Changes Caused by Bisphenols in the Chemical Coding of Neurons of the Enteric Nervous System of Mouse Stomach
Abstract
:1. Introduction
2. Materials and Methods
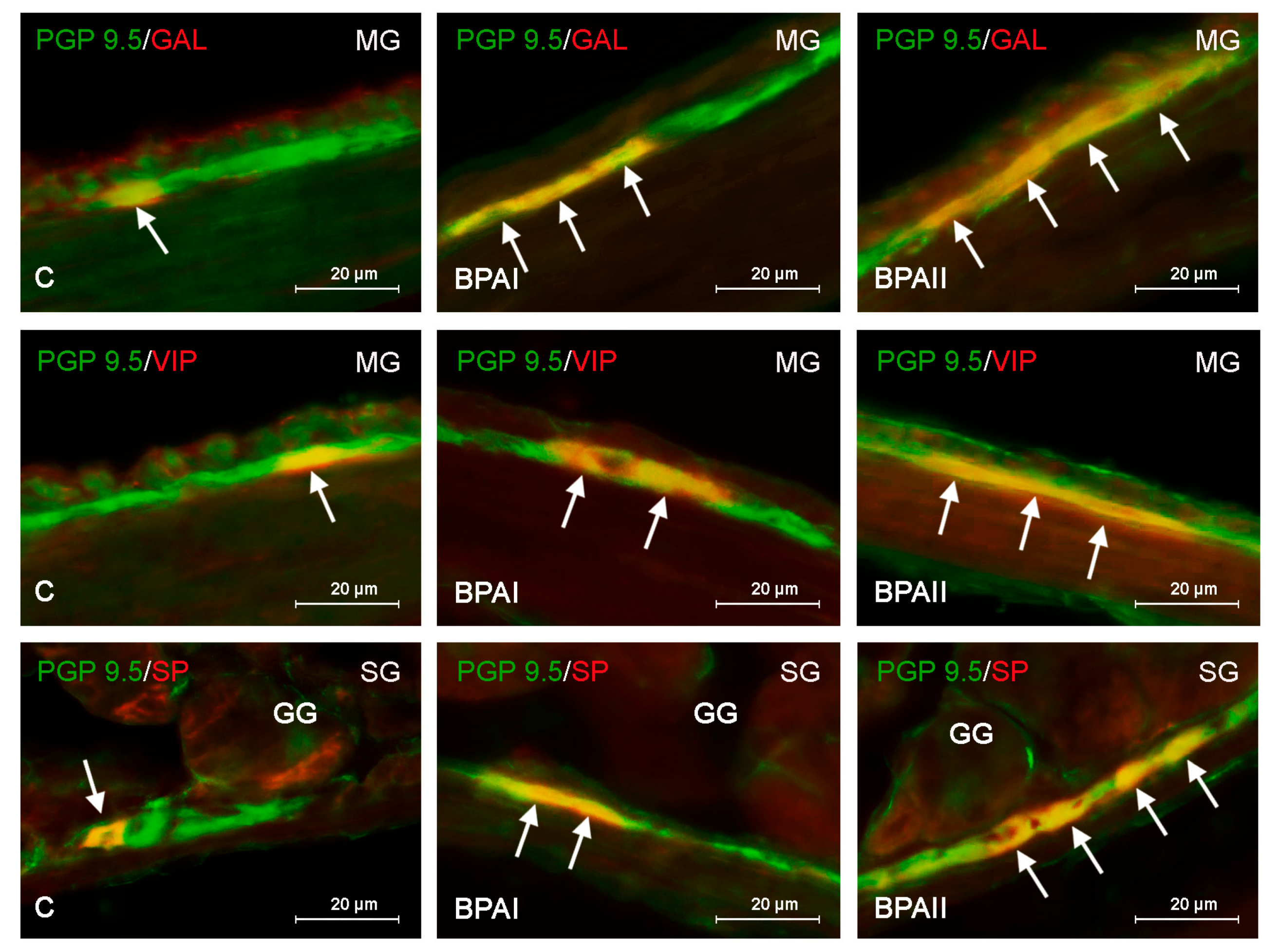
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michałowicz, J. Bisphenol A-sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, A.; Rutkowska, A.; Rachoń, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Panstw. Zakl. Hig. 2015, 66, 5–11. [Google Scholar] [PubMed]
- Frankowski, R.; Zgoła-Grześkowiak, A.; Grześkowiak, T.; Sójka, K. The presence of bisphenol A in the thermal paper in the face of changing European regulations–A comparative global research. Environ. Pollut. 2020, 265, 114879. [Google Scholar] [CrossRef] [PubMed]
- Bousoumah, R.; Leso, V.; Iavicoli, I.; Huuskonen, P.; Viegas, S.; Porras, S.P.; Santonen, T.; Frery, N.; Robert, A.; Ndaw, S. Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review. Sci. Total Environ. 2021, 783, 146905. [Google Scholar] [CrossRef]
- An, H.; Yu, H.; Wei, Y.; Liu, F.; Ye, J. Disrupted metabolic pathways and potential human diseases induced by bisphenol S. Environ. Toxicol. Pharmacol. 2021, 88, 103751. [Google Scholar] [CrossRef]
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol A: A literature review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef] [Green Version]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Bisphenol A, bisphenol F, and bisphenol S: The bad and the ugly. Where is the good? Life 2021, 11, 314. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Liu, W.; Fu, D.; Zhang, X.; Chai, J.; Tian, S.; Han, J. Development and validation of a new artificial gastric digestive system. Food Res. Int. 2019, 122, 183–190. [Google Scholar] [CrossRef]
- Szymanska, K.; Makowska, K.; Gonkowski, S. The Influence of High and Low Doses of Bisphenol A (BPA) on the Enteric Nervous System of the Porcine Ileum. Int. J. Mol. Sci. 2018, 19, 917. [Google Scholar] [CrossRef] [Green Version]
- Szymanska, K.; Gonkowski, S. Neurochemical characterization of the enteric neurons within the porcine jejunum in physiological conditions and under the influence of bisphenol A (BPA). Neurogastroenterol. Motil. 2019, 31, e13580. [Google Scholar] [CrossRef]
- Sarkar, K.; Tarafder, P.; Paul, G. Bisphenol A inhibits duodenal movement ex vivo of rat through nitric oxide-mediated soluble guanylyl cyclase and α-adrenergic signaling pathways. J. Appl. Toxicol. 2016, 36, 131–139. [Google Scholar] [CrossRef]
- Qu, W.; Zhao, Z.; Chen, S.; Zhang, L.; Wu, D.; Chen, Z. Bisphenol A suppresses proliferation and induces apoptosis in colonic epithelial cells through mitochondrial and MAPK/AKT pathways. Life Sci. 2018, 208, 167–174. [Google Scholar] [CrossRef]
- Zhao, Z.; Qu, W.; Wang, K.; Chen, S.; Zhang, L.; Wu, D.; Chen, Z. Bisphenol A inhibits mucin 2 secretion in intestinal goblet cells through mitochondrial dysfunction and oxidative stress. Biomed. Pharmacother. 2019, 111, 901–908. [Google Scholar] [CrossRef]
- Feng, L.; Chen, S.; Zhang, L.; Qu, W.; Chen, Z. Bisphenol A increases intestinal permeability through disrupting intestinal barrier function in mice. Environ. Pollut. 2019, 254, 112960. [Google Scholar] [CrossRef]
- Makowska, K.; Gonkowski, S. Bisphenol A (BPA) Affects the Enteric Nervous System in the Porcine Stomach. Animals 2020, 10, 2445. [Google Scholar] [CrossRef]
- Makowska, K.; Całka, J.; Gonkowski, S. Effects of the long-term influence of bisphenol A and bisphenol S on the population of nitrergic neurons in the enteric nervous system of the mouse stomach. Sci. Rep. 2023, 13, 331. [Google Scholar] [CrossRef]
- Makowska, K.; Gonkowski, S. Age and Sex-Dependent Differences in the Neurochemical Characterization of Calcitonin Gene-Related Peptide-Like Immunoreactive (CGRP-LI) Nervous Structures in the Porcine Descending Colon. Int. J. Mol. Sci. 2019, 20, 1024. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected roles for the second brain: Enteric nervous system as master regulator of bowel function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; de Ponti, F.; de Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 126–127, 264–272. [Google Scholar] [CrossRef]
- Phillips, R.J.; Powley, T.L. Innervation of the gastrointestinal tract: Patterns of aging. Auton. Neurosci. 2007, 136, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaroni, C.; De Ponti, F.; Cosentino, M.; Lecchini, S.; Frigo, G. Plasticity in the enteric nervous system. Gastroenterology 1999, 117, 1438–1458. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The Enteric Nervous System; Blackwell Publishing: Oxford, MA, USA, 2006. [Google Scholar]
- Vergnolle, N.; Cirillo, C. Neurons and glia in the enteric nervous system and epithelial barrier function. Physiology 2018, 33, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Puzan, M.; Hosic, S.; Ghio, C.; Koppes, A. Enteric nervous system regulation of intestinal stem cell differentiation and epithelial monolayer function. Sci. Rep. 2018, 8, 6313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-Elsoud, R.A.E.A.; Ahmed Mohamed Abdelaziz, S.; Attia Abd Eldaim, M.; Hazzaa, S.M. Moringa oleifera alcoholic extract protected stomach from bisphenol A-induced gastric ulcer in rats via its anti-oxidant and anti-inflammatory activities. Environ. Sci. Pollut. Res. Int. 2022, 29, 68830–68841. [Google Scholar] [CrossRef]
- Dobrzynska, M.M.; Gajowik, A.; Jankowska-Steifer, E.A.; Radzikowska, J.; Tyrkiel, E.J. Reproductive and developmental F1 toxicity following exposure of pubescent F0 male mice to bisphenol A alone and in a combination with X-rays irradiation. Toxicology 2018, 410, 142–151. [Google Scholar] [CrossRef]
- Rezg, R.; Abot, A.; Mornagui, B.; Aydi, S.; Knauf, C. Effects of Bisphenol S on hypothalamic neuropeptides regulating feeding behavior and apelin/APJ system in mice. Ecotoxicol. Environ. Saf. 2018, 161, 459–466. [Google Scholar] [CrossRef]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Thomas, B.F.; Keimowitz, A.R.; Brine, D.R.; Veselica, M.M.; Fail, P.A.; Chang, T.Y.; Seely, J.C.; et al. Three generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 2002, 68, 121–146. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.W.; Jeong, J.Y.; Hwang, M.S.; Jung, K.K.; Lee, K.H.; Lee, H.M. Establishment of the korean tolerable daily intake of bisphenol a based on risk assessments by an expert committee. Toxicol. Res. 2010, 26, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, M.; Wojnowska-Baryla, I.; Cydzik-Kwiatkowska, A. Bisphenol A Removal from Water and Wastewater; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-92361-1. [Google Scholar]
- Makowska, K.; Lepiarczyk, E.; Gonkowski, S. The comparison of the influence of bisphenol A (BPA) and its analogue bisphenol S (BPS) on the enteric nervous system of the distal colon in mice. Nutrients 2023, 15, 200. [Google Scholar] [CrossRef]
- Brzozowska, M.; Całka, J. Review: Occurrence and Distribution of Galanin in the Physiological and Inflammatory States in the Mammalian Gastrointestinal Tract. Front. Immunol. 2021, 11, 602070. [Google Scholar] [CrossRef]
- Currò, D.; Preziosi, P. Non-adrenergic non-cholinergic relaxation of the rat stomach. Gen. Pharmacol. 1998, 31, 697–703. [Google Scholar] [CrossRef]
- Tomita, R. Regulation of vasoactive intestinal peptide and substance P in the human pyloric sphincter. Hepatogastroenterology 2009, 56, 1403–1406. [Google Scholar]
- Lang, R.; Gundlach, A.L.; Kofler, B. The galanin peptide family: Receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol. Ther. 2007, 115, 177–207. [Google Scholar] [CrossRef]
- Botella, A.; Delvaux, M.; Frexinos, J.; Bueno, L. Comparative effects of galanin on isolated smooth muscle cells from ileum in five mammalian species. Life Sci. 1992, 50, 1253–1261. [Google Scholar] [CrossRef]
- Makowska, K. Chemically induced inflammation and nerve damage affect the distribution of vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nervous structures in the descending colon of the domestic pig. Neurogastroenterol. Motil. 2018, 30, e13439. [Google Scholar] [CrossRef]
- Arciszewski, M.B.; Ekblad, E. Effects of vasoactive intestinal petide and galanin on survival of cultured porcine myenteric neurons. Regul. Pept. 2005, 125, 185–192. [Google Scholar] [CrossRef]
- Baralić, K.; Živančević, K.; Javorac, D.; Buha Djordjevic, A.; Anđelković, M.; Jorgovanović, D.; Antonijević Miljaković, E.; Ćurčić, M.; Bulat, Z.; Antonijević, B.; et al. Multi-strain probiotic ameliorated toxic effects of phthalates and bisphenol A mixture in Wistar rats. Food Chem. Toxicol. 2020, 143, 111540. [Google Scholar] [CrossRef]
- Durcik, M.; Gramec Skledar, D.; Tomašič, T.; Trontelj, J.; Peterlin Mašič, L. Last piece in the puzzle of bisphenols BPA, BPS and BPF metabolism: Kinetics of the in vitro sulfation reaction. Chemosphere 2022, 303, 135133. [Google Scholar] [CrossRef]
- Mazur, C.S.; Marchitti, S.A.; Dimova, M.; Kenneke, J.F.; Lumen, A.; Fisher, J. Human and rat ABC transporter efflux of bisphenol a and bisphenol a glucuronide: Interspecies comparison and implications for pharmacokinetic assessment. Toxicol. Sci. 2012, 128, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Doerge, D.R.; Twaddle, N.C.; Vanlandingham, M.; Fisher, J.W. Pharmacokinetics of bisphenol A in neonatal and adult CD-1 mice: Inter-species comparisons with Sprague-Dawley rats and rhesus monkeys. Toxicol. Lett. 2011, 207, 298–305. [Google Scholar] [CrossRef] [PubMed]
- EFSA, J. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978–4599. [Google Scholar]
- Mantyh, C.R.; Vigna, S.R.; Maggio, J.E.; Mantyh, P.W.; Bollinger, R.R.; Pappas, T.N. Substance P binding sites on intestinal lymphoid aggregates and blood vessels in inflammatory bowel disease correspond to authentic NK-1 receptors. Neurosci. Lett. 1994, 178, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef] [Green Version]
- Salthun-Lassalle, B.; Traver, S.; Hirsch, E.C.; Michel, P.P. Substance P, neurokinins A and B, and synthetic tachykinin peptides protect mesencephalic dopaminergic neurons in culture via an activity-dependent mechanism. Mol. Pharmacol. 2005, 68, 1214–1224. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, H.; Yokota, H.; Kibe, R.; Sayama, Y.; Yuasa, A. Excretion of bisphenol A-glucuronide into the small intestine and deconjugation in the cecum of the rat. Biochim. Biophys. Acta 2002, 1573, 171–176. [Google Scholar] [CrossRef]
- Peillex, C.; Kerever, A.; Lachhab, A.; Pelletier, M. Bisphenol A, bisphenol S and their glucuronidated metabolites modulate glycolysis and functional responses of human neutrophils. Environ. Res. 2021, 196, 110336. [Google Scholar] [CrossRef]
- Seki, S.; Aoki, M.; Hosokawa, T.; Saito, T.; Masuma, R.; Komori, M.; Kurasaki, M. Bisphenol-A suppresses neu-rite extension due to inhibition of phosphorylation of mito-gen-activated protein kinase in PC12 cells. Chem. Biol. Interact. 2011, 194, 23–30. [Google Scholar] [CrossRef]
- Xu, X.; Xie, L.; Hong, X.; Ruan, Q.; Lu, H.; Zhang, Q.; Zhang, G.; Liu, X. Perinatal exposure to bisphenol-A inhibits synaptogenesis and affects the synaptic morphological development in offspring male mice. Chemosphere 2013, 91, 1073–1081. [Google Scholar] [CrossRef]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R. Neuro-toxic and reproductive effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Huang, Q.; Chi, Y.; Dong, S.; Fan. J. Bisphenol-A induces neurodegeneration through disturbance of intracellular calcium homeostasis in human embryonic stem cells-derived cortical neurons. Chemosphere 2019, 229, 618–630. [Google Scholar] [CrossRef]
- Young, W. Role of calcium in central nervous system injuries. J. Neurotrauma 1992, 1, S9–S25. [Google Scholar]
- Wang, W.; Wang, J.; Wang, Q.; Wu, W.; Huan, F.; Xiao, H. Bisphenol A modulates calcium currents and intracellular calcium concentration in rat dorsal root ganglion neurons. J. Membr. Biol. 2013, 246, 391–397. [Google Scholar] [CrossRef]
- Nakamura, K.; Itoh, K.; Sugimoto, T.; Fushiki, S. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci. Lett. 2007, 420, 100–105. [Google Scholar] [CrossRef]
- Song, H.; Park, J.; Buim, P.T.C.; Choi, K.; Gye, M.C.; Hong, Y.C.; Kim, J.H.; Lee, Y.J. Bisphenol A induces COX-2 through the mitogen-activated protein kinase pathway and is associated with levels of inflammation-related markers in elderly populations. Environ. Res. 2017, 158, 490–498. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J. Changes in VIP-, SP- and CGRP- like immunoreactivity in intramural neurons within the pig stomach following supplementation with low and high doses of acrylamide. Neurotoxicology 2018, 69, 47–59. [Google Scholar] [CrossRef]
- Vota, D.; Aguero, M.; Grasso, E.; Hauk, V.; Gallino, L.; Soczewski, E.; Pérez Leirós, C.; Ramhorst, R. Progesterone and VIP cross-talk enhances phagocytosis and anti-inflammatory profile in trophoblast-derived cells. Mol. Cell. Endocrinol. 2017, 443, 146–154. [Google Scholar] [CrossRef]



| Primary Antibodies | Use of Antibodies on Porcine Tissues in Previous Studies | ||||
|---|---|---|---|---|---|
| Antigen | Code | Host Species | Working Dilution | Supplier | |
| PGP 9.5 | 7863-2004 | Mouse | 1:1000 | Biogenesis Ltd., Poole, UK | [17,32] |
| VIP | VA 1285 | Rabbit | 1:2000 | Enzo Life Sciences; Farmingdale, NY, USA | [32] |
| GAL | T-5036 | Guinea Pig | 1:2000 | Peninsula Labs., San Carlos, CA, USA; | [32] |
| SP | 8450-0505 | Rat | 1:1000 | Bio-Rad (AbD Serotec), Kidlington, UK | [32] |
| VAChT | H-V006 | Rabbit | 1:2000 | Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA | [32] |
| Secondary Antibodies | |||||
| Reagents | Working dilution | Supplier | |||
| Alexa fluor 488 donkey anti-mouse IgG | 1:1000 | Invitrogen, Carlsbad, CA, USA | [17,32] | ||
| Alexa fluor 546 donkey anti-rabbit IgG | 1:1000 | Invitrogen | [17,32] | ||
| Alexa fluor 546 donkey anti-guinea pig IgG | 1:1000 | Invitrogen | [32] | ||
| Alexa fluor 546 donkey anti-rat IgG | 1:1000 | Invitrogen | [32] | ||
| Substance | Type of Enteric Ganglion | Animal Groups | ||||
|---|---|---|---|---|---|---|
| C | BPAI | BPAII | BPSI | BPSII | ||
| GAL | MG | 32.84 ± 0.46 | 37.89 ± 0.61 * | 43.60 ± 0.82 * | 32.95 ± 0.87 | 35.27 ± 0.57 * |
| SG | 30.37 ± 0.90 | 35.32 ± 0.65 * | 38.08 ± 0.84 * | 31.15 ± 0.70 | 35.01 ± 1.00 * | |
| VIP | MG | 38.85 ± 0.84 | 41.40 ± 0.40 * | 44.24 ± 1.02 * | 39.76 ± 0.57 * | 39.44 ± 0.94 * |
| SG | 31.31 ± 0.62 | 35.08 ± 1.15 * | 42.82 ± 0.18 * | 32.30 ± 0.50 | 33.62 ± 1.34 | |
| SP | MG | 20.73 ± 1.43 | 31.44 ± 3.47 * | 36.70 ± 2.08 * | 23.59 ± 0.98 | 28.03 ± 1.30 * |
| SG | 14.98 ± 0.84 | 27.92 ± 0.70 * | 33.35 ± 2.16 * | 23.52 ± 1.18 * | 30.34 ± 2.56 * | |
| VAChT | MG | 54.41 ± 0.41 | 48.05 ± 0.72 * | 39.69 ± 0.38 * | 49.88 ± 0.97 * | 45.32 ± 1.04 * |
| SG | 51.70 ± 0.64 | 46.52 ± 0.98 * | 36.63 ± 1.43 * | 48.59 ± 0.24 * | 44.18 ± 1.06 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makowska, K.; Gonkowski, S. Changes Caused by Bisphenols in the Chemical Coding of Neurons of the Enteric Nervous System of Mouse Stomach. Int. J. Environ. Res. Public Health 2023, 20, 5125. https://doi.org/10.3390/ijerph20065125
Makowska K, Gonkowski S. Changes Caused by Bisphenols in the Chemical Coding of Neurons of the Enteric Nervous System of Mouse Stomach. International Journal of Environmental Research and Public Health. 2023; 20(6):5125. https://doi.org/10.3390/ijerph20065125
Chicago/Turabian StyleMakowska, Krystyna, and Slawomir Gonkowski. 2023. "Changes Caused by Bisphenols in the Chemical Coding of Neurons of the Enteric Nervous System of Mouse Stomach" International Journal of Environmental Research and Public Health 20, no. 6: 5125. https://doi.org/10.3390/ijerph20065125





