Levels, Distributions, and Potential Risks of Hexachlorobutadiene from Two Tetrachloroethylene Factories in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
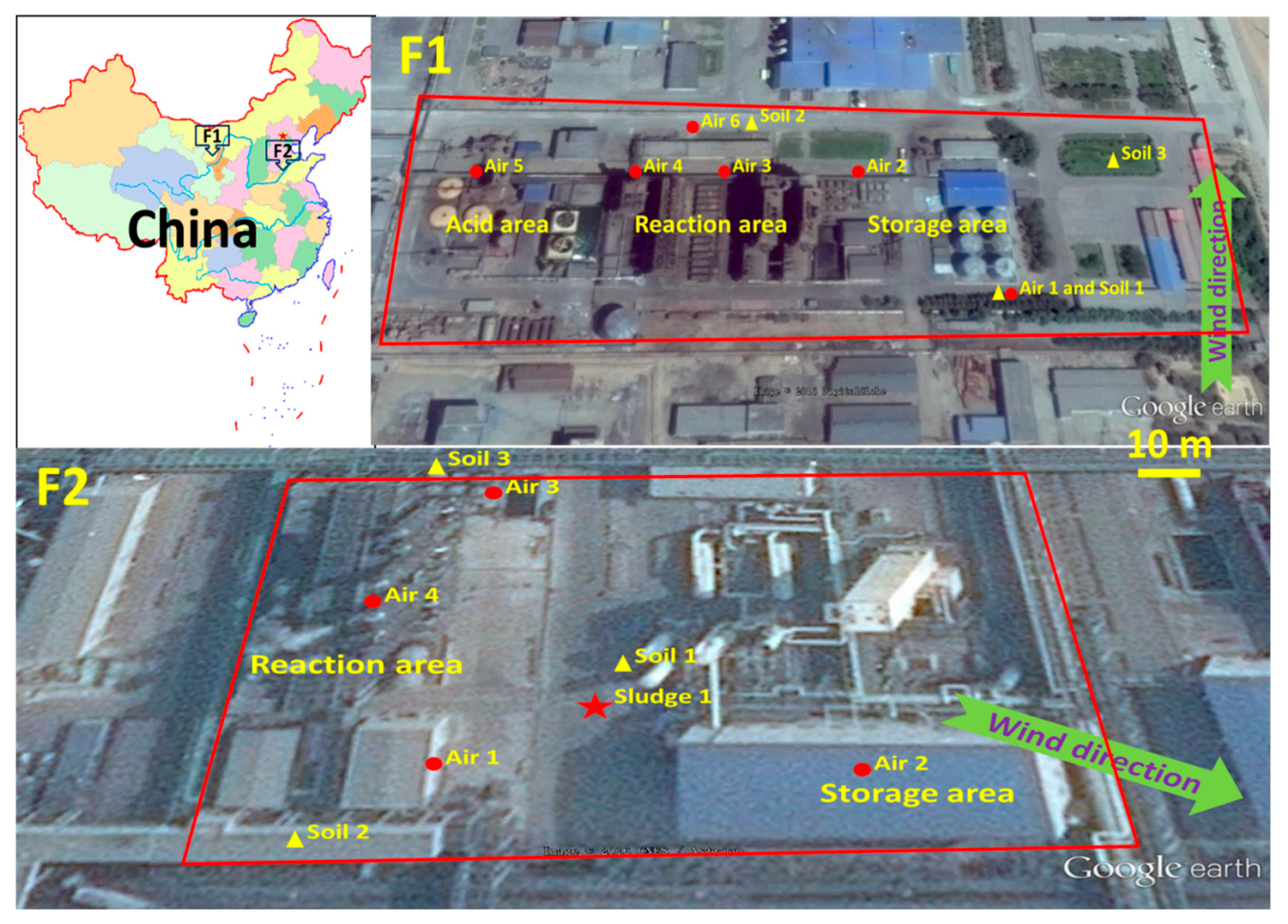
2.2. Factories and Samples
2.3. Ambient Air Sample Analysis
2.4. Soil and Sludge Sample Analysis
2.5. Residue and Product Sample Analysis
3. Results and Discussion
3.1. Levels and Distributions of HCBD in Air, Soil, and Sludge Samples
3.2. Levels of HCBD in Residues and Products
3.3. Human Health Risk Assessment
3.4. Management Needs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janz, D.M. Hexachlorobutadiene. Encycl. Toxicol. 2014, 2, 872–873. [Google Scholar]
- Nuhu, A.A.; Basheer, C.; Abu-Thabit, N.Y.; Alhooshani, K.; Al-Arfaj, A.R. Analytical method development using functionalized polysulfone membranes for the determination of chlorinated hydrocarbons in water. Talanta 2011, 87, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Juang, D.F.; Lee, C.H.; Chen, W.C.; Yuan, C.S. Do the VOCs that evaporate from a heavily polluted river threaten the health of riparian residents? Sci. Total Environ. 2010, 408, 4524–4531. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Fang, M.D. Sources and distribution of chlorobenzenes and hexachlorobutadiene in surficial sediments along the coast of southwestern Taiwan. Chemosphere 1997, 35, 2039–2050. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Sun, C.; Yu, M.; Gao, Y.; Wang, T.; Liu, J.; Jiang, G. Levels and distributions of hexachlorobutadiene and three chlorobenzenes in biosolids from wastewater treatment plants and in soils within and surrounding a chemical plant in China. Enviorn. Sci. Technol. 2014, 48, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, Q.; Cheng, J.; Qu, D.; Yang, Y.; Guo, W. Distribution and accumulation of hexachlorobutadiene in soils and terrestrial organisms from an agricultural area, East China. Ecotoxicol. Environ. Saf. 2014, 108, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, Q.; Nie, Z.; Yang, Y.; Yang, J.; Qu, D.; Cheng, J. Levels and distribution of organochlorine pesticides and hexachlorobutadiene in soils and terrestrial organisms from a former pesticide-producing area in Southwest China. Stoch. Environ. Res. Risk Assess. 2016, 30, 1249–1262. [Google Scholar] [CrossRef]
- Jurgens, M.D.; Johnson, A.C.; Jones, K.C.; Hughes, D.; Lawlor, A.J. The presence of EU priority substances mercury, hexachlorobenzene, hexachlorobutadiene and PBDEs in wild fish from four English rivers. Sci. Total Environ. 2013, 461–462, 441–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmar, J.E.; Hung, H.; Vorkamp, K.; Letcher, R.J.; Muir, D.C.G. Hexachlorobutadiene (HCBD) contamination in the Arctic environment: A review. Emerg. Contam. 2019, 5, 116–122. [Google Scholar] [CrossRef]
- The New POPs under the Stockholm Convention: Hexachlorobutadiene. UNEP. 2015. Available online: http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (accessed on 2 February 2016).
- Denier van der Gon, H.; van het Bolscher, M.; Visschedijk, A.; Zandveld, P. Emissions of persistent organic pollutants and eight candidate POPs from UNECE−Europe in 2000, 2010, and 2020 and the emission reduction resulting from the implementation of the UNECE POP protocol. Atmos. Environ. 2007, 41, 9245–9261. [Google Scholar] [CrossRef]
- Markovec, L.M.; Magee, R.J. Identification of major perchloroaromatic compounds in waste products from the production of carbon tetrachloride and tetrachloroethylene. Analyst 1984, 109, 497–501. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, W.; Zhang, L.; Li, X. Highly chlorinated unintentionally produced persistent organic pollutants generated during the methanol-based production of chlorinated methanes: A case study in China. Chemosphere 2015, 133, 1–5. [Google Scholar] [CrossRef] [PubMed]
- The Dow Chemical Company. Product Safety Assessment: Perchloroethylene 2008. Form No. 233-00398-MM-0608, 1–7. Available online: https://www.nyc.gov/assets/dep/downloads/pdf/air/perc-dow-co-safety-sheet.pdf (accessed on 5 February 2023).
- Yang, M.; Mao, H.; Li, H.; Yang, F.; Cao, F.; Wang, Y. Quantifying Concentrations and Emissions of Hexachlorobutadiene—A New Atmospheric Persistent Organic Pollutant in northern China. Environ. Res. 2023, 216, 114139. [Google Scholar] [CrossRef] [PubMed]
- Environment Canada, Canadian Environmental Protection Act, 1999, Priority Substance List Assessment Report Hexachlorobutadiene, 2000, 42. Available online: https://www.canada.ca/en/environment-climate-change/services/canadian-environmental-protection-act-registry/publications/canadian-environmental-protection-act-1999.html (accessed on 5 February 2023).
- Zhang, Y.; Yang, L.B.; Xu, J.S. Gas chromatography method for determination of hexachlorobutadiene in the air of workplace. Chin. J. Ind. Hyg. Occup. Dis. 2009, 27, 507–508. [Google Scholar]
- Sampling and Analysis of Selected Toxic Substances: Task 1B hexachlorobutadiene, EPA 560/6-76-015; USEPA; Office of Toxic Substances: Washington, DC, USA, 1976.
- POPs Review Committee. Hexachlorobutadiene: Risk Management Evaluation. 2013. UNEP/POPS/POPRC.9/13/Add.2. Available online: https://www.informea.org/en/report-persistent-organic-pollutants-review-committee-work-its-ninth-meeting-risk-management (accessed on 5 February 2023).
- Hazard Quotient Risk Calculation Tool. 2016. Available online: http://www.popstoolkit.com/tools/HHRA/NonCarcinogen.aspx (accessed on 5 February 2023).
- Risk Assessment Guidance for Superfund: Volume III-Part A, Process for Conducting Probabilistic Risk Assessment. USEPA; 2001. Available online: epa.gov/risk/risk-assessment-guidance-superfund-rags-volume-iii-part (accessed on 8 March 2023).
- Risk Assessment Guidance for Superfund Volume I: Human Health. Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment). USEPA; 2004. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/part_e_final_revision_10-03-07.pdf (accessed on 5 February 2023).
- Zhang, L.; Dong, L.; Yang, W.; Zhou, L.; Shi, S.; Zhang, X.; Niu, S.; Li, L.; Wu, Z.; Huang, Y. Passive air sampling of organochlorine pesticides and polychlorinated biphenyls in the Yangtze River Delta, China: Concentrations, distributions, and cancer risk assessment. Environ. Pollut. 2013, 181, 159–166. [Google Scholar] [CrossRef] [PubMed]
| Factory | Air | Soil and Sludge | Residue and Product | |||||
|---|---|---|---|---|---|---|---|---|
| No. | Concentration (µg/m3) | LCR (×10−6) | No. | Concentration (µg/kg) | HQ (×10−6) | Sample Name | Concentration (mg/kg) | |
| F1 | air 1 | 1.46 | 0.584 | soil 1 | 42.2 | 159 | tetrachloroethane | 2.72 × 103 |
| air 2 | 8.59 | 3.44 | soil 2 | 140 | 528 | TCE desorption column liquid | 4.49 × 104 | |
| air 3 | 491 | 196 | soil 3 | 55.3 | 208 | TCE low boiling liquid | 0.062 | |
| air 4 | 1.17 × 103 | 468 | — | — | — | pentachloroethane | 3.92 | |
| air 5 | 53.3 | 21.3 | PCE desorption column liquid | 3.80 × 103 | ||||
| air 6 | 305 | 122 | commercial TCE and PCE | ND | ||||
| F2 | air 1 | 5.53 × 103 | 2210 | soil 1 | 2.18 × 103 | 8280 | carbon tetrachloride | 12.9 |
| air 2 | 8.20 | 3.28 | soil 2 | 13.3 | 50.0 | PCE heavy fraction crystal | 7.86 × 104 | |
| air 3 | 1.96 | 0.784 | soil 3 | 4.13 | 15.0 | PCE heavy fraction liquid | 8.47 × 104 | |
| air 4 | 18.1 | 7.24 | sludge 1 | 7.27 × 104 | — | commercial PCE | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Guo, J.; Liu, M.; Liu, J.; Zhang, L. Levels, Distributions, and Potential Risks of Hexachlorobutadiene from Two Tetrachloroethylene Factories in China. Int. J. Environ. Res. Public Health 2023, 20, 5107. https://doi.org/10.3390/ijerph20065107
Liu C, Guo J, Liu M, Liu J, Zhang L. Levels, Distributions, and Potential Risks of Hexachlorobutadiene from Two Tetrachloroethylene Factories in China. International Journal of Environmental Research and Public Health. 2023; 20(6):5107. https://doi.org/10.3390/ijerph20065107
Chicago/Turabian StyleLiu, Chengyou, Jing Guo, Meng Liu, Jinlin Liu, and Lifei Zhang. 2023. "Levels, Distributions, and Potential Risks of Hexachlorobutadiene from Two Tetrachloroethylene Factories in China" International Journal of Environmental Research and Public Health 20, no. 6: 5107. https://doi.org/10.3390/ijerph20065107




