Flat versus Simulated Mountain Trail Running: A Multidisciplinary Comparison in Well-Trained Runners
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Design
2.3. Testing Procedures
2.4. Cardiopulmonary Measurements
2.5. Gait Parameters
2.6. Surface Electromyography
2.7. Data Analysis
2.7.1. Cardiopulmonary Analysis
2.7.2. Surface Electromyography
2.8. Statistics
3. Results
3.1. Metabolic and Cardiopulmonary Parameters
3.2. Gait Parameters
3.3. EMG Data
3.4. Psychological Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.C.; Brellenthin, A.G.; Thompson, P.D.; Sui, X.; Lee, I.M.; Lavie, C.J. Running as a Key Lifestyle Medicine for Longevity. Prog. Cardiovasc. Dis. 2017, 60, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.V.; Sales, M.M.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. How Much Further for the Sub-2-Hour Marathon? Open Access J. Sport. Med. 2018, 9, 139–145. [Google Scholar] [CrossRef]
- Jones, A.M.; Kirby, B.S.; Clark, I.E.; Rice, H.M.; Fulkerson, E.; Wylie, L.J.; Wilkerson, D.P.; Vanhatalo, A.; Wilkins, B.W. Physiological Demands of Running at 2-Hour Marathon Race Pace. J. Appl. Physiol. 2021, 130, 369–379. [Google Scholar] [CrossRef]
- Knechtle, B.; Nikolaidis, P.T. Physiology and Pathophysiology in Ultra-Marathon Running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Scheer, V. Participation Trends of Ultra Endurance Events. Sport. Med. Arthrosc. Rev. 2019, 27, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Ong, J.C.; Wang, G. Historical Analysis of Participation in 161km Ultramarathons in North America. Int. J. Hist. Sport. 2010, 27, 1877–1891. [Google Scholar] [CrossRef] [PubMed]
- Deelen, I.; Janssen, M.; Vos, S.; Kamphuis, C.B.M.; Ettema, D. Attractive Running Environments for All? A Cross-Sectional Study on Physical Environmental Characteristics and Runners’ Motives and Attitudes, in Relation to the Experience of the Running Environment. BMC Public Health 2019, 19, 366. [Google Scholar] [CrossRef]
- Avancini, A.; Skroce, K.; Tregnago, D.; Frada, P.; Trestini, I.; Cercato, M.C.; Bonaiuto, C.; Tarperi, C.; Schena, F.; Milella, M.; et al. “running with Cancer”: A Qualitative Study to Evaluate Barriers and Motivations in Running for Female Oncological Patients. PLoS ONE 2020, 15, e0227846. [Google Scholar] [CrossRef]
- Zamparo, P.; Perini, R.; Orizio, C.; Sacher, M.; Ferretti, G. The Energy Cost of Walking or Running on Sand. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 183–187. [Google Scholar] [CrossRef]
- Lejeune, T.M.; Willems, P.A.; Heglund, N.C. Mechanics and Energetics of Human Locomotion on Sand. J. Exp. Biol. 1998, 201, 2071–2080. [Google Scholar] [CrossRef]
- Jensen, K.; Johansen, L.; Kärkkäinen, O.P. Economy in Track Runners and Orienteers during Path and Terrain Running. J. Sport. Sci. 1999, 17, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Pinnington, H.C.; Dawson, B. The Energy Cost of Running on Grass Compared to Soft Dry Beach Sand. J. Sci. Med. Sport. 2001, 4, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Creagh, U.; Reilly, T.; Lees, A. Kinematics of Running on “off-Road” Terrain. Ergonomics 1998, 41, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Fang, Y.; Liu, D.M.S.; Wang, L.; Ren, S.; Liu, Y. Surface Effects on In-Shoe Plantar Pressure and Tibial Impact during Running. J. Sport. Health Sci. 2015, 4, 384–390. [Google Scholar] [CrossRef]
- Dixon, S.J.; Collop, A.C.; Batt, M.E. Surface Effects on Ground Reaction Forces and Lower Extremity Kinematics in Running. Med. Sci. Sport. Exerc. 2000, 32, 1919–1926. [Google Scholar] [CrossRef]
- Nicot, F.; Sabater-Pastor, F.; Samozino, P.; Millet, G.Y.; Rupp, T. Effect of Ground Technicity on Cardio-Respiratory and Biomechanical Parameters in Uphill Trail Running. Eur. J. Sport. Sci. 2022, 22, 1836–1846. [Google Scholar] [CrossRef]
- Larsen, P.; Elsoe, R.; Graven-Nielsen, T.; Laessoe, U.; Rasmussen, S. Decreased Muscle Strength Is Associated with Impaired Long-Term Functional Outcome after Intramedullary Nailing of Femoral Shaft Fracture. Eur. J. Trauma. Emerg. Surg. 2015, 41, 673–681. [Google Scholar] [CrossRef]
- Vincent, H.K.; Brownstein, M.; Vincent, K.R. Injury Prevention, Safe Training Techniques, Rehabilitation, and Return to Sport in Trail Runners. Arthrosc. Sport. Med. Rehabil. 2022, 4, e151–e162. [Google Scholar] [CrossRef]
- Lacquaniti, F.; Ivanenko, Y.P.; Zago, M. Patterned Control of Human Locomotion. J. Physiol. 2012, 590, 2189–2199. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Eckardt, N.; Kibele, A.; Arampatzis, A. Challenging Human Locomotion: Stability and Modular Organisation in Unsteady Conditions. Sci. Rep. 2018, 8, 2740. [Google Scholar] [CrossRef]
- Vernillo, G.; Giandolini, M.; Edwards, W.B.; Morin, J.B.; Samozino, P.; Horvais, N.; Millet, G.Y. Biomechanics and Physiology of Uphill and Downhill Running. Sport. Med. 2017, 47, 615–629. [Google Scholar] [CrossRef]
- Nardello, F.; Venturini, N.; Skroce, K.; Tarperi, C.; Schena, F. Kinematic and Mechanical Changes during a Long Half-Marathon Race: Males and Females at Uphill/Downhill Slopes. J. Sport. Med. Phys. Fit. 2021, 61, 350–358. [Google Scholar] [CrossRef]
- Viljoen, C.; Janse van Rensburg, D.C.C.; van Mechelen, W.; Verhagen, E.; Silva, B.; Scheer, V.; Besomi, M.; Gajardo-Burgos, R.; Matos, S.; Schoeman, M.; et al. Trail Running Injury Risk Factors: A Living Systematic Review. Br. J. Sport. Med. 2022, 56, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Minetti, A.E.; Moia, C.; Roi, G.S.; Susta, D.; Ferretti, G. Energy Cost of Walking and Running at Extreme Uphill and Downhill Slopes. J. Appl. Physiol. 2002, 93, 1039–1046. [Google Scholar] [CrossRef]
- Voloshina, A.S.; Ferris, D.P. Biomechanics and Energetics of Running on Uneven Terrain. J. Exp. Biol. 2015, 218, 711–719. [Google Scholar] [CrossRef]
- Borg, E.; Kaijser, L. A Comparison between Three Rating Scales for Perceived Exertion and Two Different Work Tests. Scand. J. Med. Sci. Sport. 2006, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Doust, J.H. A 1% Treadmill Grade Most Accurately Reflects the Energetic Cost of Outdoor Running. J. Sport. Sci. 1996, 14, 321–327. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Cerone, G.L. Tutorial. Surface EMG Detection, Conditioning and Pre-Processing: Best Practices. J. Electromyogr. Kinesiol. 2020, 54, 102440. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J.; Whipp, B.J.A. A New Method for Detecting Anaerobic Threshold by Gas Exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- di Prampero, P.E.; Atchou, G.; Brückner, J.C.; Moia, C. The Energetics of Endurance Running. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.E.H.; Mackinnon, S.N. The Energetics of Walking on Sand and Grass at Various Speeds. Ergonomics 2006, 49, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Voloshina, A.S.; Kuo, A.D.; Daley, M.A.; Ferris, D.P. Biomechanics and Energetics of Walking on Uneven Terrain. J. Exp. Biol. 2013, 216, 3963–3970. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Ivanenko, Y.P.; d’Avella, A.; Serrao, M.; Ranavolo, A.; Draicchio, F.; Cappellini, G.; Casali, C.; Lacquaniti, F. Neuromuscular Adjustments of Gait Associated with Unstable Conditions. J. Neurophysiol. 2015, 114, 2867–2882. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Ivanenko, Y.P.; Serrao, M.; Ranavolo, A.; d’Avella, A.; Draicchio, F.; Conte, C.; Casali, C.; Lacquaniti, F. Locomotor Patterns in Cerebellar Ataxia. J. Neurophysiol. 2014, 112, 2810–2821. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Bastian, A.J. Cerebellar Control of Balance and Locomotion. Neuroscientist 2004, 10, 247–259. [Google Scholar] [CrossRef]
- Mizrahi, J.; Verbitsky, O.; Isakov, E. Shock Accelerations and Attenuation in Downhill and Level Running. Clin. Biomech. 2000, 15, 15–20. [Google Scholar] [CrossRef]
- Hajiloo, B.; Anbarian, M.; Esmaeili, H.; Mirzapour, M. The Effects of Fatigue on Synergy of Selected Lower Limb Muscles during Running. J. Biomech. 2020, 103, 109692. [Google Scholar] [CrossRef]
- Gantz, A.M.; Derrick, T.R. Kinematics and Metabolic Cost of Running on an Irregular Treadmill Surface. J. Sport. Sci. 2018, 36, 1103–1110. [Google Scholar] [CrossRef]
- Soule, R.G.; Goldman, R.F. Terrain Coefficients for Energy Cost Prediction. J. Appl. Physiol. 1972, 32, 706–708. [Google Scholar] [CrossRef]
- Slater, L.V.; Simpson, A.S.; Blemker, S.S.; Hertel, J.; Saliba, S.A.; Weltman, A.L.; Hart, J.M. Biomechanical Adaptations during Running Differ Based on Type of Exercise and Fitness Level. Gait Posture 2018, 60, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lehman, W.L. Overuse Syndromes in Runners. Am. Fam. Physician 1984, 29, 157–161. [Google Scholar] [PubMed]
- Messier, S.P.; Edwards, D.G.; Martin, D.F.; Lowery, R.B.; Cannon, D.W.; James, M.K.; Curl, W.W.; Read, H.M.; Hunter, D.M. Etiology of Iliotibial Band Friction Syndrome in Distance Runners. Med. Sci. Sport. Exerc. 1995, 27, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Damavandi, M.; Eslami, M.; Pearsall, D.J. Side-Sloped Surfaces Substantially Affect Lower Limb Running Kinematics. Sport. Biomech. 2017, 16, 1171895. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n = 20 |
|---|---|
| Age (years) | 33 ± 8 |
| Body mass (kg) | 70.3 ± 6.4 |
| Body height (m) | 1.77 ± 0.06 |
| BMI (kg/m2) | 22.9 ± 1.9 |
| GET (km·h−1) | 13.0 ± 1.5 |
| RCP (km·h−1) | 15.0 ± 1.0 |
| O2max (ml·kg−1·min−1) | 63.8 ± 7.2 |
| Training time (average/week, h) | 10.5 ± 2.5 |
| N° of training sessions/week | 7 ± 1 |
| Years competing in official races | 5 ± 2 |
| 8 km·h−1 | 10 km·h−1 | 12 km·h−1 | |||
|---|---|---|---|---|---|
| Metabolic and cardiopulmonary measurements | |||||
| O2 | mlO2·kg−1·min−1 | FC | 36.61 ± 2.83 | 43.85 ± 3.69 $$$ | 51.33 ± 4.73 ###, £££ |
| URV | 36.20 ± 2.67 | 43.16 ± 2.92 $$$ | 51.57 ± 3.24 ###, £££ | ||
| pbonf = 1, d = 0.045 | pbonf = 1, d = 0.116 | pbonf = 1, d = −0.180 | |||
| BLa− | mmol·L−1 | FC | 1.35 ± 0.43 | 1.81 ± 0.78 | 3.49 ± 1.82 |
| URV | 1.31 ± 0.45 | 1.80 ± 1.02 | 3.86 ± 2.25 | ||
| pfried = 0.604 | |||||
| HR | bpm | FC | 126 ± 11 | 143 ± 13 $$$ | 159 ± 14 ###, £££ |
| URV | 127 ± 5 | 143 ± 18 $$$ | 161 ± 17 ###, £££ | ||
| pbonf = 1, d = −0.089 | pbonf = 1, d = −0.050 | pbonf = 1, d = −0.114 | |||
| Cr | J·kg−1·m−1 | FC | 4.85 ± 0.48 | 4.79 ± 0.49 | 4.75 ± 0.47 |
| URV | 4.82 ± 0.46 | 4.73 ± 0.38 | 4.82 ± 0.31 | ||
| pbonf = 1, d = 0.071 | pbonf = 1, d = 0.146 | pbonf = 1, d = −0.155 | |||
| Gait parameters | |||||
| Ground time | ms | FC | 290.2 ± 20.6 | 258.2 ± 13.0 $$$ | 234.4 ± 11.6 ###, £££ |
| URV | 293.0 ± 17.5 | 258.8 ± 13.4 $$$ | 235.0 ± 11.4 ###, £££ | ||
| pbonf = 1, d = 0.011 | pbonf = 1, d = −0.014 | pbonf = 1, d = −0.019 | |||
| Cadence | spm | FC | 162.4 ± 9.9 | 165.8 ± 9.6 $$$ | 170.4 ± 9.8 ###, £££ |
| URV | 162.9 ± 10.0 | 168.0 ± 10.3 $$$ | 173.2 ± 10.2 ###, £££ | ||
| pbonf = 1, d = −0.017 | pbonf = 1, d = −0.088 | pbonf = 1, d = −0.065 | |||
| EMG parameters | |||||
| Peaks | mV | FC | 0.267 ± 0.038 | 0.247 ± 0.026 | 0.267 ± 0.04 # |
| URV | 0.261 ± 0.066 | 0.161± 0.063 | 0.282 ± 0.034 | ||
| pbonf = 1, d = −0.186 | pbonf = 1, d = −0.166 | pbonf = 1, d = −0.108 | |||
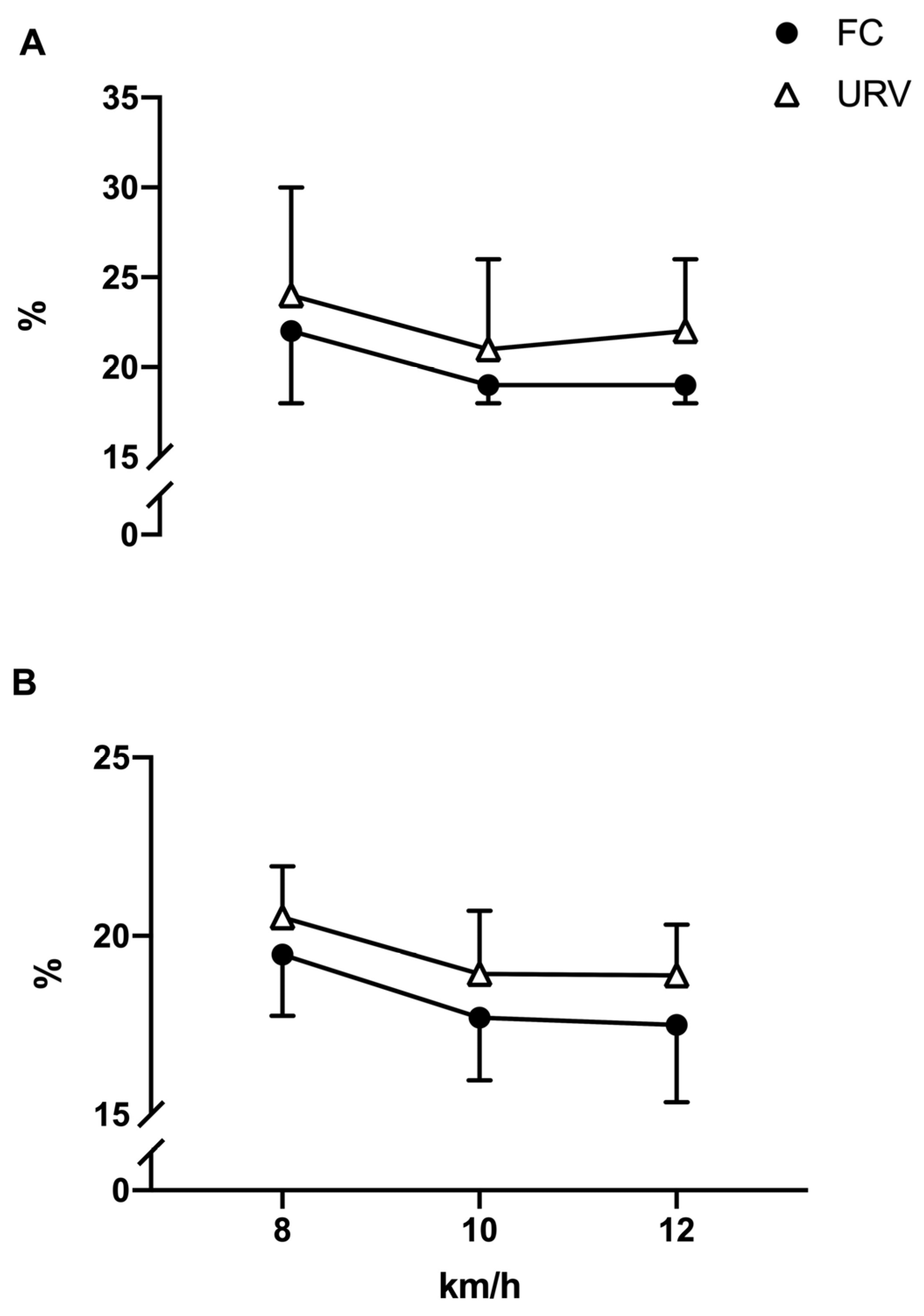
| CoV Pks | % | FC | 22 ± 4 | 19 ± 1 | 19 ± 1 |
| URV | 24 ± 6 | 21 ± 5 | 22 ± 4 | ||
| pbonf = 0.694, d = −0.226 | pbonf = 0.896, d = −0.213 | pbonf = 0.146, d = −0.297 | |||
| Width | ms | FC | 110 ± 8 | 108 ± 8 $$ | 107 ± 8 ### |
| URV | 112 ± 8 | 109 ± 8 $$ | 108 ± 7 ### | ||
| pbonf = 1, d = −0.171 | pbonf = 1, d = −0.141 | pbonf = 1, d = −0.092 | |||
| Cov Width | % | FC | 19 ± 2 | 18 ± 2 | 17 ± 2 |
| URV | 20 ± 1 | 19 ± 2 | 19 ± 1 | ||
| pbonf = 0.059, d = −0.256 | pbonf = 0.014 *, d = −0.301 | pbonf = 0.003 **, d = −0.342 | |||
| Psychological parameters | |||||
| RPE | FC | 9 ± 7 | 19 ± 13 | 35 ± 14 | |
| URV | 11 ± 9 | 22 ± 12 | 40 ± 16 | ||
| pfried = 0.454 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skroce, K.; Bettega, S.; D’Emanuele, S.; Boccia, G.; Schena, F.; Tarperi, C. Flat versus Simulated Mountain Trail Running: A Multidisciplinary Comparison in Well-Trained Runners. Int. J. Environ. Res. Public Health 2023, 20, 5189. https://doi.org/10.3390/ijerph20065189
Skroce K, Bettega S, D’Emanuele S, Boccia G, Schena F, Tarperi C. Flat versus Simulated Mountain Trail Running: A Multidisciplinary Comparison in Well-Trained Runners. International Journal of Environmental Research and Public Health. 2023; 20(6):5189. https://doi.org/10.3390/ijerph20065189
Chicago/Turabian StyleSkroce, Kristina, Simone Bettega, Samuel D’Emanuele, Gennaro Boccia, Federico Schena, and Cantor Tarperi. 2023. "Flat versus Simulated Mountain Trail Running: A Multidisciplinary Comparison in Well-Trained Runners" International Journal of Environmental Research and Public Health 20, no. 6: 5189. https://doi.org/10.3390/ijerph20065189





