Trunk Control Measurement Scale (TCMS): Psychometric Properties of Cross-Cultural Adaptation and Validation of the Spanish Version
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Study Procedures
2.3. Outcomes
2.3.1. Trunk Control Measurement Scale
2.3.2. Gross Motor Function Measure-88
2.3.3. Pediatric Disability Inventory Computer Adaptive Test
2.3.4. Cerebral Palsy Quality of Life
2.4. Sample Size
2.5. Linguistic Adaptation
2.6. Data Analysis
2.6.1. Validity
2.6.2. Test–Retest Reliability
3. Results
3.1. Participants
3.2. Internal Consistency
3.3. Convergent Validity
3.4. Discriminant Validity
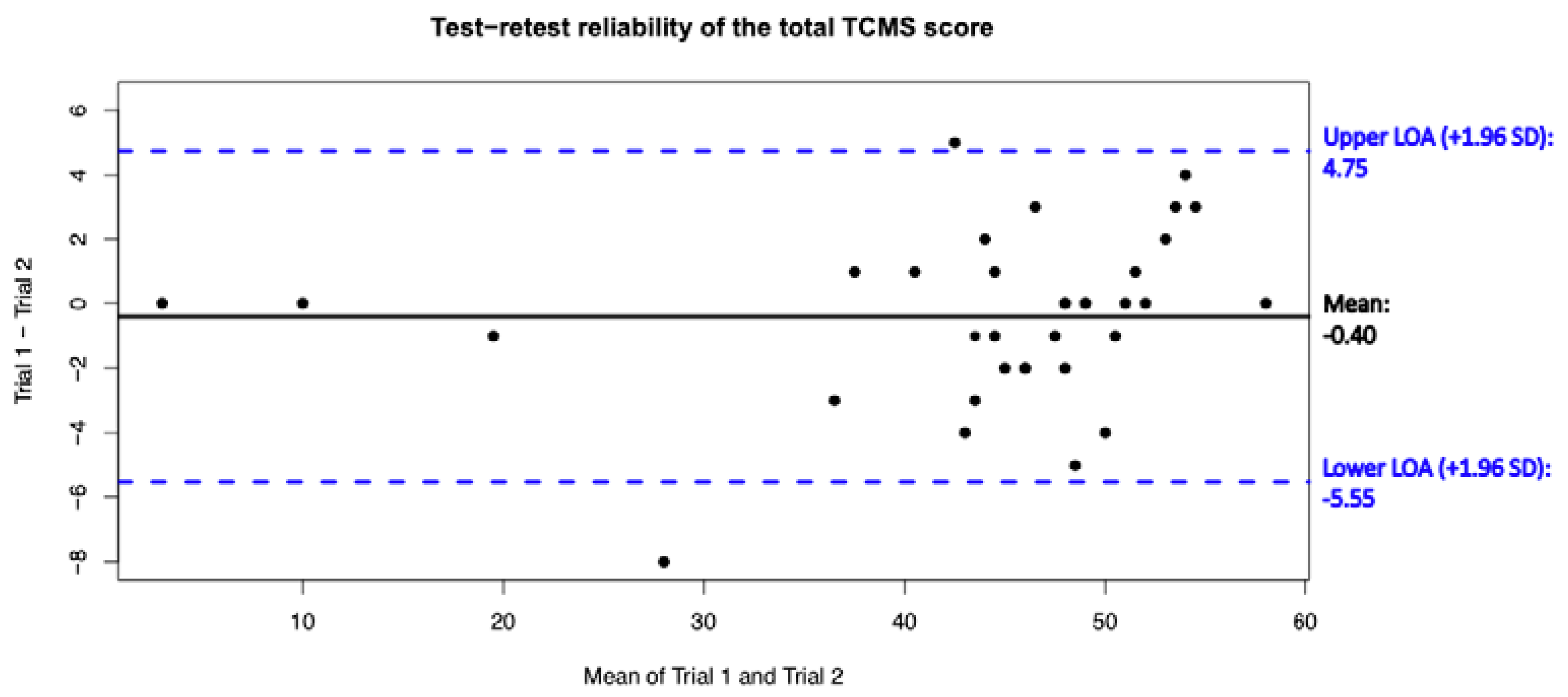
3.5. Test–Retest Reliability
4. Discussion
4.1. Participants
4.2. Internal Consistency
4.3. Convergent Validity
4.4. Discriminant Ability
4.5. Test–Retest Reliability
5. Clinical Implications
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- Brogren, E.; Hadders-Algra, M. Postural dysfunction in children with cerebral palsy: Some implications for therapeutic guidance. Neural Plast. 2005, 12, 221–228. [Google Scholar]
- Van Balen, L.C.; Dijkstra, L.J.; Hadders-Algra, M. Development of postural adjustments during reaching in typically developing infants from 4 to 18 months. Exp. Brain Res. 2012, 220, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyrman, L.; Feys, H.; Molenaers, G.; Jaspers, E.; Monari, D.; Nieuwenhuys, A.; Desloovere, K. Altered trunk movements during gait in children with spastic diplegia: Compensatory or underlying trunk control deficit? Res. Dev. Disabil. 2014, 35, 2044–2052. [Google Scholar] [CrossRef]
- Saether, R.; Helbostad, J.L.; Adde, L.; Braendvik, S.; Lydersen, S.; Vik, T.; Sæther, R.; Brændvik, S. The relationship between trunk control in sitting and during gait in children and adolescents with cerebral palsy. Dev. Med. Child Neurol. 2015, 57, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Curtis, D.J.; Butler, P.; Saavedra, S.; Bencke, J.; Kallemose, T.; Sonne-Holm, S.; Woollacott, M. The central role of trunk control in the gross motor function of children with cerebral palsy: A retrospective cross-sectional study. Dev. Med. Child Neurol. 2014, 57, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Heyrman, L.; Desloovere, K.; Molenaers, G.; Verheyden, G.; Klingels, K.; Monbaliu, E.; Feys, H. Clinical characteristics of impaired trunk control in children with spastic cerebral palsy. Res. Dev. Disabil. 2013, 34, 327–334. [Google Scholar] [CrossRef]
- Seyyar, G.K.; Aras, B.; Aras, O. Trunk control and functionality in children with spastic cerebral palsy. Dev. Neurorehabilit. 2019, 22, 120–125. [Google Scholar] [CrossRef]
- Pavão, S.L.; dos Santos, A.N.; de Oliveira, A.B.; Rocha, N.A.C.F. Functionality level and its relation to postural control during sitting-to-stand movement in children with cerebral palsy. Res. Dev. Disabil. 2014, 35, 506–511. [Google Scholar] [CrossRef]
- Marsico, P.; Mitteregger, E.; Balzer, J.; van Hedel, H.J. The Trunk Control Measurement Scale: Reliability and discriminative validity in children and young people with neuromotor disorders. Dev. Med. Child Neurol. 2017, 59, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Jackman, M.; Sakzewski, L.; Morgan, C.; Boyd, R.N.; Brennan, S.E.; Langdon, K.; Toovey, R.A.M.; Greaves, S.; Thorley, M.; Novak, I. Interventions to improve physical function for children and young people with cerebral palsy: International clinical practice guideline. Dev. Med. Child Neurol. 2021, 64, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, S.M.; Gómez-Conesa, A.; Montesinos, M.D.H. Association between gross motor function and postural control in sitting in children with Cerebral Palsy: A correlational study in Spain. BMC Pediatr. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzer, J.; Marsico, P.; Mitteregger, E.; Van Der Linden, M.L.; Mercer, T.H.; Van Hedel, H.J.A. Influence of trunk control and lower extremity impairments on gait capacity in children with cerebral palsy. Disabil. Rehabil. 2018, 40, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Pin, T.W.; Butler, P.B.; Cheung, H.-M.; Shum, S.L.-F. Segmental Assessment of Trunk Control in infants from 4 to 9 months of age- a psychometric study. BMC Pediatr. 2018, 18, 182. [Google Scholar] [CrossRef] [PubMed]
- Saether, R.; Helbostad, J.L.; Riphagen, I.I.; Vik, T. Clinical tools to assess balance in children and adults with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2013, 55, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Bañas, B.B.; Gorgon, E.J.R. Clinimetric Properties of Sitting Balance Measures for Children with Cerebral Palsy: A Systematic Review. Phys. Occup. Ther. Pediatr. 2015, 34, 313–334. [Google Scholar] [CrossRef]
- Desloovere, K.; Heyrman, L. Trunk control in children with cerebral palsy: Where are we now? Dev. Med. Child Neurol. 2014, 57, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kim, M. Reliability and responsiveness of the gross motor function measure-88 in children with cerebral palsy. Phys. Ther. 2013, 93, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Ferre-Fernández, M.; Murcia-González, M.A.; Ríos-Díaz, J. Intra- and Interrater Reliability of the Spanish Version of the Gross Motor Function Measure (GMFM-SP-88). Pediatr. Phys. Ther. 2022, 34, 193–200. [Google Scholar] [CrossRef]
- Russell, D.J.; Hart, H. Gross Motor Function Measure (GMFM-66 & GMFM-88). User’s Manual, 2nd ed.; Mac Keith Press: London, UK, 2013. [Google Scholar]
- Verheyden, G.; Nieuwboer, A.; Mertin, J.; Preger, R.; Kiekens, C.; De Weerdt, W. The Trunk Impairment Scale: A new tool to measure motor impairment of the trunk after stroke. Clin. Rehabil. 2004, 18, 326–334. [Google Scholar] [CrossRef]
- Verheyden, G.; Willems, A.-M.; Ooms, L.; Nieuwboer, A. Validity of the Trunk Impairment Scale as a Measure of Trunk Perfor-mance in People With Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2007, 88, 1304–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, H.P.; Eidem, A.; Hansen, G.; Nyquist, A.; Vik, T.; Sæther, R. Validity and Responsiveness of the Trunk Impairment Scale and Trunk Control Measurement Scale in Young Individuals with Cerebral Palsy. Phys. Occup. Ther. Pediatr. 2016, 36, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Heyrman, L.; Molenaers, G.; Desloovere, K.; Verheyden, G.; De Cat, J.; Monbaliu, E.; Feys, H. A clinical tool to measure trunk control in children with cerebral palsy: The Trunk Control Measurement Scale. Res. Dev. Disabil. 2011, 32, 2624–2635. [Google Scholar] [CrossRef] [PubMed]
- Mitteregger, E.; Marsico, P.; Balzer, J.; van Hedel, H.J. Translation and construct validity of the Trunk Control Measurement Scale in children and youths with brain lesions. Res. Dev. Disabil. 2015, 45–46, 343–352. [Google Scholar] [CrossRef]
- Ozal, C.; Ari, G.; Gunel, M.K. Inter–intra observer reliability and validity of the Turkish version of Trunk Control Measurement Scale in children with cerebral palsy. Acta Orthop. Traumatol. Turc. 2019, 53, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Ravizzotti, E.; Vercelli, S.; Pellicciari, L.; Furmanek, M.P.; Zagnoni, G.; Piscitelli, D. Reliability and Validity of the Trunk Control Measurement Scale Among Children and Adolescents with Cerebral Palsy in Tanzania. Percept. Mot. Ski. 2020, 128, 731–745. [Google Scholar] [CrossRef]
- Talu, B. Determine the Relationship Between Abdominal Muscle Strength, Trunk Control and Urinary Incontinence in Children with Diplegic Cerebral Palsy. Urol. J. 2018, 15, 180–185. [Google Scholar] [CrossRef]
- Shin, J.-W.; Song, G.-B.; Ko, J. The effects of neck and trunk stabilization exercises on cerebral palsy children’s static and dynamic trunk balance: Case series. J. Phys. Ther. Sci. 2017, 29, 771–774. [Google Scholar] [CrossRef] [Green Version]
- Velasco, M.A.; Raya, R.; Muzzioli, L.; Morelli, D.; Otero, A.; Iosa, M.; Cincotti, F.; Rocon, E. Evaluation of cervical posture improvement of children with cerebral palsy after physical therapy based on head movements and serious games. Biomed. Eng. Online 2017, 16, 74. [Google Scholar] [CrossRef] [Green Version]
- Decavele, S.; Ortibus, E.; Van Campenhout, A.; Jansen, B.; Omelina, L.; Franki, I. A randomized cross-over design evaluating the effects of using a rehabilitation-specific gaming software platform for the achievement of individual physiotherapy goals of children with severe spastic cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 35. [Google Scholar] [CrossRef] [Green Version]
- Adıguzel, H.; Elbasan, B. Effects of modified pilates on trunk, postural control, gait and balance in children with cerebral palsy: A single-blinded randomized controlled study. Acta Neurol. Belg. 2022, 122, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Keles, M.; Elbasan, B.; Apaydin, U.; Aribas, Z.; Bakirtas, A.; Kokturk, N. Effects of inspiratory muscle training in children with cerebral palsy: A randomized controlled study. Dev. Med. Child Neurol. 2018, 60, 25. [Google Scholar] [CrossRef]
- Guillemin, F.; Bombardier, C.; Beaton, D. Cross-cultural adaptation of health-related quality of life measures: Literature review and proposed guidelines. J. Clin. Epidemiol. 1993, 46, 1417–1432. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.; Santo, R.M.; Guillemin, F. A review of guidelines for cross-cultural adaptation of questionnaires could not bring out a consensus. J. Clin. Epidemiol. 2015, 68, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-Y.; Shin, W.-S. Reliability and validity of the Korean version of the Trunk Control Measurement Scale (TCMS-K) for children with cerebral palsy. Res. Dev. Disabil. 2014, 35, 581–590. [Google Scholar] [CrossRef]
- Heo, J.-Y.; Shin, H.-K. Reliability analysis of the Korean version of the trunk control measurement scale in cerebral palsy. J. Phys. Ther. Sci. 2018, 30, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Seyyar, G.K.; Aras, B.; Aras, O. Trunk control in children with Ataxic Cerebral Palsy. Percept. Mot. Ski. 2019, 126, 815–827. [Google Scholar] [CrossRef]
- Moke, L.; Severijns, P.; Schelfaut, S.; Van De Loock, K.; Hermans, L.; Molenaers, G.; Jonkers, I.; Scheys, L. Performance on Balance Evaluation Systems Test (BESTest) Impacts Health-Related Quality of Life in Adult Spinal Deformity Patients. Spine 2018, 43, 637–646. [Google Scholar] [CrossRef]
- Severijns, P.; Overbergh, T.; Scheys, L.; Moke, L.; Desloovere, K. Reliability of the balance evaluation systems test and trunk control measurement scale in adult spinal deformity. PLoS ONE 2019, 14, e0221489. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.; Knol, D.L.; Bouter, L.M.; De Vet, H.C.W. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Qual. Life Res. 2010, 19, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Franki, I.; Broeck, C.V.D.; De Cat, J.; Molenaers, G.; Vanderstraeten, G.; Desloovere, K. A study of whether video scoring is a reliable option for blinded scoring of the Gross Motor Function Measure-88. Clin. Rehabil. 2015, 29, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.; Long, T.; Kennedy, E.; Bavishi, S. The efficacy of GMFM-88 and GMFM-66 to detect changes in gross motor function in children with cerebral palsy (CP): A literature review. Disabil. Rehabil. 2013, 36, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Almeida, K.M.; Albuquerque, K.A.; Ferreira, M.L.; Aguiar, S.K.B.; Mancini, M.C. Reliability of the Brazilian Portuguese version of the Gross Motor Function Measure in children with cerebral palsy. Braz. J. Phys. Ther. 2016, 20, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferre-Fernández, M. Adaptación Transcultural y Propiedades Psicométricas de la Versión Española del Gross Motor Function Measure-88 (GMFM-88-SP). Ph.D. Thesis, Universidad Autonoma de Murcia, Murcia, Spain, 2019. [Google Scholar]
- Dumas, H.M.; Fragala-Pinkham, M.A.; Rosen, E.L.; Lombard, K.A.; Farrell, C. Pediatric Evaluation of Disability Inventory Computer Adaptive Test (PEDI-CAT) and Alberta Infant Motor Scale (AIMS): Validity and Responsiveness. Phys. Ther. 2015, 95, 1559–1568. [Google Scholar] [CrossRef]
- Mancini, M.C.; Coster, W.J.; Amaral, M.F.; Avelar, B.S.; Freitas, R.; Sampaio, R.F. New version of the Pediatric Evaluation of Disability Inventory (PEDI-CAT): Translation, cultural adaptation to Brazil and analyses of psychometric properties. Braz. J. Phys. Ther. 2016, 20, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Waters, E.; Davis, E.; Mackinnon, A.; Boyd, R.; Graham, H.K.; Lo, S.K.; Wolfe, R.; Stevenson, R.; Bjornson, K.; Blair, E.; et al. Psychometric properties of the quality of life questionnaire for children with CP. Dev. Med. Child Neurol. 2007, 49, 49–55. [Google Scholar] [CrossRef]
- Davis, E.; Mackinnon, A.; Davern, M.; Boyd, R.; Bohanna, I.; Waters, E.; Graham, H.K.; Reid, S.; Reddihough, D. Description and psychometric properties of the CP QOL-Teen: A quality of life questionnaire for adolescents with cerebral palsy. Res. Dev. Disabil. 2013, 34, 344–352. [Google Scholar] [CrossRef]
- Badia, M.; Orgaz, M.B.; Riquelme, I.; Gómez-Iruretagoyena, J. Domains of the Cerebral Palsy Quality of Life Questionnaire (CP QOL) for Children and Adolescents: Spanish Adaptation and Psychometric Properties. J. Dev. Phys. Disabil. 2020, 33, 331–349. [Google Scholar] [CrossRef]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the Process of Cross-Cultural Adaptation of Self-Report Measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef] [Green Version]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef] [Green Version]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin College Division: Boston, MA, USA, 2003. [Google Scholar]
- Pautz, N.; Olivier, B.; Steyn, F. The use of nonparametric effect sizes in single study musculoskeletal physiotherapy research: A practical primer. Phys. Ther. Sport 2018, 33, 117–124. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrout, P.; Fleiss, J. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- López-De-Uralde-Villanueva, I.; Acuyo-Osorio, M.; Prieto-Aldana, M.; La Touche, R. Reliability and minimal detectable change of a modified passive neck flexion test in patients with chronic nonspecific neck pain and asymptomatic subjects. Musculoskelet. Sci. Pract. 2017, 28, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Rosenbaum, P.L.; Walter, S.D.; Russell, D.J.; Wood, M.D.E.; Galuppi, B.E. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Class. Pap. Orthop. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Mahasup, N.; Sritipsukho, P.; Lekskulchai, R.; Keawutan, P. Inter-rater and intra-rater reliability of the gross motor function measure (GMFM-66) by Thai pediatric physical therapists. J. Med. Assoc. Thail. 2011, 94, S139–S144. [Google Scholar]
- De Carvalho Chagas, P.S.; Drumond, C.M. Commentary on “Intra-and Interrater Reliability of the Spanish Version of the Gross Motor Function Measure (GMFM-SP-88)”. Pediatr. Phys. Ther. 2022, 34, 201. [Google Scholar] [CrossRef]
- Rivera-Rujana, D.M.; Muñoz-Rodríguez, D.I.; Agudelo-Cifuentes, M.C. Reliability of the Gross Motor Function Measure-66 scale in the evaluation of children with cerebral palsy: Validation for Colombia. Bol. Med. Hosp. Infant. Mex. 2022, 79, 33–43. [Google Scholar] [CrossRef]
- Thompson, S.V.; Cech, D.J.; Cahill, S.M.; Krzak, J.J. Linking the Pediatric Evaluation of Disability Inventory-Computer Adaptive Test (PEDI-CAT) to the International Classification of Function. Pediatr. Phys. Ther. 2018, 30, 113–118. [Google Scholar] [CrossRef]
- Sakzewski, L.; Bleyenheuft, Y.; Boyd, R.N.; Novak, I.; Elliott, C.; Reedman, S.; Morgan, C.; Pannek, K.; Fripp, J.; Golland, P.; et al. Protocol for a multisite randomised trial of Hand–Arm Bimanual Intensive Training Including Lower Extremity training for children with bilateral cerebral palsy: HABIT-ILE Australia. BMJ Open 2019, 9, e032194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, M.H.; Rosenbaum, P.L.; Russell, D.J.; Palisano, R.J. Quality of life among adolescents with cerebral palsy: What does the literature tell us? Dev. Med. Child Neurol. 2007, 49, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Schiariti, V.; Selb, M.; Cieza, A.; O’Donnell, M. International Classification of Functioning, Disability and Health Core Sets for children and youth with cerebral palsy: A consensus meeting. Dev. Med. Child Neurol. 2015, 57, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Østensjø, S.; Bjorbækmo, W.; Carlberg, E.B.; Vøllestad, N.K. Assessment of everyday functioning in young children with disabilities: An ICF-based analysis of concepts and content of the Pediatric Evaluation of Disability Inventory (PEDI). Disabil. Rehabil. 2006, 28, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, V.; Rachwani, J.; Saussez, G.; Bleyenheuft, Y.; Dutkowsky, J.; Gordon, A.M.; Woollacott, M.H. The Seated Postural & Reaching Control Test (SP&R-co) in Cerebral Palsy: A Validation Study. Phys. Occup. Ther. Pediatr. 2020, 40, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, S. Trunk control in cerebral palsy: Are we ready to address the elephant in the room? Dev. Med. Child Neurol. 2015, 57, 309–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, M.B.; Loram, I.; Holmes, P.; Darby, J.; Butler, P.B. Working towards an objective segmental assessment of trunk control in children with cerebral palsy. Gait Posture 2018, 65, 45–50. [Google Scholar] [CrossRef]
- Østensjø, S.; Carlberg, E.B.; Vøllestad, N.K. Motor impairments in young children with cerebral palsy: Relationship to gross motor function and everyday activities. Dev. Med. Child Neurol. 2004, 46, 580–589. [Google Scholar] [CrossRef]
- Panibatla, S.; Kumar, V.; Narayan, A. Relationship Between Trunk Control and Balance in Children with Spastic Cerebral Palsy: A Cross-Sectional Study. J. Clin. Diagn. Res. 2017, 11, YC05–YC08. [Google Scholar] [CrossRef]
- Boonzaaijer, M.; Van Dam, E.; Van Haastert, I.C.; Nuysink, J. Concurrent Validity Between Live and Home Video Observations Using the Alberta Infant Motor Scale. Pediatr. Phys. Ther. 2017, 29, 146–151. [Google Scholar] [CrossRef]
| Spanish Version of the Trunk Control Measurement Scale (TCMS-S) | ||||
|---|---|---|---|---|
| Total | Static Sitting Balance | Selective Movement Control | Dynamic Reaching | |
| Gross Motor Function Measure-88 (GMFM) | ||||
| Total score | 0.816 ** | 0.738 ** | 0.795 ** | 0.624 ** |
| Lying and rolling | 0.575 ** | 0.537 ** | 0.544 ** | 0.577 ** |
| Sitting | 0.621 ** | 0.574 ** | 0.58 ** | 0.628 ** |
| Crawling and kneeling | 0.706 ** | 0.613 ** | 0.678 ** | 0.691 ** |
| Standing | 0.794 ** | 0.737 ** | 0.771 ** | 0.595 ** |
| Walking, running, and jumping | 0.790 ** | 0.711 ** | 0.777 ** | 0.603 ** |
| Pediatric Evaluation Disability Inventory-Computer Adaptive Test (PEDI-CAT) | ||||
| Daily activities | 0.585 ** | 0.428 ** | 0.601 ** | 0.474 ** |
| Mobility | 0.760 ** | 0.690 ** | 0.745 ** | 0.559 ** |
| Social/Cognitive | 0.380 ** | 0.239 * | 0.422 ** | 0.144 |
| Cerebral Palsy-Quality of Life (CP-QOL) | ||||
| Social well-being, acceptance, and participation | 0.175 | 0.081 | 0.214 * | 0.102 |
| Feelings about functioning | 0.576 ** | 0.519 ** | 0.580 ** | 0.406 ** |
| Emotional well-being and self-esteem | 0.268 ** | 0.255 * | 0.267 ** | 0.077 |
| Pain and impact of disability | 0.034 | 0.036 | 0.022 | 0.025 |
| School well-being | 0.153 | 0.162 | 0.151 | 0.006 |
| Access to services | 0.235 * | 0.203 * | 0.239 * | 0.219 * |
| Family health | 0.260 * | 0.237 * | 0.256 * | 0.224 * |
| TCMS-S | Mean ± SD; Median (IQR) | Between-Group Differences p-Value; r Effect Size (a) GMFCS I vs. GMFCS II (b) GMFCS II vs. GMFCS III (c) GMFCS III vs. GMFCS IV | |||
|---|---|---|---|---|---|
| GMFCS I | GMFCS II | GMFCS III | GMFCS IV | ||
| Total score | 51.08 ± 4.67; | 42.49 ± 5.79; | 30.54 ± 10.14; | 20 ± 12.56; | (a) p < 0.001; r = 0.66 |
| 51 (48.75 to 55) | 43 (38.5 to 47.5) | 32 (24 to 38) | 18 (14 to 24.5) | (b) p < 0.001; r = 0.52 | |
| (c) p = 0.068; r = 0.41 | |||||
| Static sitting balance | 18.97 ± 1.44; | 17.56 ± 2.17; | 13.08 ± 4.37; | 9.57 ± 5.16; | (a) p < 0.001; r = 0.37 |
| 20 (18 to 20) | 18 (16 to 19.5) | 14 (12 to 16) | 9 (5.5 to 14) | (b) p < 0.001; r = 0.53 | |
| (c) p = 0.110; r = 0.36 | |||||
| Selective movement control | 22.39 ± 3.6; | 15.56 ± 4.28; | 10.54 ± 4.82; | 5.71 ± 5.59; | (a) p < 0.001; r = 0.69 |
| 22 (20 to 25.25) | 16 (14 to 19) | 11 (7 to 14) | 5 (1.5 to 8) | (b) p = 0.002; r = 0.44 | |
| (c) p = 0.057; r = 0.43 | |||||
| Dynamic reaching | 9.72 ± 0.85; | 9.36 ± 0.84; | 6.92 ± 2.29; | 4.71 ± 3.5; | (a) p = 0.006; r = 0.32 |
| 10 (10 to 10) | 10 (9 to 10) | 7 (6 to 9) | 4 (2.5 to 7) | (b) p < 0.001; r = 0.52 | |
| (c) p = 0.140; r = 0.33 | |||||
| Item | Kappa (κ)/ Weighted Kappa (κw) | Value (95% CI) | Agreement (%) |
|---|---|---|---|
| Static sitting balance | |||
| Item 1 Item 2 Item 3 left Item 3 right Item 4 left Item 4 right Item 5 left Item 5 right | κ κ κ κ κw κw κw κw | 1.00 (—) 0.81 (0.55 to 1.00) 1.00 (—) 0.78 (0.38 to 1.00) 0.81 (0.68 to 0.95) 0.90 (0.77 to 1.00) 0.75 (0.54 to 0.96) 0.91 (0.79 to 1.00) | 100% 94% 100% 97% 83% 94% 86% 94% |
| Selective movement control | |||
| Item 6a Item 6b Item 7a Item 7b Item 8a left Item 8a right Item 8b left Item 8b right Item 8c left Item 8c right Item 9a left Item 9a right Item 9b left Item 9b right Item 9c left Item 9c right Item 10a Item 10b Item 11a Item 11b Item 12a Item 12b | κ κ κ κ κ κ κ κ κ κ κ κ κw κw κ κ κw κ κw κ κw κ | 0.47 (−0.15 to 1.00) 1.00 (—) 0.65 (0.03 to 1.00) 0.69 (0.44 to 0.94) 1.00 (—) 0.65 (0.03 to 1.00) 0.80 (0.58 to 1.00) 0.72 (0.47 to 0.97) 0.87 (0.705 to 1.00) 0.72 (0.47 to 0.97) 1.00 (—) 1.00 (—) 0.87 (0.73 to 1.00) 0.76 (0.58 to 0.95) 0.54 (0.26 to 0.82) 0.61 (0.36 to 0.86) 0.74 (0.54 to 0.94) 0.93 (0.80 to 1.00) 0.69 (0.44 to 0.94) 0.87 (0.63 to 1.00) 0.77 (0.61 to 0.92) 0.72 (0.42 to 1.00) | 94% 100% 97% 86% 100% 97% 91% 89% 94% 89% 100% 100% 91% 83% 77% 80% 83% 97% 86% 97% 77% 91% |
| Dynamic Reaching | |||
| Item 13 | κw | 0.88 (0.63 to 1.00) | 97% |
| Item 14 left | κw | 0.94 (0.81 to 1.00) | 97% |
| Item 14 right | κw | 0.95 (0.86 to 1.00) | 97% |
| Item 15 left | κw | 0.90 (0.69 to 1.00) | 97% |
| Item 15 right | κw | 1.00 (—) | 100% |
| TCMS-S | Mean ± SD | ICC (95% CI) | SEM | MDC95 (Score); MDC95 (%) | |
|---|---|---|---|---|---|
| Trial 1 | Trial 2 | ||||
| Total score | 43.51 ± 12.19 | 43.91 ± 11.65 | 0.98 (0.95 to 0.99) | 1.86 | 5.15; 12% |
| Static sitting balance | 8.89 ± 2.35 | 8.94 ± 2.33 | 0.99 (0.98 to 0.99) | 0.24 | 0.66; 7% |
| Selective movement control | 17.11 ± 4.54 | 17.37 ± 4.16 | 0.94 (0.89 to 0.97) | 1.02 | 2.83; 16% |
| Dynamic reaching | 17.51 ± 6.19 | 17.6 ± 6.05 | 0.95 (0.91 to 0.98) | 1.35 | 3.75; 21% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Ruiz, J.; Estrada-Barranco, C.; Martín-Gómez, C.; Egea-Gámez, R.M.; Valera-Calero, J.A.; Martín-Casas, P.; López-de-Uralde-Villanueva, I. Trunk Control Measurement Scale (TCMS): Psychometric Properties of Cross-Cultural Adaptation and Validation of the Spanish Version. Int. J. Environ. Res. Public Health 2023, 20, 5144. https://doi.org/10.3390/ijerph20065144
López-Ruiz J, Estrada-Barranco C, Martín-Gómez C, Egea-Gámez RM, Valera-Calero JA, Martín-Casas P, López-de-Uralde-Villanueva I. Trunk Control Measurement Scale (TCMS): Psychometric Properties of Cross-Cultural Adaptation and Validation of the Spanish Version. International Journal of Environmental Research and Public Health. 2023; 20(6):5144. https://doi.org/10.3390/ijerph20065144
Chicago/Turabian StyleLópez-Ruiz, Javier, Cecilia Estrada-Barranco, Carlos Martín-Gómez, Rosa M. Egea-Gámez, Juan Antonio Valera-Calero, Patricia Martín-Casas, and Ibai López-de-Uralde-Villanueva. 2023. "Trunk Control Measurement Scale (TCMS): Psychometric Properties of Cross-Cultural Adaptation and Validation of the Spanish Version" International Journal of Environmental Research and Public Health 20, no. 6: 5144. https://doi.org/10.3390/ijerph20065144
APA StyleLópez-Ruiz, J., Estrada-Barranco, C., Martín-Gómez, C., Egea-Gámez, R. M., Valera-Calero, J. A., Martín-Casas, P., & López-de-Uralde-Villanueva, I. (2023). Trunk Control Measurement Scale (TCMS): Psychometric Properties of Cross-Cultural Adaptation and Validation of the Spanish Version. International Journal of Environmental Research and Public Health, 20(6), 5144. https://doi.org/10.3390/ijerph20065144






