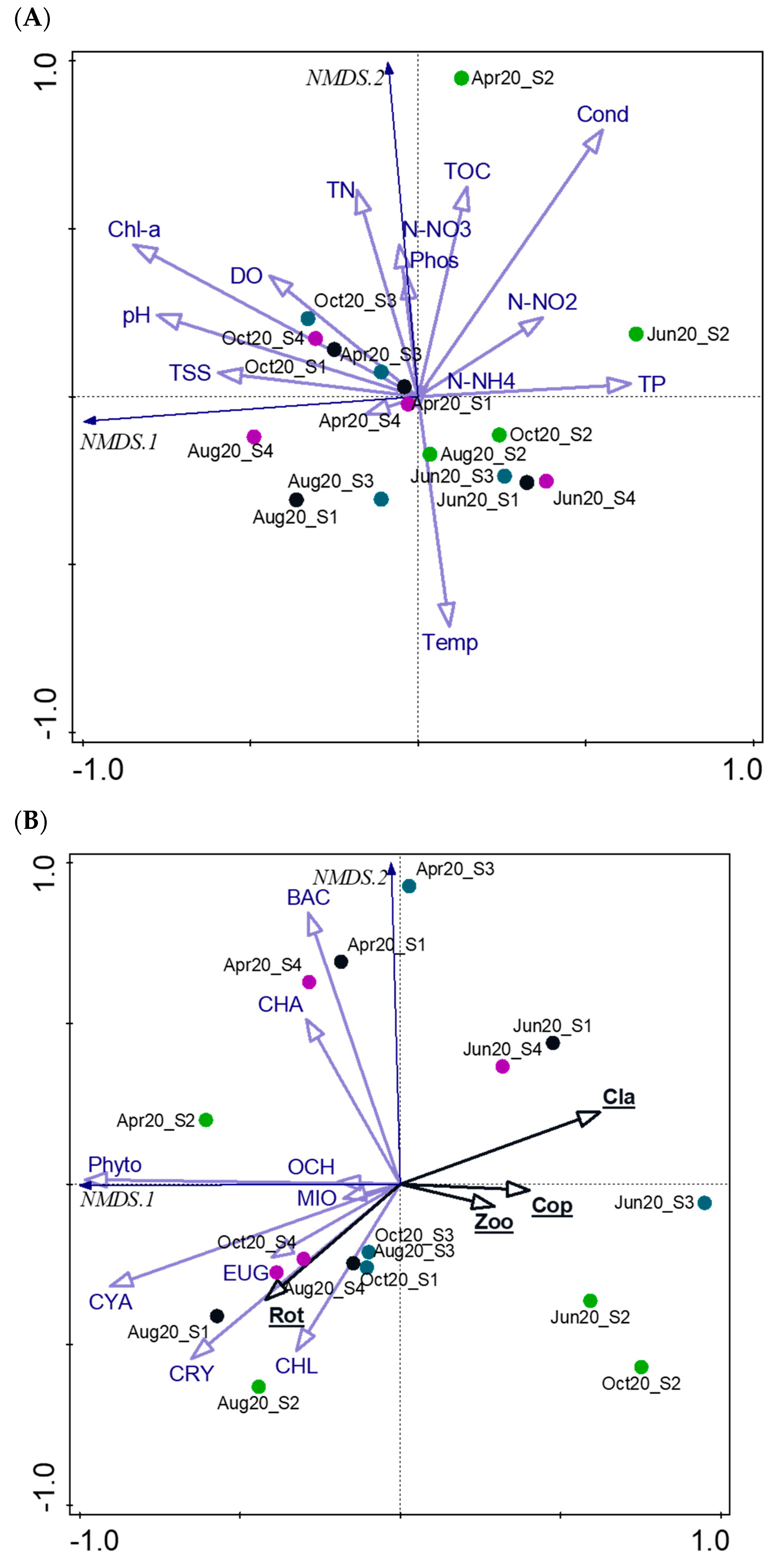
3.3. Ecological Implications: Phytoplankton–Zooplankton–Environment
3.4. Potential Toxin-Producing Cyanobacteria, Including Invasive Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paerl, H.W.; Fulton, R.S. Ecology of Harmful Cyanobacteria. In Ecology of Harmful Algae; Granéli, E., Turner, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 189, pp. 95–109. [Google Scholar]
- Kobos, J.; Błaszczyk, A.; Hohlfeld, N.; Toruńska-Sitarz, A.; Krakowiak, A.; Hebel, A.; Stryk, K.; Grabowska, M.; Toporowska, M.; Kokociński, M.; et al. Cyanobacteria and cyanotoxins in Polish freshwater bodies. Oceanol. Hydrobiol. Stud. 2013, 42, 358–378. [Google Scholar] [CrossRef] [Green Version]
- Toporowska, M.; Pawlik-Skowrońska, B.; Kalinowska, R. Mass Development of Diazotrophic Cyanobacteria (Nostocales) and Production of Neurotoxic Anatoxin-a in a Planktothrix (Oscillatoriales) Dominated Temperate Lake. Water Air Soil Pollut. 2016, 227, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yang, Z.; Yu, Y.; Shi, X. Interannual and Seasonal Shift between Microcystis and Dolichospermum: A 7-Year Investigation in Lake Chaohu, China. Water 2020, 12, 1978. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Christophoridis, C.; Zervou, S.K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and diversity of cyanotoxins in Greek lakes. Sci. Rep. 2018, 14, 17877. [Google Scholar] [CrossRef] [Green Version]
- Znachor, P.; Nedom, J.; Hejzlar, J.; Seďa, J.; Komárková, J.; Kolář, V.; Mrkvička, T.; Boukal, D.S. Changing environmental conditions underpin long-term patterns of phytoplankton in a freshwater reservoir. Sci. Total Environ. 2020, 710, 135626. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A.; Kalinowska, K.; Bogacka-Kapusta, E.; Stawecki, K.; Traczuk, P. Persistent blooms of filamentous cyanobacteria in a cormorant-affected aquatic ecosystem: Ecological indicators and consequences. Ecol. Indic. 2021, 124, 107421. [Google Scholar] [CrossRef]
- Wilk-Woźniak, E.; Solarz, W.; Najberek, K.; Pociecha, A. Alien cyanobacteria: An unsolved part of the ‘‘expansion and evolution’’ jigsaw puzzle? Hydrobiologia 2016, 764, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Budzyńska, A.; Rosińska, J.; Pełechata, A.; Toporowska, M.; Napiórkowska-Krzebietke, A.; Kozak, A.; Messyasz, B.; Pęczuła, W.; Kokociński, M.; Szeląg-Wasielewska, E.; et al. Environmental factors driving the occurrence of the invasive cyanobacterium Sphaerospermopsis aphanizomenoides (Nostocales) in temperate lakes. Sci. Total Environ. 2019, 650, 1338–1347. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, H.K.; Kim, I.S. Invasion and toxin production by exotic nostocalean cyanobacteria (Cuspidothrix, Cylindrospermopsis, and Sphaerospermopsis) in the Nakdong River, Korea. Harmful Algae 2020, 100, 101954. [Google Scholar] [CrossRef]
- Meriggi, C.; Drakare, S.; Polaina Lacambra, E.; Johnson, R.K.; Laugen, A.T. Species distribution models as a tool for early detection of the invasive Raphidiopsis raciborskii in European lakes. Harmful Algae 2022, 113, 102202. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Park, H.-K.; Kim, I.-S. Assessment of the Appearance and Toxin Production Potential of Invasive Nostocalean Cyanobacteria Using Quantitative Gene Analysis in Nakdong River, Korea. Toxins 2022, 14, 294. [Google Scholar] [CrossRef]
- Sivonen, K. Cyanobacterial Toxins. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Elsevier Publishers: Oxford, UK, 2009; pp. 290–307. [Google Scholar]
- Hodoki, Y.; Ohbayashi, K.; Kobayashi, Y.; Okuda, N.; Nakano, S. Detection and identification of potentially toxic cyanobacteria: Ubiquitous distribution of Microcystis aeruginosa and Cuspidothrix issatschenkoi in Japanese lakes. Harmful Algae 2012, 16, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Cirés, S.; Ballot, A. A review of the phylogeny, ecology and toxin production of bloom-forming Aphanizomenon spp. and related species within the Nostocales (cyanobacteria). Harmful Algae 2016, 54, 21–43. [Google Scholar] [CrossRef]
- Mirasbekov, Y.; Abdimanova, A.; Sarkytbayev, K.; Samarkhanov, K.; Abilkas, A.; Potashnikova, D.; Arbuz, G.; Issayev, Z.; Vorobjev, I.A.; Malashenkov, D.V.; et al. Combining Imaging Flow Cytometry and Molecular Biological Methods to Reveal Presence of Potentially Toxic Algae at the Ural River in Kazakhstan. Front. Mar. Sci. 2021, 8, 680482. [Google Scholar] [CrossRef]
- Ballot, A.; Scherer, P.I.; Wood, S.A. Variability in the anatoxin gene clusters of Cuspidothrix issatschenkoi from Germany, New Zealand, China and Japan. PLoS ONE 2018, 13, e0200774. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- De la Cruz, A.A.; Hiskia, A.; Kaloudis, T.; Chernoff, N.; Hill, D.; Antoniou, M.G.; He, X.; Loftin, K.; O’Shea, K.; Zhao, C.; et al. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. Process. Impacts 2013, 15, 1979–2003. [Google Scholar] [CrossRef]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [Green Version]
- Sabour, B.; Loudiki, M.; Oudra, B.; Vasconcelos, V.; Oubraim, S.; Fawzi, B. Contributed Article Dynamics and toxicity of Anabaena aphanizomenoides (Cyanobacteria) waterblooms in the shallow brackish Oued Mellah lake (Morocco). Aquat. Ecosyst. Health Manag. 2005, 8, 95–104. [Google Scholar] [CrossRef]
- Dziga, D.; Maksylewicz, A.; Maroszek, M.; Budzyńska, A.; Kozak, A.; Rosińska, J.; Napiórkowska-Krzebietke, A.; Toporowska, M.; Grabowska, M.; Meriluoto, J. The biodegradation of microcystins in temperate freshwater bodies with previous cyanobacterial history. Ecotoxicol. Environ. Saf. 2017, 145, 420–430. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A.; Dunalska, J. Phytoplankton-based recovery requirement for urban lakes in the implementation of the Water Framework Directive’s ecological targets. Oceanol. Hydrobiol. Stud. 2015, 44, 109–119. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A.; Dunalska, J.; Grochowska, J.; Łopata, M.; Brzozowska, R. Intensity and thresholds of cyanobacterial blooms—An approach to determine the necessity to restore urban lakes. Carpath. J. Earth Env. 2015, 10, 123–132. [Google Scholar]
- Baoying, N.; Yuanqing, H. Tourism Development and Water Pollution: Case Study in Lijiang Ancient Town, China Population. Resour. Environ. 2007, 17, 123–127. [Google Scholar] [CrossRef]
- Lenart-Boroń, A.M.; Boroń, P.M.; Prajsnar, J.A.; Guzik, M.W.; Żelazny, M.S.; Pufelska, M.D.; Chmiel, M.J. COVID-19 lockdown shows how much natural mountain regions are affected by heavy tourism. Sci. Total Environ. 2022, 806, 151355. [Google Scholar] [CrossRef]
- Kiersztyn, B.; Chróst, R.; Kaliński, T.; Siuda, W.; Bukowska, A.; Kowalczyk, G.; Grabowska, K. Structural and functional microbial diversity along a eutrophication gradient of interconnected lakes undergoing anthropopressure. Sci. Rep. 2019, 9, 11144. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.J.; Balomenou, C.; Nijkamp, P.; Poulaki, P.; Lagos, D. The Sustainability of Yachting Tourism: A Case Study on Greece. Int. J. Res. Tour. Hosp. 2016, 2, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Dokulil, M.T. Environmental Impacts of Tourism on Lakes. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Gill, S.S., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2014; pp. 81–88. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommung der quantitativen Phytoplankton-Methodik. Mitt. Internat. Verein. Limnol. 1958, 9, 1–38. [Google Scholar]
- Napiórkowska-Krzebietke, A.; Kobos, J. Assessment of the cell biovolume of phytoplankton widespread in coastal and inland water bodies. Water Res. 2016, 104, 532–546. [Google Scholar] [CrossRef]
- Huber-Pestalozzi, G. Das phytoplankton des Süβwassers. Systematik und Biologie. 7 Teil, 1 Häfte: Chlorophyceae (Grünalgen) Ordnung Chlorococcales. In Die Binnengewässer Einzeldarstellungen aus der Limnologie und Ihren Nachbargebieten; Thienemann, A., Ed.; E. Schweizerbart’sche Verlasbuchhandlung: Stuttgart, Germany, 1983; pp. 1–1044. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 1. Teil: Naviculaceae. In Süβwasserflora von Mitteleuropa; Pascher, A., Ed.; VEB Gustaw Fischer Verlag: Jena, Germany, 1986; pp. 1–876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 2. Teil: Epithemiaceae, Surirellaceae. In Süβwasserflora von Mitteleuropa; Pascher, A., Ed.; Gustaw Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1988; pp. 1–596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süβwasserflora von Mitteleuropa; Pascher, A., Ed.; Gustaw Fischer Verlag: Stuttgart, Germany; Jena, Germany, 1991; pp. 1–576. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil: Oscillatoriales. In Süβwasserflora von Mitteleuropa; Pascher, A., Ed.; Gustaw Fischer Verlag: Stuttgart, Germany; Jena, Germany, 2005; pp. 1–759. [Google Scholar]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 20 October 2022).
- Flössner, D. Krebstiere (Crustacea), Kiemen- und Blattfüßer (Branchiopoda), Fischläuse (Branchiura); VEB Gustav Fischer Verlag: Jena, Germany, 1972. [Google Scholar]
- Radwan, S.; Bielańska-Grajner, I.; Ejsmont-Karabin, J. Część ogólna, Monogononta—Część systematyczna. 32.A. In Wrotki (Rotifera). Fauna Słodkowodna Polski. 32; Radwan, S., Ed.; Polskie Towarzystwo Hydrobiologiczne, Uniwersytet Łódzki, Oficyna Wydawnicza Tercja: Łód’z, Poland, 2004; pp. 1–146. (In Polish) [Google Scholar]
- Rybak, J.I.; Błędzki, L.A. Widłonogi, Copepoda: Cyclopoida, Klucz do Oznaczania; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2005. [Google Scholar]
- Ejsmont-Karabin, J. The usefulness of zooplankton as lake ecosystem indicators: Rotifer Trophic State Index. Pol. J. Ecol. 2012, 60, 339–350. [Google Scholar]
- Bottrell, H.H.; Duncan, A.; Gliwicz, Z.M.; Grygierek, E.; Herzig, A.; Hillbright, I.A.; Kurasawa, H.; Larsson, P.; Weglenska, T. A review of some problems in zooplankton production studies. Norw. J. Zool. 1976, 24, 419–456. [Google Scholar]
- Dunalska, J. Elaboration of the Results of Study on Physicochemical Parameters of Lake Sztynorckie—Report. 2020. (In Polish) [Google Scholar]
- Napiórkowska-Krzebietke, A.; Chybowski, Ł.; Prus, P.; Adamczyk, M. Assessment Criteria and Ecological Classification of Polish Lakes and Rivers: Limitations and Current State. In Polish River Basins and Lakes—Part II Biological Status and Water Management; Korzeniewska, E., Harnisz, M., Eds.; The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2020; Volume 87, pp. 295–325. [Google Scholar]
- Regulation of the Minister of Infrastructure of June 25, 2021 on the Classification of Ecological Status, Ecological Potential and Chemical Status and the Method of Classifying the Status of Surface Water Bodies, as Well as Environmental Quality Standards for Priority Substances. Dz.U. 2021 poz. 1475. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001475 (accessed on 7 March 2023). (In Polish)
- Ejsmont-Karabin, J.; Karabin, A. The suitability of zooplankton as lake ecosystem indicators: Crustacean trophic state index. Pol. J. Ecol. 2013, 61, 561–573. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 5, 417–428. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A. Phytoplankton response to fish-induced environmental changes in a temperate shallow pond-type lake. Arch. Pol. Fish. 2017, 25, 211–264. [Google Scholar] [CrossRef] [Green Version]
- Napiórkowska-Krzebietke, A. Cyanobacterial bloom intensity in the ecologically relevant state of lakes—An approach to Water Framework Directive implementation. Oceanol. Hydrobiol. Stud. 2015, 44, 97–108. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Żelazna-Wieczorek, J.; Skrobek, I.; Ziułkiewicz, M.; Adamski, M.; Kamiński, A.; Żmudzki, P. Persistent Cyanobacteria Blooms in Artificial Water Bodies—An Effect of Environmental Conditions or the Result of Anthropogenic Change. Int. J. Environ. Res. Public Health 2022, 19, 6990. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Hercog, K.; Štampar, M.; Filipič, M.; Cameán, A.M.; Jos, Á.; Žegura, B. Genotoxic Effects of Cylindrospermopsin, Microcystin-LR and Their Binary Mixture in Human Hepatocellular Carcinoma (HepG2) Cell Line. Toxins 2020, 12, 778. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Jiang, S.C. Can cyanotoxins penetrate human skin during water recreation to cause negative health effects? Harmful Algae 2020, 98, 101872. [Google Scholar] [CrossRef]
- Mehnert, G.; Leunert, F.; Cirés, S.; Jöhnk, K.D.; Rücker, J.; Nixdorf, B.; Wiedner, C. Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions. J. Plankton Res. 2010, 32, 1009–1021. [Google Scholar] [CrossRef]
- Wood, S.A.; Rasmussen, J.P.; Holland, P.T.; Campbell, R.; Crowe, A.L. First report of the cyanotoxin anatoxin-a from Aphanizomenon issatschenkoi (Cyanobacteria). J. Phycol. 2007, 43, 356–365. [Google Scholar] [CrossRef]
- Savadova, K.; Mazur-Marzec, H.; Karosienė, J.; Kasperovičienė, J.; Vitonytė, I.; Toruńska-Sitarz, A.; Koreivienė, J. Effect of Increased Temperature on Native and Alien Nuisance Cyanobacteria from Temperate Lakes: An Experimental Approach. Toxins 2018, 10, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziesemer, S.; Meyer, S.; Edelmann, J.; Vennmann, J.; Gudra, C.; Arndt, D.; Effenberg, M.; Hayas, O.; Hayas, A.; Thomassen, J.S.; et al. Target Mechanisms of the Cyanotoxin Cylindrospermopsin in Immortalized Human Airway Epithelial Cells. Toxins 2022, 14, 785. [Google Scholar] [CrossRef] [PubMed]
- Napiórkowska-Krzebietke, A.; Hutorowicz, A. Long-term changes of phytoplankton in Lake Mamry Północne. Oceanol. Hydrobiol. Stud. 2005, 34, 217–228. [Google Scholar]


| Date | Species | Functional Classification |
|---|---|---|
| April | Limnothrix redekei (30%) | S1 |
| Pseudanabaena limnetica (24%) | S1 | |
| Ulnaria acus (20%) | D | |
| Planktolyngbya limnetica (12%) | S1 | |
| Ulnaria ulna (5%) | D | |
| June | Limnothrix redekei (48%) | S1 |
| Pseudanabaena limnetica (10%) | S1 | |
| Aphanizomenon gracile (8%) | H1 | |
| Planktolyngbya limnetica (5%) | S1 | |
| August | Pseudanabaena limnetica (27%) | S1 |
| Aphanizomenon gracile (23%) | H1 | |
| Planktolyngbya limnetica (13%) | S1 | |
| Raphidiopsis raciborskii (8%) | SN | |
| Limnothrix redekei (7%) | S1 | |
| October | Pseudanabaena limnetica (28%) | S1 |
| Cryptomonas erosa (16%) | Y | |
| Raphidiopsis raciborskii (15%) | SN | |
| Planktolyngbya limnetica (12%) | S1 | |
| Limnothrix redekei (11%) | S1 | |
| Ulnaria acus (5%) | D |
| Date | Species | Functional Classification |
|---|---|---|
| April | Polyarthra longiremis (47%) | Eutrophy |
| Keratella quadrata (12%) | Eutrophy | |
| Asplanchna priodonta (9%) | Mesotrophy | |
| Paracyclops sp. (6%) | - | |
| June | Bosmina longispina (31%) | Mesotrophy |
| Bosmina longirostris (22%) | Eutrophy | |
| Macrocylops albidus (15%) | Mesotrophy | |
| Thermocyclops sp. (9%) | Eutrophy | |
| August | Polyarthra longiremis (40%) | Eutrophy |
| Anuraeopsis fisa (22%) | Eutrophy | |
| Macrocylops albidus (21%) | Mesotrophy | |
| Cyclopoida nauplii (13%) | Mesotrophy | |
| Trichocerca capucina (6%) | Mesotrophy | |
| Trichocerca pusilla (6%) | Eutrophy | |
| October | Asplanchna priodonta (36%) | Mesotrophy |
| Polyarthra euryptera (23%) | Mesotrophy | |
| Mesocyclops leuckartii (16%) | Mesotrophy | |
| Eudiaptomus gracilis (8%) | Eutrophy |
| Date | Sampling Site | Sphaerospermopsis aphanizomenoides | Cuspidothrix issatschenkoi | Raphidiopsis raciborskii |
|---|---|---|---|---|
| April | S1 | − | − | − |
| S2 | − | − | − | |
| S3 | − | − | − | |
| S4 | − | + | − | |
| S5 | − | + | − | |
| June | S1 | − | − | − |
| S2 | − | − | − | |
| S3 | − | − | − | |
| S4 | + | − | − | |
| S5 | + | − | − | |
| August | S1 | + | + | + |
| S2 | + | + | + | |
| S3 | + | + | + | |
| S4 | + | + | + | |
| S5 | + | + | + | |
| October | S1 | − | + | − |
| S2 | − | + | − | |
| S3 | + | + | − | |
| S4 | − | + | + | |
| S5 | − | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napiórkowska-Krzebietke, A.; Dunalska, J.A.; Bogacka-Kapusta, E. Ecological Implications in a Human-Impacted Lake—A Case Study of Cyanobacterial Blooms in a Recreationally Used Water Body. Int. J. Environ. Res. Public Health 2023, 20, 5063. https://doi.org/10.3390/ijerph20065063
Napiórkowska-Krzebietke A, Dunalska JA, Bogacka-Kapusta E. Ecological Implications in a Human-Impacted Lake—A Case Study of Cyanobacterial Blooms in a Recreationally Used Water Body. International Journal of Environmental Research and Public Health. 2023; 20(6):5063. https://doi.org/10.3390/ijerph20065063
Chicago/Turabian StyleNapiórkowska-Krzebietke, Agnieszka, Julita Anna Dunalska, and Elżbieta Bogacka-Kapusta. 2023. "Ecological Implications in a Human-Impacted Lake—A Case Study of Cyanobacterial Blooms in a Recreationally Used Water Body" International Journal of Environmental Research and Public Health 20, no. 6: 5063. https://doi.org/10.3390/ijerph20065063





