Possible Sources of Trace Metals in Obese Females Living in Informal Settlements near Industrial Sites around Gauteng, South Africa
Abstract
1. Introduction
2. Methodology
2.1. Study Design and Setting
2.2. Sampling and Study Population
2.3. Trace Metal Determination
2.4. Data Analysis
2.5. Quality Control and Assurance
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivas-Arancibia, S.; Balderas-Miranda, J.; Belmont-Zúñiga, L.; Martínez-Jáquez, M.; Hernández-Orozco, E.; Cornejo-Trejo, V.; Reséndiz-Ramos, C.; Cruz-García, I.; Espinosa-Caleti, I.; Valdés-Fuentes, M.; et al. Oxidative stress, antioxidant defenses, COVID-19 and pollution. Med. Res. Arch. 2020, 8. [Google Scholar] [CrossRef]
- Li, T.; Yu, L.; Yang, Z.; Shen, P.; Lin, H.; Shui, L.; Tang, M.; Jin, M.; Chen, K.; Wang, J. Associations of Diet Quality and Heavy Metals with Obesity in Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES). Nutrients 2022, 14, 4038. [Google Scholar] [CrossRef] [PubMed]
- Limaye, S.; Salvi, S. Obesity and asthma: The role of environmental pollutants. Immunol. Allergy Clin. 2014, 34, 839–855. [Google Scholar]
- World Health Organization. Human Biomonitoring: Facts and Figures; WHO Regional Office for Europe: Copenhagen, Denmark, 2015. [Google Scholar]
- Heart and Stroke Foundation South Africa. Cardiovascular Disease Statistics Reference Document; HSFSA: Cape Town, South Africa, 2016; Volume 3. [Google Scholar]
- Joubert, J.; Norman, R.; Bradshaw, D.; Goedecke, J.H.; Steyn, N.P.; Puoane, T. Estimating the burden of disease attributable to excess body weight in South Africa in 2000. S. Afr. Med. J. 2007, 97, 683–690. [Google Scholar] [PubMed]
- Steyn, K.; Damasceno, A. Lifestyle and related risk factors for chronic diseases. Dis. Mortal. Sub-Sahar. Afr. 2006, 2, 247–265. [Google Scholar]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Sampaio, M.S.; Reddy, P.N.; Kuo, H.T.; Poommipanit, N.; Cho, Y.W.; Shah, T.; Bunnapradist, S. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation 2010, 89, 1117–1125. [Google Scholar] [CrossRef]
- Turan, Ö.; Özdemir, H.; Demir, G. Deposition of heavy metals on coniferous tree leaves and soils near heavy urban traffic. Front. Life Sci. Relat. Technol. 2020, 1, 35–41. [Google Scholar]
- TürksoY, R.; Terzioğlu, G.; Yalçin, İ.E.; Türksoy, Ö.; Demir, G. Removal of heavy metals from textile industry wastewater. Front. Life Sci. Relat. Technol. 2021, 2, 44–50. [Google Scholar] [CrossRef]
- Imseng, M.; Wiggenhauser, M.; Müller, M.; Keller, A.; Frossard, E.; Wilcke, W.; Bigalke, M. The fate of Zn in agricultural soils: A stable isotope approach to anthropogenic impact, soil formation, and soil−plant cycling. Environ. Sci. Technol. 2019, 53, 4140. [Google Scholar] [CrossRef]
- Shi, T.R.; Ma, J.; Wu, F.Y.; Ju, T.N.; Gong, Y.W.; Zhang, Y.Y.; Wu, X.; Hou, H.; Zhao, L.; Shi, H.D. Mass balance-based inventory of heavy metals inputs to and outputs from agricultural soils in Zhejiang Province, China Sci. Total Environ. 2019, 649, 1269–1280. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, J.; Wang, L.; Liang, T.; Guo, Q.; Guan, Y.; Rinklebe, J. Elucidating the differentiation of soil heavy metals under different land uses with geographically weighted regression and self-organizing map. Environ. Pollut. 2020, 260, 114065. [Google Scholar] [CrossRef]
- Cai, P.; Cai, G.; Yang, J.; Li, X.; Lin, J.; Li, S.; Zhao, L. Distribution, risk assessment, and quantitative source apportionment of heavy metals in surface sediments from the shelf of the northern South China Sea. Mar. Pollut. Bull. 2023, 187, 114589. [Google Scholar] [CrossRef]
- George, A.; Shen, B.; Kang, D.; Yang, J.; Luo, J. Emission control strategies of hazardous trace elements from coal-fired power plants in China. J. Environ. Sci. 2020, 93, 66–90. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xu, T.; Tian, Y.; Zhong, Y.; Ali, F.A.Z.; Yang, X.; Lu, B. Health benefits and phenolic compounds of Moringa oleifera leaves: A comprehensive review. Phytomedicine 2019, 93, 153771. [Google Scholar] [CrossRef]
- Mohammed, R.S.; Ibrahim, W.; Sabry, D.; El-Jaafary, S.I. Occupational metals exposure and cognitive performance among foundry workers using tau protein as a biomarker. Neurotoxicology 2020, 76, 10–16. [Google Scholar] [CrossRef]
- Gill, M. Heavy metal stress in plants: A review. Int. J. Adv. Res. 2014, 2, 1043–1055. [Google Scholar]
- Bomberg, M.; Ahonen, L. Geomicrobes: Life in terrestrial deep subsurface. Front. Microbiol. 2017, 8, 103. [Google Scholar] [CrossRef]
- Muhammad Shehata, I.; Masood, W.; Nemr, N.; Anderson, A.; Bhusal, K.; Edinoff, A.N.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. The possible application of ketamine in the treatment of depression in Alzheimer’s disease. Neurol. Int. 2022, 14, 310–321. [Google Scholar] [CrossRef]
- Vincēviča-Gaile, Z. Impact of Environmental Conditions on Micro-and Macroelement Content in Selected Food from Latvia. Ph.D. Thesis, [In Latvia] University of Latvia, Riga, Latvia, 2014; pp. 1–182. [Google Scholar]
- Street, R.A.; Goessler, W.; Naidoo, S.; Shezi, B.; Cele, N.; Rieger, J.; Ettinger, K.; Reddy, T.; Mathee, A. Exposure to lead and other toxic metals from informal foundries producing cookware from scrap metal. Environ. Res. 2020, 191, 109860. [Google Scholar] [CrossRef]
- Lion, G.N.; Olowoyo, J.O. Population health risk due to dietary intake of toxic heavy metals from Spinacia oleracea harvested from soils collected in and around Tshwane, South Africa. S. Afr. J. Bot. 2013, 88, 178–182. [Google Scholar] [CrossRef]
- Lion, G.N.; Olowoyo, J.O. Health risk assessment of dust samples (indoor and outdoor) collected from houses around a mining area in Brits, South Africa. Curr. Top. Toxicol. 2021, 17, 99–111. [Google Scholar]
- Nriagu, J.O.; Bhattacharya, P.; Mukherjee, A.B.; Bundschuh, J.; Zevenhoven, R.; Loeppert, R.H. Arsenic in soil and groundwater: An overview. Trace Met. Other Contam. Environ. 2007, 9, 3–60. [Google Scholar]
- Olowoyo, J.O.; Magoloi, Z.G.; Lion, G.N.; Ogunbanjo, G. Toxic trace metals in blood of occupationally exposed casual mine workers living in shacks around mining area in Brits, South Africa. Trace Elem. Electrolytes 2019, 37, 1–11. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Molecular, clinical and environmental toxicology. Environ. Toxicol. 2012, 3, 133–164. [Google Scholar]
- Nallagouni, C.; Reddy, K.P. Aluminum and fluoride impacts cortex and hippocampus structure in rats: Protective role of resveratrol. Int. J. Appl. Biol. Pharm. Technol. 2017, 8, 89–97. [Google Scholar]
- Tajahmadi, S.; Shamloo, A.; Shojaei, A.; Sharifzadeh, M. Adsorption Behavior of a Gd-Based Metal–Organic Framework toward the Quercetin Drug: Effect of the Activation Condition. ACS Omega 2022, 7, 41177–41188. [Google Scholar] [CrossRef]
- Etsuyankpa, M.B.; Ahmad, A.S.; Mustapha, S.; Baba, N.M.; Ndamitso, M.M.; Elabor, R. Levels of selected heavy metals in blood and urine of workers of Alkali Kongo Plaza GSM Market within Lafia Metropolis, Nasarawa State, Nigeria. Environ. Chall. 2022, 9, 100649. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, J.; Du, B.; Liu, H.; Zhang, W.; Liang, J.; Zhang, W.; You, L.; Zhou, J. Health risks to local residents from the exposure of heavy metals around the largest copper smelter in China. Ecotoxicol. Environ. Saf. 2022, 171, 329–336. [Google Scholar] [CrossRef]
- Nkosi, N.Z.; Manzi, M.S.; Westgate, M.; Roberts, D.; Durrheim, R.J.; Ogasawara, H.; Ziegler, M.; Rickenbacher, M.; Liebenberg, B.; Onstott, T.C.; et al. Physical property studies to elucidate the source of seismic reflectivity within the ICDP DSeis seismogenic zone: Klerksdorp goldfield, South Africa. Int. J. Rock Mech. Min. Sci. 2022, 155, 105082. [Google Scholar] [CrossRef]
- Zupunski, L.; Street, R.; Ostroumova, E.; Winde, F.; Sachs, S.; Geipel, G.; Nkosi, V.; Bouaoun, L.; Haman, T.; Schüz, J.; et al. Environmental exposure to uranium in a population living in close proximity to gold mine tailings in South Africa. J. Trace Elem. Med. Biol. 2023, 77, 127141. [Google Scholar] [CrossRef]
- Martin, S.; Griswold, W. Human health effects of heavy metals. Environ. Sci. Technol. Briefs Citiz. 2009, 15, 1–6. [Google Scholar]
- Brunetto, G.; Miotto, A.; Ceretta, C.A.; Schmitt, D.E.; Heinzen, J.; de Moraes, M.P.; Canton, L.; Tiecher, T.L.; Comin, J.J.; Girotto, E. Mobility of copper and zinc fractions in fungicide-amended vineyard sandy soils. Arch. Agron. Soil Sci. 2014, 60, 609–624. [Google Scholar] [CrossRef]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Potential human risk of dissolved heavy metals in gold mine waters of Gauteng Province, South Africa. J. Toxicol. Environ. Health Sci. 2018, 10, 56–63. [Google Scholar]
- Olowoyo, J.O.; Lion, G.N. Urban farming as a possible source of trace metals in human diets. S. Afr. J. Sci. 2016, 112, 30–35. [Google Scholar] [CrossRef]
- Okereafor, G.; Makhatha, M.; Mekuto, L.; Mavumengwana, V. Evaluation of trace elemental levels as pollution indicators in an abandoned gold mine dump in Ekurhuleni Area, South Africa. In Trace Metals in the Environment—New Approaches and Recent Advances; IntechOpen: London, UK, 2019. [Google Scholar]
- Molloy, I.; Chen, H.; Li, T.; Wang, Q.; Li, N.; Bertino, E.; Calo, S.; Lobo, J. Mining roles with multiple objectives. ACM Trans. Inf. Syst. Secur. 2010, 13, 1–35. [Google Scholar] [CrossRef]
- Africa Mining, IQ. Africa’s Mining Portal: Online Mining Information Service. 2022. Available online: https://projectsiq.co.za/?gclid=Cj0KCQjwk7ugBhDIARIsAGuvgPYNnMO7sBTYX-j9eS5kYlpmzErfa8VcHLWXs-D9yqjuegtJ-NbUy5IaAv1MEALw_wcB (accessed on 1 February 2023).
- Hendryx, M.; Zullig, K.J.; Luo, J. Impacts of coal use on health. Annu. Rev. Public Health 2020, 41, 397–415. [Google Scholar] [CrossRef]
- Ortega-Flores, P.A.; Serviere-Zaragoza, E.; De Anda-Montañez, J.A.; Freile-Pelegrín, Y.; Robledo, D.; Méndez-Rodríguez, L.C. Trace elements in pelagic Sargassum species in the Mexican Caribbean: Identification of key variables affecting arsenic accumulation in S. fluitans. Sci. Total Environ. 2022, 806, 150657. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Finkelman, R.B.; French, D.; Graham, I.T.; Yang, Y.; Li, J.; Yang, P. Leaching behavior of trace elements from fly ashes of five Chinese coal power plants. Int. J. Coal Geol. 2020, 219, 103381. [Google Scholar] [CrossRef]
- Flues, M.; Sato, I.M.; Scapin, M.A.; Cotrim, M.E.B.; Camargo, I.M.C. Toxic elements mobility in coal and ashes of Figueira coal power plant, Brazil. Fuel 2013, 103, 430–436. [Google Scholar] [CrossRef]
- Silva, L.F.; Ward, C.R.; Hower, J.C.; Izquierdo, M.; Waanders, F.; Oliveira, M.L.; Li, Z.; Hatch, R.S.; Querol, X. Mineralogy and leaching characteristics of coal ash from a major Brazilian power plant. Coal Combust. Gasif. Prod. 2010, 2, 51–65. [Google Scholar] [CrossRef]
- Okedeyi, O.O.; Dube, S.; Awofolu, O.R.; Nindi, M.M. Assessing the enrichment of heavy metals in surface soil and plant (Digitaria eriantha) around coal-fired power plants in South Africa. Environ. Sci. Pollut. Res. 2014, 21, 4686–4696. [Google Scholar] [CrossRef] [PubMed]
- Vorster, T.; Mthombeni, J.; TeWaterNaude, J.; Phillips, J.I. The Association between the Histological Subtypes of Mesothelioma and Asbestos Exposure Characteristics. Int. J. Environ. Res. Public Health 2022, 19, 14520. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, J.; Martinello, K.D.B. Coal as an energy source and its impacts on human health. Energy Geosci. 2021, 2, 113–120. [Google Scholar] [CrossRef]
- Nasab, H.; Rajabi, S.; Eghbalian, M.; Malakootian, M.; Hashemi, M.; Mahmoudi-Moghaddam, H. Association of As, Pb, Cr, and Zn urinary heavy metals levels with predictive indicators of cardiovascular disease and obesity in children and adolescents. Chemosphere 2022, 294, 133664. [Google Scholar] [CrossRef]
- Fan, J.G.; Kim, S.U.; Wong, V.W.S. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef]
- Griffin, W.M.; Michalek, J.; Matthews, H.S.; Hassan, M.N.A. Availability of biomass residues for co-firing in Peninsular Malaysia: Implications for cost and GHG emissions in the electricity sector. Energies 2014, 7, 804–823. [Google Scholar] [CrossRef]
- De Ridder, J.H.; Coetzee, D. Childhood obesity in South Africa: Are we sitting on a time bomb? Glob. J. Health Phys. Educ. Pedagog. 2013, 2, 239–249. [Google Scholar]
- Lion, G.N.; Ogunbanjo, G.A.; Olowoyo, J.O. Concentration of Trace Metals in Blood and the Relationship with Reproductive Hormones (Estradiol and Progesterone) of Obese Females Living Around a Mining Area in Brits, South Africa. Nat. Environ. Pollut. Technol. 2020, 19, 1163–1171. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, S.Y.; Lee, Y.J. Facile one-pot hydrothermal synthesis of cubic spinel-type manganese ferrite/biochar composites for environmental remediation of heavy metals from aqueous solutions. Bioresour. Technol. 2018, 261, 1–9. [Google Scholar] [CrossRef]
- ElMohr, M.; Faris, M.; Bakry, S.; Hozyen, H.; Elshaer, F.M. Effect of passive smoking on heavy metals concentration in blood and follicular fluid of patients on going ICSI. Indian J. Sci. Technol. 2020, 13, 2035–2040. [Google Scholar] [CrossRef]
- Olufunsho, O.O.; Tope-Ajayi, A.A.; Alabi, G.O. Effect of wood smoke on selected blood antioxidant parameters, essential and heavy metal levels in women. Eur. Sci. J. 2015, 11, 238–244. [Google Scholar]
- Raimi, I.O.; Komolafe, B.F.; Agboola, O.O.; Mugivhisa, L.L.; Olowoyo, J.O. Influence of wind direction on the level of trace metals in plants collected around a Quarry Site in South Africa. Pol. J. Environ. Stud. 2019, 28, 3385–3393. [Google Scholar] [CrossRef]
- Dey, S.; Tripathy, B.; Kumar, M.S.; Das, A.P. Ecotoxicological consequences of manganese mining pollutants and their biological remediation. Environ. Chem. Ecotoxicol. 2023, 5, 55–61. [Google Scholar] [CrossRef]
- Pesch, B.; Weiss, T.; Kendzia, B.; Henry, J.; Lehnert, M.; Lotz, A.; Heinze, E.; Käfferlein, H.U.; Van Gelder, R.; Berges, M.; et al. Levels and predictors of airborne and internal exposure to manganese and iron among welders. J. Expo Sci. Environ. Epidemiol. 2012, 22, 291–298. [Google Scholar] [CrossRef]
- Aguera, R.G.; da Silva Freires, C.; de Oliveira, L.O.; Monteiro, L.R.; Lini, R.S.; Romoli, J.C.Z.; Freire, B.M.; Nerilo, S.B.; Junior, M.M.; Batista, B.L.; et al. Risk evaluation of occupational exposure of southern Brazilian flower farmers to pesticides potentially leading to cholinesterase inhibition and metals exposure. Environ. Toxicol. Pharmacol. 2022, 93, 103874. [Google Scholar] [CrossRef]
- Alexander, C.S. Cobalt-beer cardiomyopathy: A clinical and pathologic study of twenty-eight cases. Am. J. Med. 1972, 53, 395–417. [Google Scholar] [CrossRef]
- Mannino, D.M.; Homa, D.M.; Matte, T.; Hernandez-Avila, M. Active and passive smoking and blood lead levels in U.S. adults: Data from the Third National Health and Nutrition Examination Survey. Nicotine Tob. Res. 2005, 7, 557–564. [Google Scholar] [CrossRef]
- Mohanty, N.; Patra, B.N. Polypyrrole-Sodium Alginate Nanocomposites for Enhanced Removal of Toxic Organic and Metal Pollutants from Wastewater. Mater. Today Commun. 2023, 34, 105325. [Google Scholar] [CrossRef]
- Cameán, A.; López-Artíguez, M.; Roca, I.; Herce-Pagliai, C.; Menéndez, M.; Repetto, M. Determination of cobalt, manganese, and alcohol content in beers. J. Food Prot. 1998, 61, 129–131. [Google Scholar] [CrossRef]
- Eren, H.; Uzun, H.; Andac, M.; Bilir, S. Potentiometric monitoring of cobalt in beer sample by solid contact ion selective electrode. J. Food Drug Anal. 2014, 22, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Talan, D.; Huang, Q. A review study of rare Earth, Cobalt, Lithium, and Manganese in Coal-based sources and process development for their recovery. Miner. Eng. 2022, 189, 107897. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.; Dai, M.; Fang, S.; Liao, Y.; Yu, Z.; Ma, X. Co-pyrolysis kinetics of sewage sludge and bagasse using multiple normal distributed activation energy model (M-DAEM). Bioresour. Technol. 2018, 259, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.A.; Younus, M.M.; Kannan, Y.J.; Nooruldeen, Z.E.; Al-Nuseirat, A. Pharmaceutical regulations in Iraq: From medicine approval to post-marketing. East. Mediterr. Health J. 2021, 27, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Arsenic in Drinking Water Update; National Academy Press: Washington, DC, USA, 2001; Volume 7, pp. 557–564. [Google Scholar]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Shekar, P.R.; Nagaraju, D.; Ganesh, V.; Rao, K.K. Microhardness studies on as-grown (111) faces of some alkaline earth nitrates. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2009, 44, 652–656. [Google Scholar] [CrossRef]
- Patrick, L. Lead Toxicity, a review of the literature. Part I: Exposure, Evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar]
- Alli, L.A. Blood level of cadmium and lead in occupationally exposed persons in Gwagwalada, Abuja, Nigeria. Interdiscip. Toxicol. 2015, 8, 146–150. [Google Scholar] [CrossRef]
- Devoy, J.; Géhin, A.; Müller, S.; Melczer, M.; Remy, A.; Antoine, G.; Sponne, I. Evaluation of chromium in red blood cells as an indicator of exposure to hexavalent chromium. Toxicol. Lett. 2016, 255, 63–70. [Google Scholar] [CrossRef]
- Sanchez, T. Effects of mercury, lead, arsenic and zinc to human renal oxidative stress and functions: A review. Arch. Med. 2018, 4. [Google Scholar] [CrossRef]
- Ge, X.; Wang, F.; Zhong, Y.; Lv, Y.; Jiang, C.; Zhou, Y.; Li, D.; Xia, B.; Su, C.; Cheng, H.; et al. Manganese in blood cells as anexposure biomarker in manganese-exposed workers healthy cohort. J. Trace Elem. Med. Biol. 2018, 45, 41–47. [Google Scholar] [CrossRef]
- Okonkwo, S.I.; Idakwo, S.O.; Ameh, E.G. Heavy metal contamination and ecological risk assessment of soils around the pegmatite mining sites at Olode area, Ibadan southwestern Nigeria. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100424. [Google Scholar] [CrossRef]
- Phakoago, M.V.; Ekosse, G.E.; Odiyo, J.O. The prevalence of geophagic practices and causative reasons for geophagia in Sekhukhune area, Limpopo Province, South Africa. Trans. R. Soc. South Afr. 2019, 74, 19–26. [Google Scholar] [CrossRef]

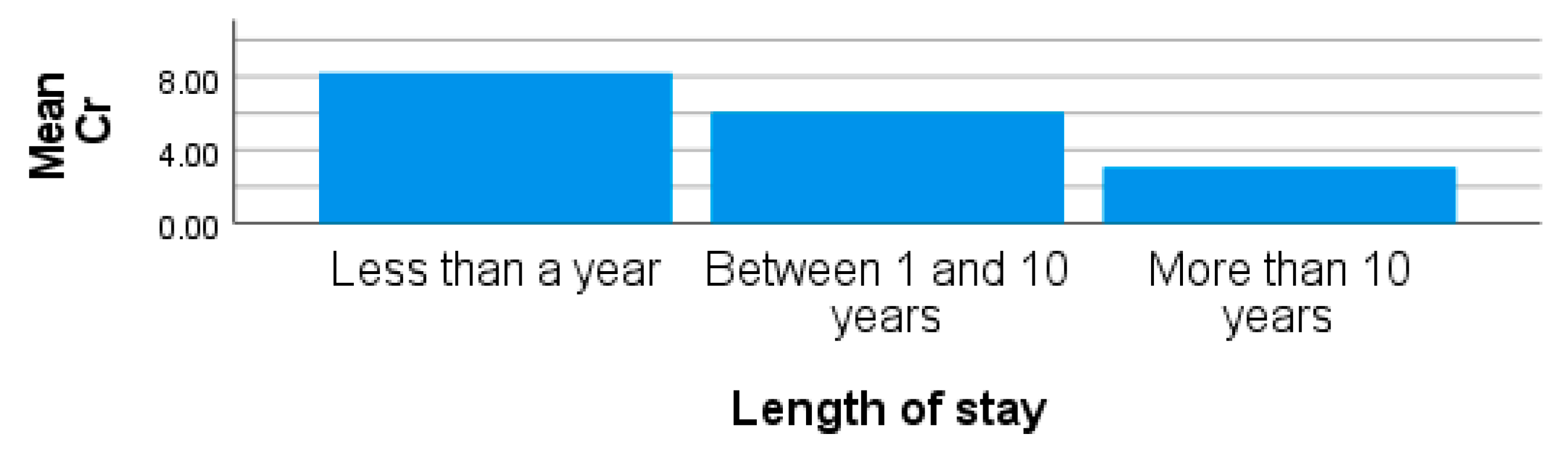

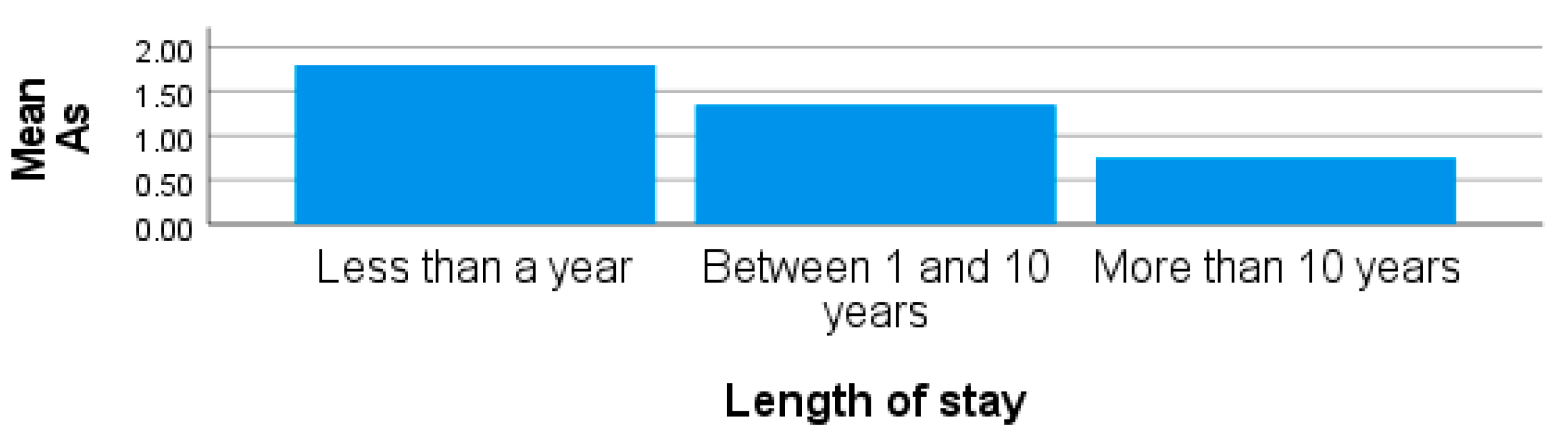
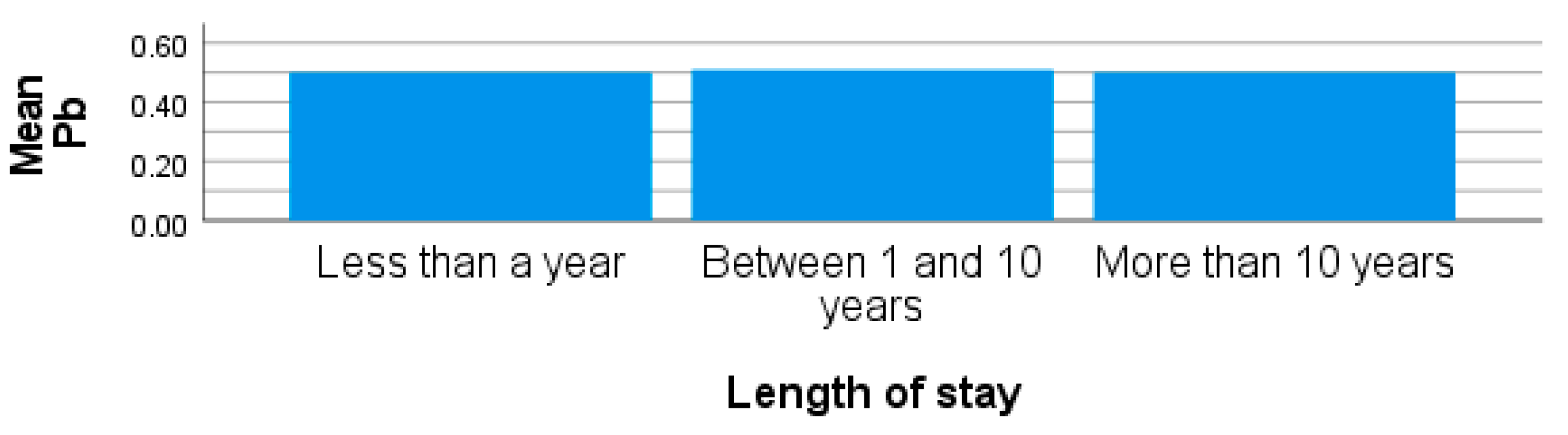
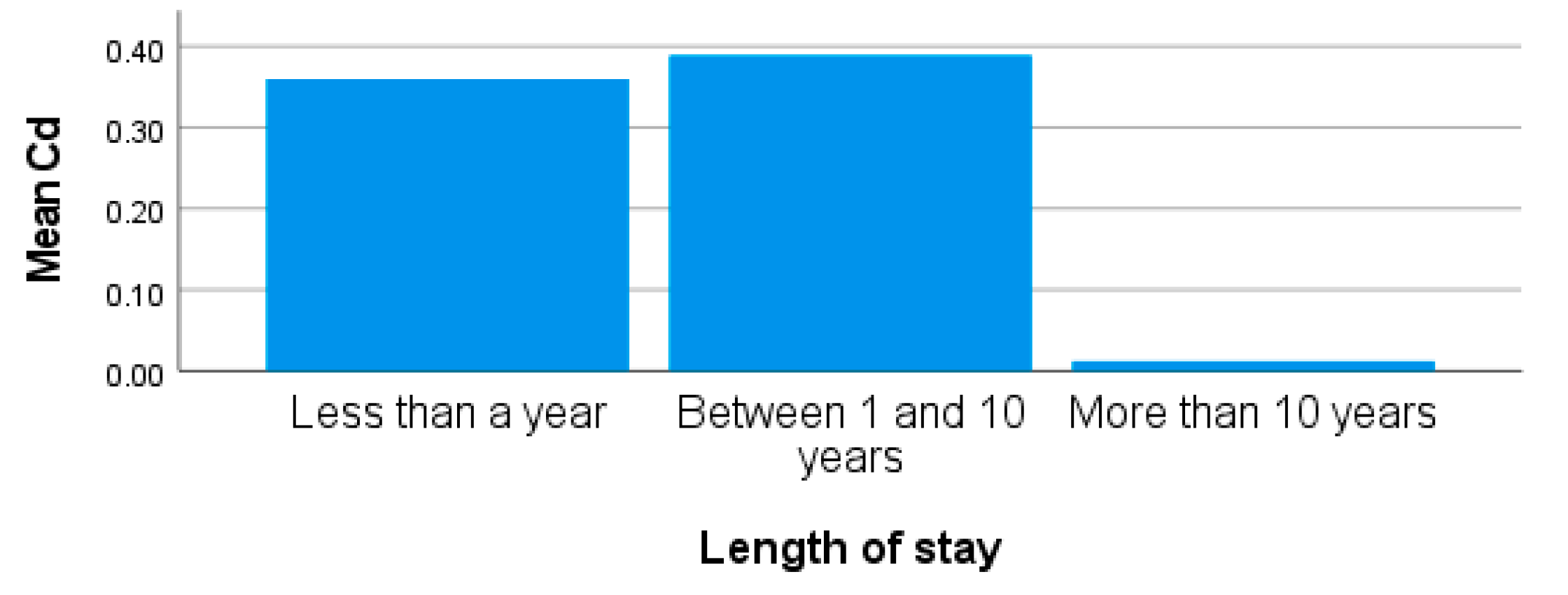
| Metals | Concentrations [µg/L; x̅ ± SD (Range)] | |||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | WHO | |
| Mn * | 12.97 ± 6.04 (6.79–33.99) | 11.41 ± 4.35 (5.50–24.40) | 11.37 ± 4.73 (4.72–22.95) | 12.60 |
| Cr | 3.70 ± 4.11 (0.41–19.01) | ND | 6.16 ± 6.30 (1.58–29.29) | 0.23 |
| Co | 2.19 ± 4.43 (0.20–18.84) | 1.08 ± 1.34 (0.02–5.50) | 2.40 ± 8.92 (0.09–50.88) | 0.30 |
| As | 0.84 ± 0.41 (0.27–2.20) | 0.95 ± 0.99 (0.03–5.70) | 1.37 ± 0.67 (0.70–3.43) | 1.00 |
| Cd | 0.20 ± 0.20 (0.02–1.10) | 0.25 ± 0.26 (0.01–0.90) | 0.38± 0.55 (0.01–2.28) | 1.12 |
| Pb * | 6.70 ± 3.40 (5.00–18.40) | 19.41 ± 11.84 (6.70–60.20) | 0.51± 0.05 (0.50–0.78) | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lion, G.N.; Olowoyo, J.O. Possible Sources of Trace Metals in Obese Females Living in Informal Settlements near Industrial Sites around Gauteng, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 5133. https://doi.org/10.3390/ijerph20065133
Lion GN, Olowoyo JO. Possible Sources of Trace Metals in Obese Females Living in Informal Settlements near Industrial Sites around Gauteng, South Africa. International Journal of Environmental Research and Public Health. 2023; 20(6):5133. https://doi.org/10.3390/ijerph20065133
Chicago/Turabian StyleLion, Gladness Nteboheng, and Joshua Oluwole Olowoyo. 2023. "Possible Sources of Trace Metals in Obese Females Living in Informal Settlements near Industrial Sites around Gauteng, South Africa" International Journal of Environmental Research and Public Health 20, no. 6: 5133. https://doi.org/10.3390/ijerph20065133
APA StyleLion, G. N., & Olowoyo, J. O. (2023). Possible Sources of Trace Metals in Obese Females Living in Informal Settlements near Industrial Sites around Gauteng, South Africa. International Journal of Environmental Research and Public Health, 20(6), 5133. https://doi.org/10.3390/ijerph20065133






