Defensins of Lucilia sericata Larvae and Their Influence on Wound Repair Processes in Practical Assessment—A Study of Three Cases
Abstract
:1. Introduction
2. Materials and Methods
3. Results
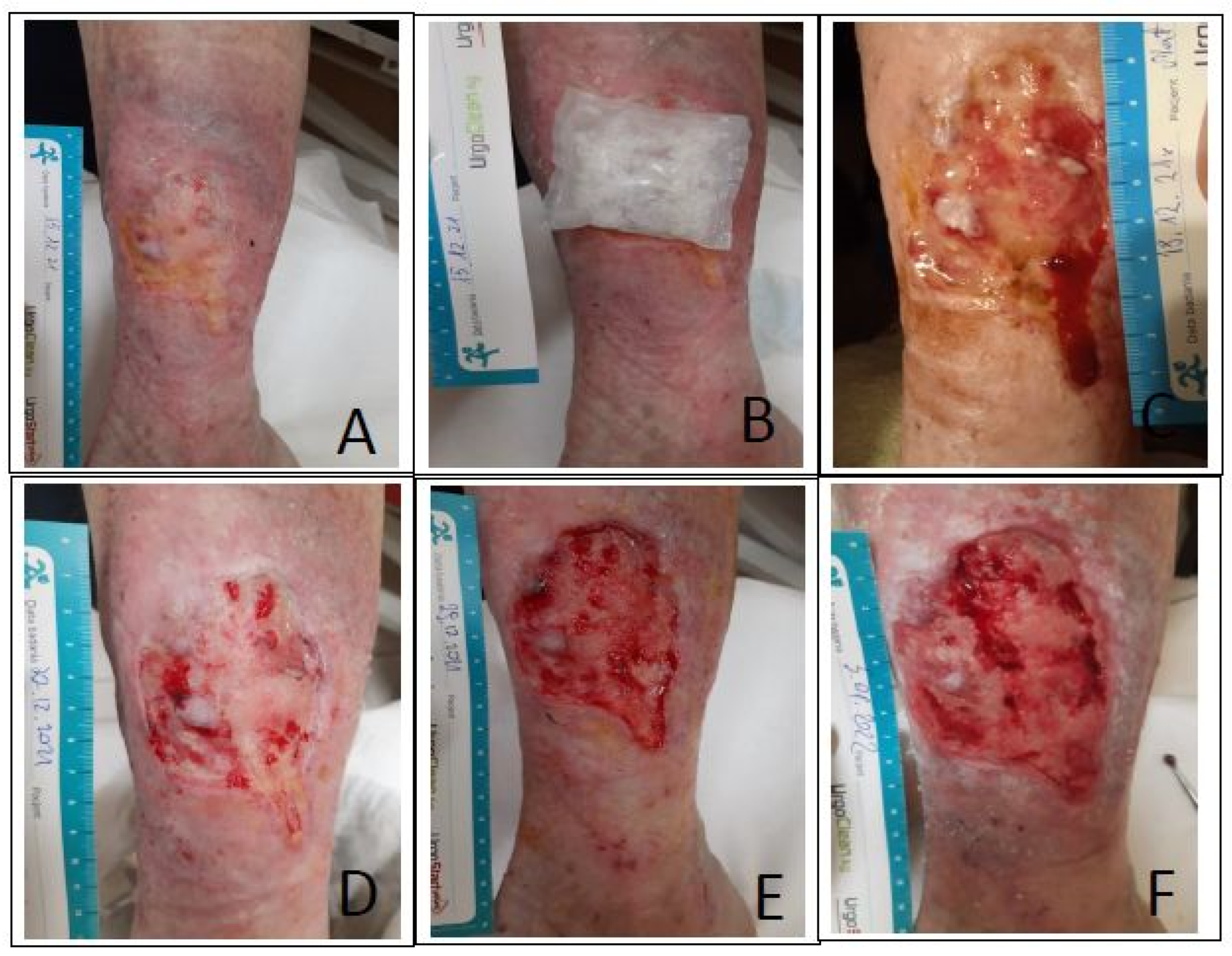
Description of the Cases
- Case I:
- Case II:
- Case III:
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Goff-Pronost, M.; Bénédicte, M.; Jean-Pierre, B.; Teot, L.; Hervé Benateau, H.; Dompmartin, A. Real-World Clinical Evaluation and Costs of Telemedicine for Chronic Wound Management. Int. J. Technol. Assess. Health Care 2018, 34, 567. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Atkin, L.; Bućko, Z.; Conde Montero, E.; Cutting, K.; Moffatt, C.; Probst, A.; Romanelli, M.; Schultz, G.S.; Tettelbach, W. Implementing TIMERS: The race against hard-to-heal wounds. J. Wound Care 2019, 28 (Suppl. 3), 1–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mospan, B.; Junka, A.; Bartoszewicz, M. Nowe oblicze znanych związków w postępowaniu miejscowym w ranach przewlekłych. [A new character of known compounds in topical treatment of chronic wounds]. Leczenie Ran 2018, 15, 79–83. (In Polish) [Google Scholar] [CrossRef]
- Bartoszewicz, M.; Krasowski, G.; Banasiewicz, T.; Lipiński, P.; Bielecki, K.; Chrapusta, A.; Korzon-Burakowska, A.; Kucharzewski, M.; Mospan, B.; Konrady, Z.; et al. Wskaźnik terapeutyczny miejscowego zakażenia rany (TILI) jako przydatne narzędzie w efektywnej pielęgnacji ran niegojących się dla lekarzy i pielęgniarek podstawowej opieki zdrowotnej, lekarzy rodzinnych i personelu zakładów opiekuńczo–leczniczych. [The therapeutic index of local wound infection (TILI) as a useful tool in the effective care of non-healing wounds for doctors and nurses of primary care, family doctors and staff of care and treatment facilities]. Forum Zakażeń 2020, 11, 285–295. (In Polish) [Google Scholar]
- Czyżewska-Dors, E.; Dors, A.; Pomorska-Mól, M. Właściwości biofilmu bakteryjnego warunkujące oporność na antybiotyki oraz metody jego zwalczania. [Properties of bacterial biofilm determining resistance to antibiotics and methods of combating it]. Życie Weterynaryjne 2018, 93, 765–771. (In Polish) [Google Scholar]
- Maciejewska, M.; Bauer, M.; Dawgul, M. Nowoczesne metody zwalczania biofilmu bakteryjnego. [Modern methods of combating bacterial biofilm]. Post. Mikrobiol. 2016, 55, 3–11. (In Polish) [Google Scholar]
- Thaarup, I.C.; Iversen, A.K.S.; Lichtenberg, M.; Bjarnsholt, T.; Jakobsen, T.H. Biofilm Survival Strategies in Chronic Wounds. Microorganisms. 2022, 10, 775. [Google Scholar] [CrossRef]
- Li, Y.-H.; Tian, X. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral. Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.; Shai, S.; Shahane, A.; Kaushik, K.S. Recent Advances in Non-Conventional Antimicrobial Approaches for Chronic Wound Biofilms: Have We Found the ’Chink in the Armor’? Biomedicines 2019, 7, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoszewicz, M.; Banasiewicz, T.; Bielecki, K. Zasady postępowania miejscowego i ogólnego w ranach/owrzodzeniach objętych procesem infekcji. [Principles of local and general management in wounds/ulcers covered by the infection process]. Forum Zakażeń 2019, 10, 1–30. (In Polish) [Google Scholar] [CrossRef]
- Karolewska, K.; Wójcik, U.; Sadowska, B.; Różalska, B. Przegląd nowoczesnych technik obrazowych i analitycznych w badaniu cech biofilmów. [Review of modern imaging and analytical techniques in the study of biofilm features]. Forum Zakażeń 2018, 9, 63–71. (In Polish) [Google Scholar] [CrossRef]
- Franklin, M.J.; Chang, C.; Akiyama, T.; Bothner, B. New Technologies for Studying Biofilms. Microbiol Spectr. 2015, 3, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Mrozikiewicz-Rakowska, B.; Mieczkowski, M.; Głażewski, T.; Czupryniak, L. Antyseptyki w leczeniu ran przewlekłych-Aktualne pytania. [Antiseptics in the treatment of chronic wounds-Current questions]. Leczenie Ran 2020, 17, 29–36. (In Polish) [Google Scholar] [CrossRef]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Assadian, O. Consensus on Wound antisepsis: Update 2018. Ski. Pharm. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- Mohd Zubir, M.Z.; Holloway, S.; Mohd Noor, N. Maggot Therapy in Wound Healing: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 21, 6103. [Google Scholar] [CrossRef]
- Sherman, R.A. Mechanisms of maggot-induced wound healing: What do we know, and where do we go from here? Evid. Based. Complement. Alternat. Med. 2014, 2014, 592419. [Google Scholar]
- Yan, L.; Chu, J.; Li, M.; Wang, X.; Zong, J.; Zhang, X.; Song, M.; Wang, S. Pharmacological Properties of the Medical Maggot: A Novel Therapy Overview. Evid Based Complement Altern. Med. 2018, 2018, 4934890. [Google Scholar] [CrossRef] [Green Version]
- Opletalová, K.; Blaizot, X.; Mourgeon, B.; Chêne, Y.; Creveuil, C.; Combemale, P.; Laplaud, A.-L.; Sohyer-Lebreuilly, I.; Dompmartin, A. Maggot therapy for wound debridement: A randomized multicenter trial. Arch. Derm. 2012, 148, 432–438. [Google Scholar]
- Bazaliński. Skuteczność terapii biologicznej z wykorzystaniem larw Lucilia sericata w leczeniu ran przewlekłych u chorych w opiece długoterminowej i paliatywnej. In Efficacy of Biological Therapy with Lucilia Sericata Larvae in the Treatment of Chronic Wounds in Patients in Long-Term and Palliative Care; Uniwersytet Rzeszowski: Rzeszów, Poland, 2019; ISBN 978-83-7996-642-4. (In Polish) [Google Scholar]
- Szewczyk, M.T.; Cwajda-Białasik, J.; Mościcka, P.; Cierzniakowska, K.; Bazaliński, D.; Jawień, A.; Spannbauer, A.; Polak, A.; Sopata, M.; Kozłowska, E.; et al. Treatment of pressure ulcers—Recommendations of the Polish Wound Management Association. Part II. Leczenie Ran. 2020, 17, 151–184. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Woo, K.; Ayello, E. Increased bacterial burden and infection: NERDS and STONES. Wounds 2007, 3, 25–46. [Google Scholar]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Andersen, C.; Black, J.; Fife, C.; Lantis, J.I.; Niezgoda, J.; Snyder, R.; Sumpio, B.; Tettelbach, W.; Treadwell, T.; et al. Management of chronic wounds: Diagnosis, preparation, treatment, and follow-up. Wounds 2017, 29, 19–36. [Google Scholar]
- Sherman, R.A.; Pechter, E.A. Maggot therapy: A review of the therapeutic applications of fly larvae in human medicine, especially for treating osteomyelitis. Med. Vet. Entomol. 1988, 2, 225–230. [Google Scholar] [CrossRef]
- Bazaliński, D.; Kózka, M.; Karnas, M.; Więch, P. Effectiveness of Chronic Wound Debridement with the Use of Larvae of Lucilia Sericata. J. Clin. Med. 2019, 8, 1845. [Google Scholar] [CrossRef] [Green Version]
- Hurlow, J.J.; Humphreys, G.J.; Bowling, F.L.; McBain, A.J. Diabetic foot infection: A critical complication. Int. Wound J. 2018, 15, 814–821. [Google Scholar] [CrossRef]
- Akbas, F.; Ozaydin, A.; Polat, E.; Onaran, I. Lucilia sericata Larval Secretions Stimulating Wound Healing Effects on Rat Dermal Fibroblast Cells. Rec. Nat. Prod. 2020, 14, 340–354. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, K.; Chen, J.; Wu, L.; Lu, H.; Wang, A.; Wang, J. A systematic review of maggot debridement therapy for chronically infected wounds and ulcers. Int J Infect Dis. 2014, 25, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Szczepanowski, Z.; Tukiendorf, A.; Krasowski, G. Further Data on Wound Healing Rates After Application of Lucilia sericata. Int. J. Low. Extrem. Wounds. 2021, 20, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, J.; Zhang, J.; Wang, W.; Sun, J.; Wang, A. Maggot debridement Therapy promotes diabetic foot. Wound healing by up-regulating endothelial cell activity. J. Diabetes Its Complicat. 2016, 30, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Van der Plas, M.J.; Jukema, G.N.; Wai, S.W.; Dogterom-Ballering, H.C.; Lagendijk, E.L.; Van Gulpen, C.; Nibbering, P.H. Maggot excretions/secretions are differentially effective against biofilms of Staphylococus aureus and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 61, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Margolin, L.; Gialanella, P. Assessment of the antimicrobial properties of maggots. Int. Wound J. 2010, 3, 202–204. [Google Scholar] [CrossRef]
- Bexfield, A.; Bond, A.E.; Roberts, E.C.; Dudley, E.; Nigam, Y.; Thomas, S.; Ratcliffe, N.A. The antibacterial activity against MRSA strains and other bacteria of a <500Da fraction from maggot excretions/secretions of Luciliasericata (Diptera: Calliphoridae). Microbes Infect. 2008, 4, 325–333. [Google Scholar]
- Zhang, Z.; Wang, J.; Zhang, B.; Liu, H.; Song, W.; He, J.; Lv, D.; Wang, S.; Xu, X. Activity of antibacterial protein from maggots against staphylococcus aureus in vitro and in vivo. Int. J. Mol. Med. 2013, 5, 1159–1165. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, D.I.; Brown, A.P. Degradation of MSCRAMM target macromolecules in VLU slough by Lucilia sericata chymotrypsin 1 (ISP) persists in the presence of tissue gelatin as activity. Int. Wound J. 2015, 4, 414–421. [Google Scholar] [CrossRef]
- Brown, A.; Horobin, A.; Blount, D.G.; Hill, P.J.; English, J.; Rich, A.; Pritchard, D.I. Blow fly Lucilia sericata nuclease digests DNA associated with Wound slough/eschar and with Pseudomonas aeruginosa biofilm. Med. Vet. Entomol. 2012, 4, 432–439. [Google Scholar] [CrossRef]
- Wang, T.Y.; Wang, W.; Li, F.F.; Chen, Y.C.; Jiang, D.; Chen, Y.D.; Wang, A.P. Maggot excretions/secretions promote diabetic Wound angiogenesis via miR18a/19a—TSP-1 axis. Diabetes Res. Clin. Pr. 2020, 165, 108140. [Google Scholar] [CrossRef]
- Nigam, Y.; Morgan, C. Does magot Therapy promote Wound healing? The clinical and cellular evidence. JEADV 2016, 30, 776–782. [Google Scholar]
- Cazander, G.; Pawiroredjo, J.S.; Vandenbroucke-Grauls, C.M.J.E.; Schreurs, M.W.J.; Jukema, G.N. Synergism between maggot excretions and antibiotics. Wound Repair Regen. 2010, 6, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowska, M.; Kowalski, M.; Koziej, M.; Antoszewski, B. Elevated serum levels of cathelicidin and β-defensin 2 are associated with basal cell carcinoma. Cent. Eur. J. Immunol. 2021, 46, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Bowling, F.L.; Salgami, E.V.; Boulton, A.J. Larval therapy: A novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers. Diabet. Care. 2007, 30, 370–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, F.; Dryburgh, N.; Donaldson, J.; Mitchell, M. Debridement for surgical wounds. Cochrane Database Syst. Rev. 2011, 11, CD006214. [Google Scholar]
- Wolcott, R. Disrupting the biofilm matrix improves Wound healing outcomes. J. Wound Care 2015, 24, 366–371. [Google Scholar] [CrossRef]
- Szczepanowski, Z.; Grabarek, B.O.; Boroń, D.; Tukiendorf, A.; Kulik-Parobczy, I.; Miszczyk, L. Microbiological effects in patients with leg ulcers and diabetic foot treated with Lucilia sericata larvae. Int Wound J. 2022, 19, 135–143. [Google Scholar] [CrossRef]
- Nezakati, E.; Hasani, M.H.; Zolfaghari, P.; Rashidan, M.; Sohrabi, M.B. Effects of Lucilia sericata Maggot Therapy in Chronic Wound Treatment: A Randomized Clinical Trial. Chronic Wound Care Manag. Res. 2020, 7, 11–17. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.; Rizzuto, A.; Rossi, A.; Perri, P.; Barbetta, A.; Abdalla, K.; Caroleo, S.; Longo, C.; Amantea, B.; Sammarco, G.; et al. Skin grafting for the treatment of chronic leg ulcers—A systematic review in evidence-based medicine. Int. Wound J. 2017, 14, 149–157. [Google Scholar] [CrossRef]
- Burnett, L.N.; Carr, E.; Tapp, D.; Raffin Bouchal, S.; Horch, J.D.; Biernaskie, J.; Gabriel, V. Patient experiences living with split thickness skin grafts. Burns 2014, 40, 1097–1105. [Google Scholar] [CrossRef]
- Sherman, R.A. Maggot versus conservative debridement therapy for the treatment of pressure ulcers. Wound Repair Regen. 2002, 10, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A. Maggot Therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care 2003, 26, 446–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gieroń, M.; Słowik-Rylska, M.; Kręcisz, B. Effectiveness of maggot debridement therapy in treating chronic wounds–Review of current literature Medical Studies. Stud. Med. 2018, 34, 325–331. [Google Scholar] [CrossRef]



| Wound Surface (cm2) | Tissue Damage | RYB | Infection acc. to NERDS/STONES [23] | CRP (0–5 mg/L) | Exudation | ABI R/L | |
|---|---|---|---|---|---|---|---|
| Case I Woman, age 64 | 55 | full thickness of the skin | Yellow | YES/NO | 25 | medium | (0.9)/(1.0) |
| Case II Woman, age 87 | 78 | full thickness of the skin | Yellow | YES/NO | 76 | large | (0.8)/(0.8) |
| Case III Woman, age 68 | 86 | full thickness of the skin | Yellow | YES/NO | 84 | large | (0.8)/(0.9) |
| Wound Surface (cm2) | Tissue Damage | RYB | Infekcja wg NERDS/STONES | CRP (0–5 mg/L) | Exudation | ABI R/L | |
|---|---|---|---|---|---|---|---|
| Case I Woman, age 64 | 50 | full thickness of the skin | Red | NO/NO | 15 | small | (0.9)/(1.0) |
| Case II Woman, age 87 | 78 | full thickness of the skin | Red/Yellow | YES/NO | 56 | medium | (0.8)/(0.8) |
| Case III Woman, age 68 | 62 | full thickness of the skin | Red | NO/NO | 24 | small | (0.8)/(0.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaliński, D.; Przybek-Mita, J.; Lisowicz, K.; Skórka, M.; Więch, P. Defensins of Lucilia sericata Larvae and Their Influence on Wound Repair Processes in Practical Assessment—A Study of Three Cases. Int. J. Environ. Res. Public Health 2023, 20, 5357. https://doi.org/10.3390/ijerph20075357
Bazaliński D, Przybek-Mita J, Lisowicz K, Skórka M, Więch P. Defensins of Lucilia sericata Larvae and Their Influence on Wound Repair Processes in Practical Assessment—A Study of Three Cases. International Journal of Environmental Research and Public Health. 2023; 20(7):5357. https://doi.org/10.3390/ijerph20075357
Chicago/Turabian StyleBazaliński, Dariusz, Joanna Przybek-Mita, Katarzyna Lisowicz, Mateusz Skórka, and Paweł Więch. 2023. "Defensins of Lucilia sericata Larvae and Their Influence on Wound Repair Processes in Practical Assessment—A Study of Three Cases" International Journal of Environmental Research and Public Health 20, no. 7: 5357. https://doi.org/10.3390/ijerph20075357





