Variability of Micro- and Macro-Elements in Anaerobic Co-Digestion of Municipal Sewage Sludge and Food Industrial By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Substrates
2.2. BMP Assays
2.3. Analytical Methods
3. Results and Discussion
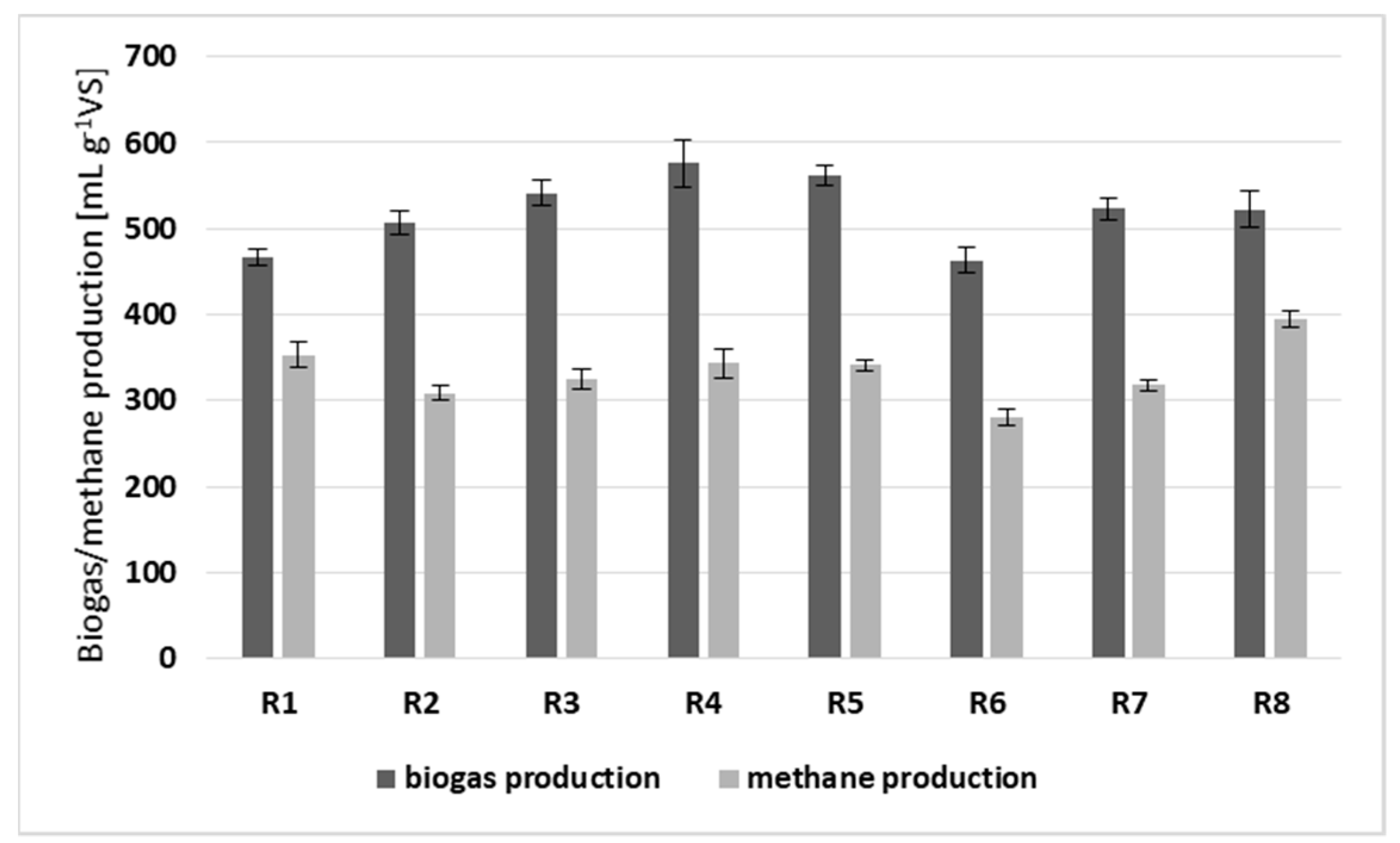
3.1. Digestion Efficiency
3.2. The Variation of Micro- and Macro-Elements within AD
3.2.1. Macro-Elements Content
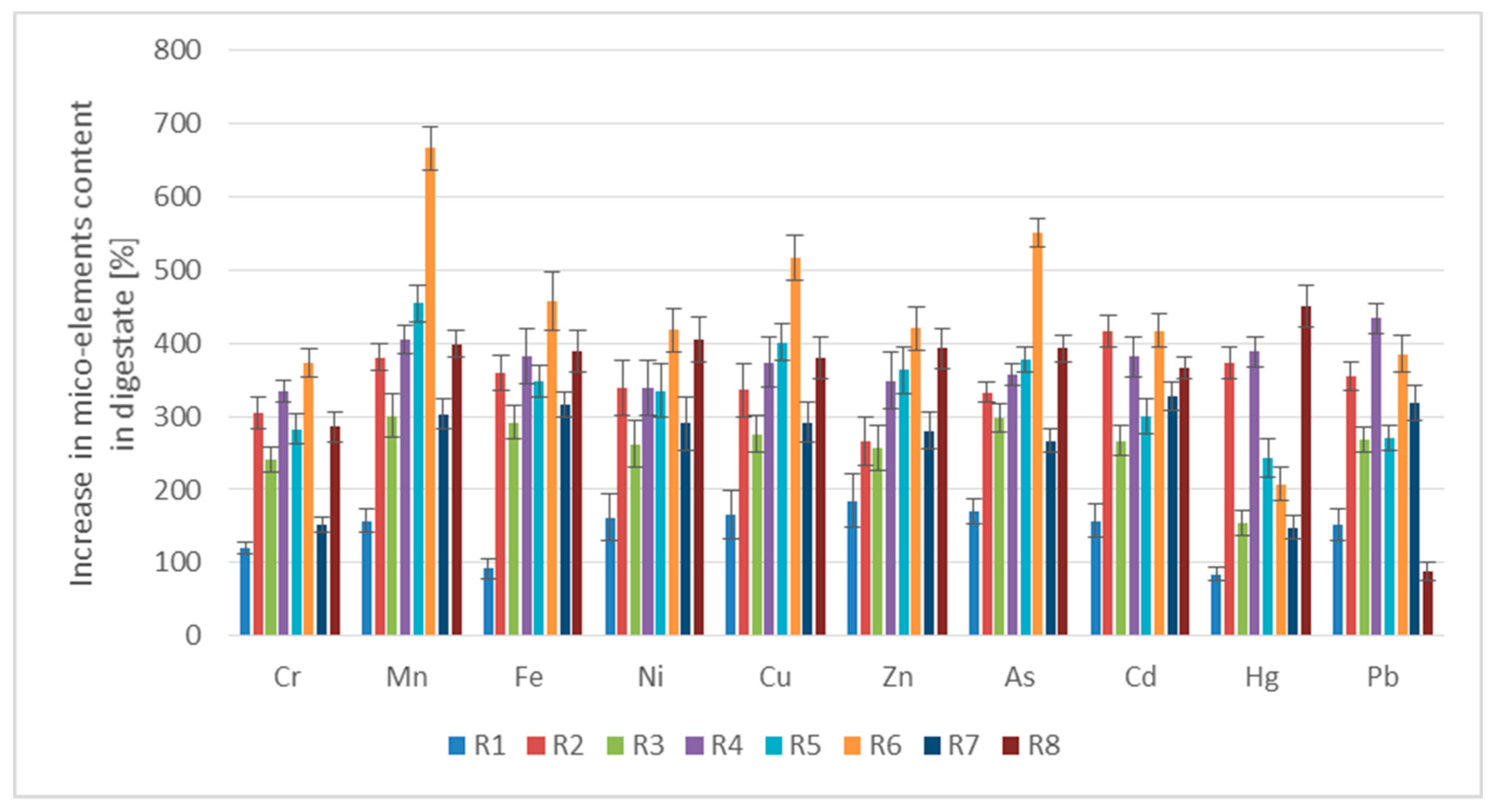
3.2.2. Content of Micro-Elements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mata-Álvarez, J.; Dosta, J.; Romero-Guiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energ. Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.Y.; Cho, J.; Kwon, E.E.; Kim, J.Y. Anaerobic digestion as an alternative disposal for phytoremediated biomass from heavy metal contaminated sites. Environ. Pollut. 2018, 243, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, J.A.; Sharma, V.K. Stimulation and inhibition of anaerobic processes by heavy metals—A review. Biol. Wastes 1990, 31, 45–67. [Google Scholar] [CrossRef]
- Jin, K.; Ran, Y.; Alengebawy, A.; Yang, G.; Jia, S.; Ai, P. Agro-environmental sustainability of using digestate fertilizer for solanaceous and leafy vegetables cultivation: Insights on fertilizer efficiency and risk assessment. J. Environ. 2020, 320, 115895. [Google Scholar] [CrossRef]
- Ai, P.; Jin, K.; Alengebawy, A.; Elsayed, M.; Ran, Y. Effect of application of different biogas fertilizer on eggplant production: Analysis of fertilizer value and risk assessment. Environ. Technol. Innovat. 2020, 19, 101019. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, D.; Zeng, X.; Li, L.; Xiao, Z. Effect of the direct use of biomass in agricultural soil on heavy metals—Activation or immobilization? Environ. Pollut. 2020, 272, 115989. [Google Scholar] [CrossRef]
- Suciu, N.A.; Lamastra, L.; Trevisan, M. PAHs content of sewage sludge in Europe and its use as soil fertilizer. Waste Manag. 2015, 41, 119–127. [Google Scholar] [CrossRef]
- Homepage—FoodDrinkEurope. Available online: https://www.fooddrinkeurope.eu/ (accessed on 20 March 2023).
- Rezzadori, K.; Benedetti, S.; Amante, E.R. Proposals for the residues recovery: Orange waste as raw material for new products. Food Bioprod. 2012, 90, 606–614. [Google Scholar] [CrossRef]
- Bachmann, S.A.; Calvete, T.; Féris, L.A. Potential applications of brewery spent grain: Critical an overview. J. Environ. Chem. Eng. 2021, 10, 106951. [Google Scholar] [CrossRef]
- Barros, R.S.; Contreras, M.P.; Morris, F.R.; Chamorro, M.V.; Arrieta, A.R. Evaluation of the methanogenic potential of anaerobic digestion of agro-industrial wastes. Heliyon 2023, 9, e14317. [Google Scholar] [CrossRef]
- Bedoić, R.; Spehar, A.B.; Puljko, J.; Čuček, L.; Ćosić, B.; Pukšec, T.; Duić, N. Opportunities and challenges: Experimental and kinetic analysis of anaerobic co-digestion of food waste and rendering industry streams for biogas production. Renew. Sustain. Energy Rev. 2020, 130, 109951. [Google Scholar] [CrossRef]
- Carlini, M.; Monarca, D.; Castellucci, S.; Mennuni, A.; Casini, L.; Selli, S. Beer spent grains biomass for biogas production: Characterization and anaerobic digestion-oriented pre-treatments. Energy Rep. 2021, 7, 921–929. [Google Scholar] [CrossRef]
- Martínez, E.J.; Rosas, J.G.; Sotres, A.; Morán, A.; Cara, J.; Sánchez, M.E.; Gómez, X. Codigestion of sludge and citrus peel wastes: Evaluating the effect of biochar addition on microbial communities. Biochem. Eng. J. 2018, 137, 314–325. [Google Scholar] [CrossRef]
- Bougrier, C.; Dognin, D.; Laroche, C.; Gonzalez, V.; Benali-Raclot, D.; Cacho Rivero, J.A. Anaerobic digestion of Brewery Spent Grains: Trace elements addition requirement. Bioresour. Technol. 2018, 247, 1193–1196. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Bernard, M.; Polit, M.; Hadj-Sadok, Z.; Pengov, M.; Dochain, D.; Estaben, M.; Labat, P. Advanced monitoring and control of anaerobic wastewater treatment plants: Software sensors and controllers for an anaerobic digester. Water Sci. Technol. 2001, 43, 175–182. [Google Scholar] [CrossRef]
- Issah, A.A.; Kabera, T. Impact of volatile fatty acids to alkalinity ratio and volatile solids on biogas production under thermophilic conditions. Waste Manag. Res. J. Sustain. Circ. Econ. 2021, 39, 871–878. [Google Scholar] [CrossRef]
- Ahring, B.K.; Angelidaki, I. Monitoring and controlling the biogas process. In Proceedings of the 8th International Conference on Anaerobic Digestion, Sendai, Japan, 25–29 May 1997; Sendai International Center: Miyagi, Japan; pp. 40–50. [Google Scholar]
- Szaja, A.; Montusiewicz, A. Enhancing the co-digestion efficiency of sewage sludge and cheese whey using brewery spent grain as an additional substrate. Bioresour. Technol. 2019, 291, 121863. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A.; Pasieczna-Patkowska, S.; Lebiocka, M. Technological and Energetic Aspects of Multi-Component Co-Digestion of the Beverage Industry Wastes and Municipal Sewage Sludge. Energies 2022, 15, 5395. [Google Scholar] [CrossRef]
- Ruiz, B.; Flotats, X. Effect of limonene on batch anaerobic digestion of citrus peel waste. Biochem. Eng. J. 2016, 109, 9–18. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, M.; Lee, J.; Shin, J.; Shin, S.G.; Lee, J. Effect of magnetite supplementation on mesophilic anaerobic digestion of phenol and benzoate: Methane production rate and microbial communities. Bioresour. Technol. 2022, 350, 126943. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Fernández, R.; Gutiérrez, M.C.; Siles, J.A. Thermophilic anaerobic digestion of pre-treated orange peel: Modelling of methane production. Process. Saf. Environ. Prot. 2018, 117, 245–254. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Mäkelä, P.S. Recycling sludge on cropland as fertilizer—Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Mineral substances and macronutrients in the anaerobic conversion of biomass: An impact evaluation. Eng. Life Sci. 2012, 12, 287–294. [Google Scholar] [CrossRef]
- Banks, C.J.; Heaven, S. Optimisation of biogas yields from anaerobic digestion by feedstock type. In The Biogas Handbook; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 131–165. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon-nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Fermoso, F.G.; van Hullebusch, E.D.; Guibaud, G.; Collins, G.; Svensson, B.H.; Carliell-Marquet, C.; Vink, J.P.; Esposito, G.; Frunzo, L. Fate of Trace Metals in Anaerobic Digestion. Adv. Biochem. Eng. Biotechnol. 2015, 151, 171–195. [Google Scholar] [CrossRef]
- Hussain, A.; Kumar, P.; Mehrotra, I. Nitrogen and phosphorus requirement in anaerobic process: A review. Environ. Eng. Manag. J. 2015, 14, 769–780. [Google Scholar] [CrossRef]
- Fernandez, N.; Forster, C.F. The anaerobic digestion of simulated coffee waste using thermophilic and mesophilic upflow filters. Process. Saf. Environ. Protect. 1994, 72, 15–20. [Google Scholar]
- Lo, H.M.; Chiu, H.Y.; Lo, S.W.; Lo, F.C. Effects of different SRT on anaerobic digestion of MSW dosed with various MSWI ashes. Bioresour. Technol. 2012, 125, 233–238. [Google Scholar] [CrossRef]
- Tan, L.; Qu, Y.; Zhou, J.; Ma, F.; Li, A. Dynamics of microbial community for X-3B wastewater decolorization coping with high-salt and metal ions conditions. Bioresour. Technol. 2009, 100, 3003–3009. [Google Scholar] [CrossRef]
- Thiele, J.H.; Wu, W.-M.; Jain, M.K.; Zeikus, J.G. Ecoengineering high rate anaerobic digestion systems: Analysis of improved syntrophic biomethanation catalysts. Biotechnol. Bioeng. 1990, 35, 990–999. [Google Scholar] [CrossRef]
- Huang, J.; Pinder, K.L. Effects of calcium on development of anaerobic acidogenic biofilms. Biotechnol. Bioeng. 1995, 45, 212–218. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, H.; Zhi, X.; Shen, J. Increased performance of continous stirred tank reactor with calcium supplementation. Int. J. Hydrog. Energy 2009, 35, 2622–2626. [Google Scholar] [CrossRef]
- Dewil, R.; Baeyens, J.; Roels, J.; Steene, B.V. Evolution of the Total Sulphur Content in Full-Scale Wastewater Sludge Treatment. Environ. Eng. Sci. 2009, 26, 867–872. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Vila, J.; Mata-Álvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Andreoli, C.V.; Pegorini, E.S.; Fernandes, F.; Santos, H.F. Land application of sewage sludge. In Sludge Treatment and Disposal; Von Sperling, M., Andreoli, C.V., Fernandes, F., Eds.; IWA Publishing: London, UK, 2007; pp. 162–206. [Google Scholar]
- European Commission. Communication From the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: A New Circular Economy Action Plan for a Cleaner and More Competitive Europe; COM/2019/640 Final; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Urbaniak, M.; Wyrwicka, A.; Tołoczko, W.; Serwecińska, L.; Zieliński, M. The effect of sewage sludge application on soil properties and willow (Salix sp.) cultivation. Sci. Total Environ. 2017, 15, 66–75. [Google Scholar] [CrossRef]
- Koszel, M.; Lorencowicz, E. Agricultural use of biogas digestate as a replacement fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 119–124. [Google Scholar] [CrossRef]
- Kazimierowicz, J. Organic waste used in agricultural biogas plants. J. Ecol. Eng. 2014, 15, 88–92. [Google Scholar] [CrossRef]
- Nowak, M.; Kacprzak, M.; Grobelak, A. Sewage sludge as a substitute for soil in the process of remediation and reclamation of sites contaminated with heavy metals. Eng. Environ. Prot. 2010, 13, 121–131. (In Polish) [Google Scholar]
- Stefaniuk, M.; Oleszczuk, P. Addition of biochar to sewage sludge decreases freely dissolved PAHs content and toxicity of sewage sludge-amended soil. Environ. Pollut. 2016, 218, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P. Evaluation of sewage sludge and slow pyrolyzed sewage sludge-derived biochar for adsorption of phenanthrene and pyrene. Bioresour. Technol. 2015, 192, 618–626. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Anjum, N.A.; Umar, S.; Singh, S.; Nazar, R.; Khan, N.A. Sulfur assimilation and cadmium tolerance in plants. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 271–302. [Google Scholar]
- Zhang, D.; Du, G.; Chen, D.; Shi, G.; Rao, W.; Li, X.; Jiang, Y.; Liu, S.; Wang, D. Effect of elemental sulfur and gypsum application on the bioavailability and redistribution of cadmium during rice growth. Sci. Total Environ. 2019, 20, 1460–1467. [Google Scholar] [CrossRef]
- Han, F.; Yun, S.; Zhang, C.; Xu, H.; Wang, Z. Steel slag as accelerant in anaerobic digestion for nonhazardous treatment and digestate fertilizer utilization. Bioresour. Technol. 2019, 282, 331–338. [Google Scholar] [CrossRef]
- Zhang, C.; Yun, S.; Li, X.; Wang, Z.; Xu, H.; Du, T. Low-cost composited accelerants for anaerobic digestion of dairy manure: Focusing on methane yield, digestate utilization and energy evaluation. Bioresour. Technol. 2018, 263, 517–524. [Google Scholar] [CrossRef]
- Djabou, A.S.M.; Qin, Y.; Thaddee, B.; Figueiredo, P.G.; Feifei, A.; Carvalho, L.J.C.B.; Omokolo, D.N.; Li, K.; Niemenak, N.; Chen, S. Effects of Calcium and Magnesium Fertilization on Antioxidant Activities during Cassava Postharvest Physiological Deterioration. Crop Sci. 2018, 58, 1385–1392. [Google Scholar] [CrossRef]
- Lominchar, M.A.; Santos, A.; de Miguel, E.; Romero, A. Remediation of aged diesel contaminated soil by alkaline activated persulfate. Sci. Total Environ. 2018, 622, 41–48. [Google Scholar] [CrossRef]
- Zeng, X.; Zou, D.; Wang, A.; Zhou, Y.; Liu, Y.; Li, Z.; Liu, F.; Wang, H.; Zeng, Q.; Xiao, Z. Remediation of cadmium-contaminated soils using Brassica napus: Effect of nitrogen fertilizers. J. Environ. Manag. 2020, 255, 109885. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B. Anaerobic Co-digestion efficiency under the stress exerted by different heavy metals concentration: An energy nexus analysis. Energy Nexus 2022, 7, 100099. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and bioaccumulation—The prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef]
- Shamsollahi, H.R.; Alimohammadi, M.; Momeni, S.; Naddafi, K.; Nabizadeh, R.; Khorasgani, F.C.; Yousefi, M. Assessment of the Health Risk Induced by Accumulated Heavy Metals from Anaerobic Digestion of Biological Sludge of the Lettuce. Biol. Trace Elem. Res. 2018, 188, 514–520. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.; Gul, E.; Yang, Q.; Yang, H.; Bartocci, P.; Fantozzi, F. Effect of Heavy Metals in the Performance of Anaerobic Digestion of Olive Mill Waste. Processes 2020, 8, 1146. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. Mansour Biogas production as affected by heavy metals in the anaerobic digestion of sludge. Egypt. J. Pet. 2014, 23, 409–417. [Google Scholar] [CrossRef]
- Naja, G.; Murphy, V.; Volesky, B. Biosorption, Metals. In Wiley Encyclopedia of Industrial Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Guo, Q.; Majeed, S.; Xu, R.; Zhang, K.; Kakade, A.; Khan, A.; Hafeez, F.Y.; Mao, C.; Liu, P.; Li, X. Heavy metals interact with the microbial community and affect biogas production in anaerobic digestion: A review. J. Environ. Manag. 2019, 240, 266–272. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Chai, Y.; Wang, L.; Mi, X.; Zhang, L.; Ware, M.A. Biogas properties and enzymatic analysis during anaerobic fermentation of Phragmites australis straw and cow dung: Influence of nickel chloride supplement. Biodegradation 2017, 28, 15–25. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Li, J.; Hou, Y.; Shi, L.; Lian, C.; Shen, Z.; Chen, Y. Evaluation of biogas production potential of trace element-contaminated plants via anaerobic digestion. Ecotoxicol. Environ. Saf. 2021, 208, 111598. [Google Scholar] [CrossRef] [PubMed]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Niu, Q.; Nie, W.; Li, X.; Yang, C. Effects of heavy metals and antibiotics on performances and mechanisms of anaerobic digestion. Bioresour. Technol. 2022, 361, 127683. [Google Scholar] [CrossRef] [PubMed]
- Facchin, V.; Cavinato, C.; Fatone, F.; Pavan, P.; Cecchi, F.; Bolzonella, D. Effect of trace element supplementation on the mesophilic anaerobic digestion of foodwaste in batch trials: The influence of inoculum origin. Biochem. Eng. J. 2013, 70, 71–77. [Google Scholar] [CrossRef]
- Karlsson, A.; Einarsson, P.; Schnürer, A.; Sundberg, C.; Ejlertsson, J.; Svensson, B.H. Impact of trace element addition on degradation efficiency of volatile fatty acids, oleic acid and phenyl acetate and on microbial populations in a biogas digester. J. Biosci. Bioeng. 2012, 114, 446–452. [Google Scholar] [CrossRef]
- Altaş, L. Inhibitory effect of heavy metals on methane-producing anaerobic granular sludge. J. Hazard. Mater. 2009, 162, 1551–1556. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Z.; Zhao, Y.; Zhang, Y.; Guo, S.; Cui, Z.; Wang, X. Effects of molybdenum, selenium and manganese supplementation on the performance of anaerobic digestion and the characteristics of bacterial community in acidogenic stage. Bioresour. Technol. 2018, 266, 166–175. [Google Scholar] [CrossRef]
- Perry, R.D.; Silver, S. Cadmium and manganese transport in Staphylococcus aureas membrane vesicles. J. Bacteriol. 1982, 150, 973–976. [Google Scholar] [CrossRef]
- Fisher, F.; Bauxbaum, L.; Toth, K.; Eisenstadt, E.; Silver, S. Regulation of manganese accumulation and exchange in Bacillus subtilis W23. J. Bacteriol. 1973, 113, 1373–1380. [Google Scholar] [CrossRef]
- Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, when Sewage Sludge is Used in Agriculture. EU Directive 86/278/EEC. Available online: http://data.europa.eu/eli/dir/1986/278/2022-01-01 (accessed on 28 February 2023).
- US EPA. 40FR Part 503—Standards for Use and Disposal of Sewage Sludge: Final Rules. Fed. Regist. 1993, 58, 9248–9415. Available online: http://www.ecfr.gov/cgi-bin/retrieveECFR?gp=2&SID=3ba5c96eb4bfc5bfdfa86764a30e9901&ty=HTML&h=L&n=pt40.30.503&r=PART (accessed on 28 February 2023).

| Parameter | Unit | Orange Peels (OP) | Orange Pulp (PL) | Brewery Spent Grain (BSG) |
|---|---|---|---|---|
| Chemical oxygen demand (COD) | g/L | 10.2 ± 0.3 | 11.8 ± 0.23 | 33.6± 0.68 |
| Volatile fatty acids (VFA) | mg/L | 611 ± 20.2 | 432.5 ± 15.2 | 608 ± 10.9 |
| pH | - | 4.35 ± 0.2 | 4.33 ± 0.1 | 5.01 ± 0.3 |
| Alkalinity (ALK) | mg/L | 27.1 ± 7.28 | 35.3 ± 6.08 | 242.0 ± 2.83 |
| Total solids (TS) | g/kg | 235.09 ± 0.50 | 155.18 ± 0.23 | 937.78 ± 0.32 |
| Volatile solids (VS) | g/kg | 224.41 ± 0.87 | 149.28 ± 0.47 | 878.21 ± 0.77 |
| Macro-elements | ||||
| Total nitrogen (TN) | mg/L | 92.1 ± 4.2 | 111.0 ± 6.1 | 739.4 ± 51.4 |
| g/kg | 0.39 ± 0.05 | 0.72 ± 0.04 | 0.79 ± 0.01 | |
| Sodium (Na) | mg/L | 57.0 ± 3.2 | 45.7 ± 2.3 | 82.9 ± 5.3 |
| g/kg | 0.24 ± 0.05 | 0.29 ± 0.01 | 0.88 ± 0.03 | |
| Magnesium (Mg) | mg/L | 171.7 ± 7.7 | 115.8 ± 8.7 | 259.8 ± 7.9 |
| g/kg | 0.73 ± 0.02 | 0.75 ± 0.01 | 0.70 ± 0.035 | |
| Phosphorus (P) | mg/L | 199.7 ± 4.9 | 369.8 ± 4.7 | 419.1 ± 49.7 |
| g/kg | 0.85 ± 0.05 | 2.74 ± 0.02 | 4.47 ± 0.61 | |
| Potassium (K) | mg/L | 2349 ± 36.1 | 1687 ± 10.1 | 1131 ± 8.7 |
| g/kg | 9.99 ± 0.6 | 10.87 ± 1.3 | 10.21 ± 0.37 | |
| Calcium (Ca) | mg/L | 1688 ± 13.1 | 887.8 ± 13.7 | 1248 ± 21.7 |
| g/kg | 7.18 ± 1.3 | 5.72 ± 0.05 | 1.33 ± 0.09 | |
| Sulfur (S) | mg/L | 203.3 ± 11.7 | 156.4 ± 9.8 | 207.8 ± 12.7 |
| g/kg | 0.86 ± 0.05 | 1.01 ± 0.02 | 2.21 ± 0.03 | |
| Micro-elements | ||||
| Chromium (Cr) | mg/L | 0.108 ± 0.05 | 0.114 ± 0.02 | 2.96 ± 0.05 |
| mg/kg | 0.46 ± 0.01 | 0.73 ± 0.01 | 3.16 ± 0.01 | |
| Manganese (Mn) | mg/L | 1.23 ± 0.06 | 0.525 ± 0.01 | 34.4 ± 0.3 |
| mg/kg | 5.21 ± 0.01 | 3.39 ± 0.1 | 3.73 ± 0.3 | |
| Iron (Fe) | mg/L | 39.75 ± 3.3 | 17.37 ± 1.2 | 144.5 ± 7.8 |
| mg/kg | 169.10 ± 10.7 | 111.90 ± 10.7 | 154.1 ± 6.9 | |
| Nickel (Ni) | mg/L | 0.21 ± 0.01 | 0.19 ± 0.05 | 0.57 ± 0.04 |
| mg/kg | 0.91 ± 0.20 | 1.24 ± 0.07 | 0.61 ± 0.03 | |
| Copper (Cu) | mg/L | 2.71 ± 0.01 | 1.43 ± 0.03 | 3.69 ± 0.03 |
| mg/kg | 11.51 ± 0.05 | 9.18 ± 0.7 | 3.94 ± 0.03 | |
| Zinc (Zn) | mg/L | 6.35 ± 0.04 | 6.17 ± 0.5 | 4.62 ± 5.1 |
| mg/kg | 27.01 ± 1.2 | 39.77 ± 4.2 | 5.85 ± 4.9 | |
| Arsenic (As) | mg/L | 0.003 ± 0.001 | 0.027 ± .0.001 | 0.050 ± 0.001 |
| mg/kg | 0.013 ± 0.001 | 0.175 ± 0.002 | 0.053 ± 0.001 | |
| Cadmium (Cd) | mg/L | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.015 ± 0.001 |
| mg/kg | 0.008 ± 0.003 | 0.011 ± 0.003 | 0.016 ± 0.001 | |
| Mercury (Hg) | mg/L | 0.150 ± 0.004 | 0.16 ± 0.02 | 0.37 ± 0.02 |
| mg/kg | 0.640 ± 0.04 | 1.02 ± 0.001 | 0.80 ± 0.03 | |
| Lead (Pb) | mg/L | 0.049 ± 0.001 | 0.029 ± 0.001 | 0.026 ± 0.01 |
| mg/kg | 0.21 ± 0.001 | 0.19 ± 0.01 | 0.28 ± 0.01 | |
| Reactor | Volatile Solid Removal (VRS) % | VFA/ALK - | pH - |
|---|---|---|---|
| R1 | 55.6 ± 4.7 | 0.052 ± 0.003 | 7.44 ± 0.01 |
| R2 | 69.5 ± 4.1 | 0.058 ± 0.002 | 7.46 ± 0.01 |
| R3 | 59.8 ± 4.0 | 0.048 ± 0.003 | 7.42 ± 0.02 |
| R4 | 69.8 ± 4.6 | 0.056 ± 0.001 | 7.49 ± 0.03 |
| R5 | 60.9 ± 3.2 | 0.048 ± 0.001 | 7.46 ± 0.01 |
| R6 | 63.3 ± 1.8 | 0.058 ± 0.002 | 7.43 ± 0.01 |
| R7 | 57.3 ± 1.1 | 0.066 ± 0.001 | 7.45 ± 0.02 |
| R8 | 59.7 ± 1.3 | 0.062 ± 0.001 | 7.44 ± 0.02 |
| Reactor | Unit | N | Na | Mg | P | K | Ca | S |
|---|---|---|---|---|---|---|---|---|
| Feedstock | ||||||||
| R1 | mg/L | 1074 ± 12.7 | 119.6 ± 5.1 | 146.5 ± 12.4 | 106.1 ± 8.8 | 182.9 ± 30.1 | 156.5 ± 7.7 | 317.9 ± 19.7 |
| g/kg | 23.1 ± 8.1 | 1.7 ± 1.3 | 2.4 ± 0.1 | 3.7 ± 3.3 | 4.2 ± 3.4 | 2.5 ± 1.4 | 7.7 ± 3.5 | |
| R2 | mg/L | 1348 ± 14.5 | 120.3 ± 3.2 | 217.4 ± 7.2 | 331.2 ± 9.2 | 304.3 ± 27.5 | 186.7 ± 8.7 | 405.8 ± 9.2 |
| g/kg | 28.3 ± 7.8 | 1.8 ± 0.05 | 4.8 ± 1.1 | 7.0 ± 1.5 | 6.4 ± 2.2 | 3.9 ± 1.4 | 7.5 ± 2.3 | |
| R3 | mg/L | 1335 ± 10.7 | 121.6 ± 7.6 | 254.9 ± 9.9 | 340.4 ± 4.6 | 342.9 ± 14.3 | 207.7 ± 10.4 | 404.3 ± 7.6 |
| g/kg | 27.6 ± 6.9 | 1.9 ± 0.07 | 5.3 ± 1.5 | 7.4 ± 1.6 | 7.1 ± 2.3 | 4.3 ± 1.7 | 7.0 ± 2.4 | |
| R4 | mg/L | 1269 ± 8.7 | 123.7 ± 8.7 | 234.1 ± 10.4 | 335.3 ± 7.7 | 296.6 ± 20.1 | 200.9 ± 9.7 | 427.3 ± 7.9 |
| g/kg | 26.5 ± 7.7 | 2.7 ± 0.07 | 4.9 ± 0.7 | 7.0 ± 2.1 | 6.2 ± 1.3 | 4.2 ± 1.1 | 8.9 ± 2.5 | |
| R5 | mg/L | 1338 ± 9.1 | 124.9 ± 10.3 | 183.4 ± 5.4 | 332.8 ± 6.9 | 301.0 ± 14.2 | 210.0 ± 10.4 | 423.2 ± 5.5 |
| g/kg | 26.3 ± 8.1 | 2.3 ± 0.04 | 3.6 ± 0.3 | 4.6 ± 1.9 | 3.9 ± 1.4 | 4.1 ± 0.9 | 8.3 ± 1.7 | |
| R6 | mg/L | 1499 ± 13.7 | 126.2 ± 11.4 | 164.9 ± 7.3 | 198.4 ± 4.5 | 201.4 ± 9.8 | 185.6 ± 12.7 | 440.0 ± 4.5 |
| g/kg | 27.8 ± 5.5 | 2.0 ± 0.03 | 3.1 ± 0.4 | 3.7 ± 1.2 | 6.0 ± 1.4 | 3.4 ± .08 | 8.3 ± 1.4 | |
| R7 | mg/L | 1507 ± 10.7 | 126.1 ± 7.9 | 247.8 ± 12.1 | 342.8 ± 12.7 | 357.5 ± 12.7 | 200.9 ± 14.8 | 441.0 ± 7.0 |
| g/kg | 28.9 ± 6.1 | 2.4 ± 0.03 | 4.4 ± 0.4 | 9.9 ± 1.8 | 10.6 ± 1.7 | 4.5 ± 1.1 | 9.5 ± 1.7 | |
| R8 | mg/L | 1571 ± 10.3 | 129.7 ± 10.1 | 256.2 ± 10.3 | 364.2 ± 10.3 | 391.2 ± 13.4 | 215.6 ± 12.7 | 444.7 ± 6.9 |
| g/kg | 30.0 ± 7.8 | 2.5 ± 0.02 | 4.3 ± 0.5 | 6.0 ± 1.9 | 6.1 ± 2.1 | 4.5 ± 1.0 | 10.7 ± 1.9 | |
| Digestate | ||||||||
| R1 | mg/L | 1492 ± 8.8 | 261.4 ± 10.2 | 472.9 ± 10.1 | 519.2 ± 15.7 | 426.7 ± 20.1 | 323.8 ± 6.7 | 886.2 ± 10.8 |
| g/kg | 66.7 ± 10.1 | 11.7 ± 1.2 | 21.1 ± 4.1 | 23.2 ± 3.2 | 19.1 ± 2.8 | 14.5 ± 1.4 | 39.6 ± 3.1 | |
| R2 | mg/L | 1769 ± 9.3 | 299.0 ± 7.8 | 558.6 ± 19.1 | 609.4 ± 17.2 | 508.6 ± 22.1 | 381.1 ± 12.7 | 1079.9 ± 10.4 |
| g/kg | 85.0 ± 12.4 | 14.4 ± 3.1 | 26.8 ± 3.9 | 29.3 ± 1.2 | 24.4 ± 3.1 | 18.3 ± 1.3 | 51.9 ± 4.0 | |
| R3 | mg/L | 1663 ± 7.8 | 280.8 ± 14.5 | 543.2 ± 17.3 | 597.1 ± 14.2 | 486.8 ± 17.8 | 376.3 ± 14.7 | 1047.7 ± 12.1 |
| g/kg | 74.8 ± 14.4 | 12.6 ± 2.7 | 24.4 ± 2.2 | 26.9 ± 4.2 | 21.9 ± 2.7 | 16.9 ± 1.8 | 47.2 ± 3.8 | |
| R4 | mg/L | 1653 ± 15.3 | 243.0 ± 16.7 | 499.2 ± 15.1 | 581.1 ± 17.3 | 428.6 ± 15.6 | 365.2 ± 13.6 | 999.2 ± 13.1 |
| g/kg | 88.3 ± 13.2 | 13.0 ± 3.4 | 26.7 ± 4.6 | 31.0 ± 3.4 | 22.9 ± 2.9 | 19.5 ± 1.7 | 53.3 ± 4.7 | |
| R5 | mg/L | 1748 ± 12.8 | 265.6 ± 17.9 | 496.2 ± 14.3 | 572.1 ± 15.1 | 430.7 ± 17.7 | 371.7 ± 14.9 | 965.2 ± 14.2 |
| g/kg | 77.2 ± 10.4 | 11.7 ± 2.7 | 21.9 ± 5.2 | 25.3 ± 4.1 | 19.0 ± 2.5 | 16.4 ± 2.0 | 42.7 ± 5.0 | |
| R6 | mg/L | 1169 ± 8.8 | 280.4 ± 10.3 | 505.1 ± 12.4 | 575.1 ± 15.1 | 466.0 ± 16.4 | 360.9 ± 15.7 | 969.3 ± 14.3 |
| g/kg | 53.1 ± 7.7 | 12.7 ± 3.1 | 22.9 ± 5.1 | 26.1 ± 3.4 | 21.2 ± 1.8 | 16.4 ± 1.7 | 44.0 ± 3.3 | |
| R7 | mg/L | 1330 ± 10.1 | 242.9 ± 11.3 | 495.0 ± 14.4 | 591.0 ± 22.5 | 453.9 ± 13.4 | 362.2 ± 12.1 | 954.8 ± 9.8 |
| g/kg | 49.4 ± 8.9 | 9.0 ± 1.5 | 18.4 ± 4.3 | 26.0 ± 2.8 | 24.4 ± 1.9 | 13.5 ± 1.3 | 35.5 ± 3.4 | |
| R8 | mg/L | 1247 ± 10.5 | 269.2 ± 7.9 | 507.1 ± 20.1 | 551.0 ± 24.0 | 459.3 ± 17.5 | 343.9 ± 17.1 | 943.4 ± 8.9 |
| g/kg | 63.1 ± 7.6 | 13.6 ± 3.7 | 25.7 ± 7.8 | 27.9 ± 2.4 | 23.2 ± 2.1 | 17.4 ± 1.4 | 47.7 ± 4.1 | |
| Reactor | Unit | Cr | Mn | Fe | Ni | Cu | Zn | As | Cd | Hg | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feedstock | |||||||||||
| R1 | mg/L | 3.31 ± 0.1 | 2.39 ± 0.01 | 116.9 ± 12.2 | 1.10 ± 0.01 | 26.21 ± 2.3 | 70.31 ± 5.6 | 0.24 ± 0.01 | 0.15 ± 0.01 | 0.58 ± 0.05 | 0.78 ± 0.20 |
| mg/kg | 71.12 ± 5.4 | 51.42 ± 2.2 | 2512 ± 60.1 | 23.58 ± 1.3 | 562.9 ± 10.3 | 1510 ± 37.8 | 5.21 ± 0.05 | 3.14 ± 0.7 | 12.54 ± 1.2 | 16.82 ± 2.1 | |
| R2 | mg/L | 2.29 ± 0.05 | 1.67 ± 0.04 | 62.25 ± 7.8 | 0.85 ± 0.5 | 20.96 ± 1.3 | 64.68 ± 4.3 | 0.20 ± 0.03 | 0.10 ± 0.01 | 0.35 ± 0.07 | 0.57 ± 0.01 |
| mg/kg | 48.11 ± 3.6 | 35.08 ± 2.1 | 1307 ± 10.7 | 17.79 ± 2.2 | 440.2 ± 12.4 | 1358 ± 27.1 | 4.14 ± 0.9 | 2.08 ± 0.05 | 7.25 ± 0.7 | 11.91 ± 2.1 | |
| R3 | mg/L | 2.60 ± 0.03 | 1.87 ± 0.01 | 69.29 ± 5.5 | 0.95 ± 0.04 | 22.60 ± 5.6 | 61.99 ± 3.6 | 0.21 ± 0.02 | 0.13 ± 0.02 | 0.55 ± 0.04 | 0.65 ± 0.05 |
| mg/kg | 53.80 ± 2.2 | 38.70 ± 1.4 | 1432 ± 15.7 | 19.72 ± 5.4 | 467.1 ± 14.3 | 1281 ± 26.3 | 4.29 ± 0.7 | 2.63 ± 0.07 | 11.40 ± 2.1 | 13.52 ± 2.5 | |
| R4 | mg/L | 2.39 ± 0.01 | 1.71 ± 0.02 | 66.32 ± 8.1 | 0.89 ± 0.05 | 20.83 ± 3.6 | 57.79 ± 4.7 | 0.20 ± 0.01 | 0.11 ± 0.03 | 0.36 ± 0.02 | 0.57 ± 0.09 |
| mg/kg | 50.00 ± 3.7 | 35.71 ± 1.7 | 1385 ± 25.1 | 18.52 ± 3.4 | 435.1 ± 14.7 | 1207 ± 45.1 | 4.11 ± 0.5 | 2.24 ± 0.04 | 7.61 ± 0.7 | 11.96 ± 2.1 | |
| R5 | mg/L | 2.25 ± 0.02 | 1.30 ± 0.02 | 62.82 ± 5.4 | 0.78 ± 0.05 | 18.01 ± 3.6 | 60.81 ± 3.7 | 0.16 ± 0.01 | 0.12 ± 0.01 | 0.41 ± 0.03 | 0.69 ± 0.07 |
| mg/kg | 44.19 ± 3.1 | 25.50 ± 1.5 | 1234 ± 14.7 | 15.38 ± 2.5 | 353.6 ± 25.6 | 1194 ±44.3 | 3.07 ± 0.75 | 2.35 ± 0.03 | 8.06 ± 1.3 | 13.50 ± 2.7 | |
| R6 | mg/L | 1.95 ± 0.01 | 1.06 ± 0.02 | 54.97 ± 7.7 | 0.72 ± 0.08 | 15.58 ± 3.6 | 51.59 ± 3.7 | 0.13 ± 0.02 | 0.09 ± 0.01 | 0.51 ± 0.05 | 0.53 ± 0.06 |
| mg/kg | 36.24 ± 1.3 | 19.70 ± 1.2 | 1020 ± 25.6 | 13.28 ± 3.6 | 289.2 ± 10.1 | 957.5 ± 17.9 | 2.44 ± 0.07 | 1.74 ± 0.04 | 9.50 ± 2.1 | 9.92 ± 0.5 | |
| R7 | mg/L | 2.97 ± 0.03 | 1.61 ± 0.02 | 57.99 ± 4.7 | 0.76 ± 0.05 | 18.46 ± 3.4 | 50.56 ± 3.7 | 0.17 ± 0.01 | 0.09 ± 0.02 | 0.41 ± 0.07 | 0.50 ± 0.04 |
| mg/kg | 56.88 ± 5.1 | 30.93 ± 4.7 | 1112 ±36.7 | 14.59 ± 2.4 | 354.0 ± 9.7 | 969.7 ± 22.4 | 3.31 ± 0.03 | 1.77 ± 0.07 | 7.92 ± 1.4 | 9.56 ± 1.1 | |
| R8 | mg/L | 2.45 ± 0.01 | 1.65 ± 0.2 | 60.93 ± 5.4 | 0.80 ± 0.03 | 19.61 ± 3.6 | 52.39 ± 4.1 | 0.18 ± 0.01 | 0.10 ± 0.02 | 0.31 ± 0.04 | 0.52 ± 0.03 |
| mg/kg | 46.71 ± 3.2 | 31.46 ± 3.2 | 1163 ± 19.3 | 15.18 ± 1.6 | 374.5 ± 12.6 | 1000 ± 40.2 | 3.41 ± 0.02 | 1.89 ± 0.04 | 5.95 ± 0.75 | 9.90 ± 1.7 | |
| Digestate | |||||||||||
| R1 | mg/L | 3.50 ± 0.5 | 2.96 ± 0.01 | 108.2 ± 7.9 | 1.38 ± 0.02 | 33.48 ± 2.7 | 96.25 ± 8.8 | 0.32 ± 0.02 | 0.18 ± 0.02 | 0.52 ± 0.06 | 0.95 ± 0.05 |
| mg/kg | 156.58 ± 10.4 | 132.51 ± 21.3 | 4836 ± 44.1 | 61.67 ± 3.2 | 1496.7 ± 36.1 | 4303 ±45.7 | 14.11 ± 3.2 | 8.09 ± 0.4 | 23.07 ± 1.3 | 42.38 ± 1.3 | |
| R2 | mg/L | 4.05 ± 0.07 | 3.51 ± 0.2 | 125.0 ± 10.7 | 1.63 ± 0.04 | 39.90 ± 5.1 | 103.6 ± 7.8 | 0.37 ± 0.04 | 0.22 ± 0.02 | 0.71 ± 0.03 | 1.13 ± 0.01 |
| mg/kg | 194.56 ± 11.7 | 168.58 ± 17.2 | 6008 ± 75.3 | 78.09 ± 3.4 | 1917 ± 45.2 | 4980 ± 39.7 | 17.93 ± 2.2 | 10.76 ± 1.1 | 34.29 ± 2.1 | 54.10 ± 2.3 | |
| R3 | mg/L | 4.07 ± 0.09 | 3.45 ± 0.05 | 124.7 ± 14.3 | 1.58 ± 0.05 | 39.02 ± 5.4 | 101.8 ± 10.2 | 0.38 ± 0.04 | 0.21 ± 0.03 | 0.64 ± 0.03 | 1.11 ± 0.05 |
| mg/kg | 183.10 ± 9.7 | 155.26 ± 10.2 | 5612 ± 36.7 | 71.30 ± 4.1 | 1756 ± 47.2 | 4581 ± 56.3 | 17.10 ± 2.1 | 9.66 ± 1.1 | 28.95 ± 2.8 | 49.75 ± 2.2 | |
| R4 | mg/L | 4.07 ± 0.07 | 3.38 ± 0.07 | 125.2 ± 7.18 | 1.52 ± 0.06 | 38.57 ± 4.1 | 101.5 ± 10.7 | 0.35 ± 0.07 | 0.20 ± 0.01 | 0.70 ± 0.03 | 1.20 ± 0.03 |
| mg/kg | 217.47 ± 14.7 | 180.53 ± 26.3 | 6685. ±32.2 | 81.38 ± 6.7 | 2059 ± 10.2 | 5417 ± 53.3 | 18.83 ± 2.3 | 10.79 ± 2.1 | 37.16 ± 2.2 | 63.83 ± 2.1 | |
| R5 | mg/L | 3.83 ± 0.7 | 3.20 ± 0.07 | 124.9 ± 7.2 | 1.51 ± 0.04 | 40.10 ± 5.1 | 125.1 ± 3.8 | 0.33 ± 0.08 | 0.21 ± 0.02 | 0.63 ± 0.07 | 1.13 ± 0.05 |
| mg/kg | 169.23 ± 14.5 | 141.47 ± 12.3 | 5518 ± 44.3 | 66.88 ± 4.2 | 1772 ± 35.1 | 5530 ± 40.5 | 14.65 ± 2.3 | 9.42 ± 1.2 | 27.62 ± 1.9 | 50.10 ± 2.3 | |
| R6 | mg/L | 3.78 ± 0.8 | 3.33 ± 0.05 | 125.1 ± 12.5 | 1.51 ± 0.04 | 39.32 ± 4.1 | 109.7 ± 7.7 | 0.35 ± 0.07 | 0.20 ± 0.02 | 0.64 ± 0.03 | 1.06 ± 0.07 |
| mg/kg | 171.40 ± 16.7 | 151.00 ± 14.1 | 5677 ± 45 | 68.75 ± 3.8 | 1785 ± 38.7 | 4980 ± 55.1 | 15.90 ± 3.2 | 8.98 ± 0.75 | 29.16 ± 2.2 | 48.17 ± 3.5 | |
| R7 | mg/L | 3.86 ± 0.6 | 3.36 ± 0.04 | 124.5 ± 10.3 | 1.53 ± 0.05 | 37.30 ± 2.1 | 99.24 ± 7.2 | 0.33 ± 0.01 | 0.20 ± 0.01 | 0.53 ± 0.03 | 1.02 ± 0.02 |
| mg/kg | 143.65 ± 20.1 | 124.78 ± 10.2 | 4629 ± 51.3 | 56.90 ± 4.7 | 1387 ± 24.1 | 3689 ± 41.3 | 12.15 ± 2.1 | 7.57 ± 0.65 | 19.62 ± 0.8 | 39.90 ± 1.1 | |
| R8 | mg/L | 3.56 ± 0.04 | 3.10 ± 0.07 | 112.3 ± 8.1 | 1.51 ± 0.04 | 35.48 ± 3.7 | 97.43 ± 7.7 | 0.33 ± 0.06 | 0.17 ± 0.02 | 0.55 ± 0.02 | 0.97 ± 0.05 |
| mg/kg | 180.09 ± 10.3 | 157.05 ± 12.1 | 5683 ± 31.2 | 76.63 ± 6.4 | 1796 ± 45.2 | 4931 ± 37.5 | 16.79 ± 2.2 | 8.81 ± 0.9 | 23.74 ± 1.5 | 18.60 ± 1.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaja, A.; Montusiewicz, A.; Lebiocka, M. Variability of Micro- and Macro-Elements in Anaerobic Co-Digestion of Municipal Sewage Sludge and Food Industrial By-Products. Int. J. Environ. Res. Public Health 2023, 20, 5405. https://doi.org/10.3390/ijerph20075405
Szaja A, Montusiewicz A, Lebiocka M. Variability of Micro- and Macro-Elements in Anaerobic Co-Digestion of Municipal Sewage Sludge and Food Industrial By-Products. International Journal of Environmental Research and Public Health. 2023; 20(7):5405. https://doi.org/10.3390/ijerph20075405
Chicago/Turabian StyleSzaja, Aleksandra, Agnieszka Montusiewicz, and Magdalena Lebiocka. 2023. "Variability of Micro- and Macro-Elements in Anaerobic Co-Digestion of Municipal Sewage Sludge and Food Industrial By-Products" International Journal of Environmental Research and Public Health 20, no. 7: 5405. https://doi.org/10.3390/ijerph20075405
APA StyleSzaja, A., Montusiewicz, A., & Lebiocka, M. (2023). Variability of Micro- and Macro-Elements in Anaerobic Co-Digestion of Municipal Sewage Sludge and Food Industrial By-Products. International Journal of Environmental Research and Public Health, 20(7), 5405. https://doi.org/10.3390/ijerph20075405






