Relationship between Vitamin D and Immunity in Older People with COVID-19
Abstract
:1. Introduction
- To analyse, among the pleiotropic effects of this hormone, how its strong multimodal modulatory effect on the immune system is able to affect the pathophysiology of this disease;
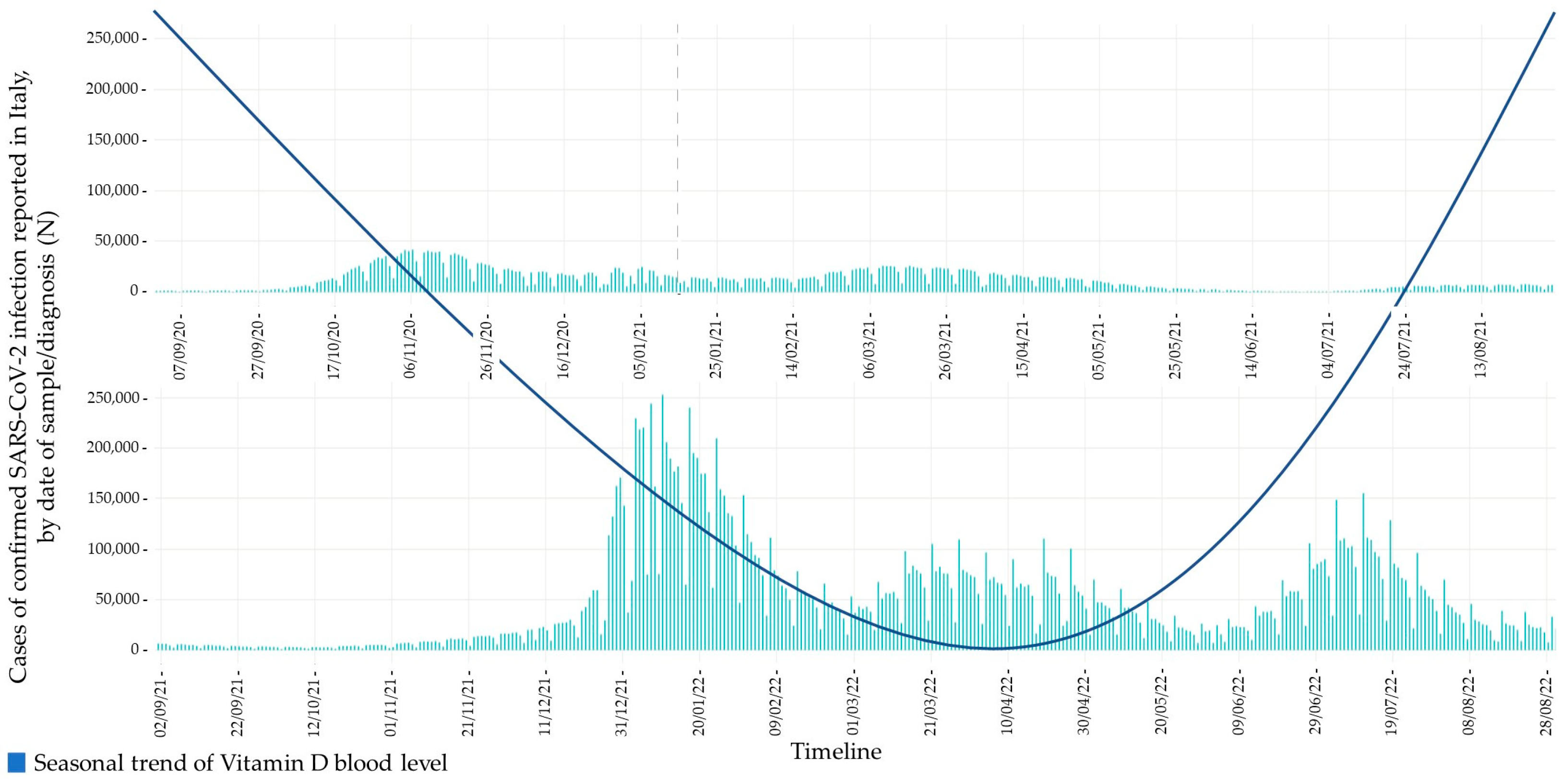
- To outline a possible relationship between its circannual fluctuations in blood levels and the epidemiological trend of COVID-19, particularly in the elderly population.
2. Epidemiology of Vitamin D Deficiency
3. Immunological Effects of Vitamin D
3.1. Innate Response
3.2. Adaptive Response
4. Epidemiology of COVID-19 in Older Persons
5. Immunological Pathophysiology of COVID-19
5.1. Innate Response
5.2. Adaptive Response
5.2.1. Antibodies Production
5.2.2. Cell-Mediated Response
5.2.3. Immunological Memory
6. Role of Vitamin D in COVID-19
- Strengthening the respiratory epithelial barrier gap junctions [96].
- Inducing the transcription of antimicrobial peptides such as cathelicidin and defensins that can reduce viral replication [25].
- Modulating the inflammatory response by both reduction of pro-inflammatory cytokines responsible for pulmonary epithelial damage and stimulating the production of anti-inflammatory cytokines [93].
- Modulating adaptive immunity towards a Th2 driven response [96].
| Authors | Immune System Response | Type of Study | Main Conclusion |
|---|---|---|---|
| De Smet et al., 2020 [91] | Stimulation of expression of cathelicidin and beta-defensin in respiratory epithelia. Protolerogenic and anti-inflammatory cytokine synthesis by inhibiting T helper 1 (Th1) proliferation and switching Th1 CD4 T cells and M1-polarised macrophages towards a type II immunity. | Retrospective observational study on 186 subjects with polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection hospitalised from 1 March 2020 to 7 April 2020 for COVID-19 pneumonia. | Individuals with vitamin D deficiency have higher relative risk of testing positive for SARS-CoV-2, compared with those with normal level of vitamin D. |
| Fasano et al., 2020 [92] | Statistical correlation. | Single-centre case-control survey. | Patients taking vitamin D supplements were less likely to develop COVID-19. |
| Annweiler et al., 2020 [95] | Prevention of respiratory epithelium infections. Enhanced production of antimicrobial peptides. Modulation of the cytokine storm involved in lung injury. | Quasi-experimental study conducted in one geriatric acute care unit dedicated to COVID-19 patients. | Vitamin D supplementation was associated with less severe COVID-19 and better survival in frail elderly. Vitamin D was able to reduce lung permeability in the animal models of ARDS in which rats supplemented with vitamin D had milder ARDS symptoms and moderate lung damage compared with controls. |
| Ali et al., 2020 [96] | Strengthening the respiratory epithelial barrier gap junctions and modulation of adaptive immunity towards a Th2 driven response. | Randomised trials and meta-analysis. | Vitamin D supplementation has been shown to have protective effects against respiratory tract infections. |
| Grant et al., 2020 [37] | Increasing antioxidant factors gene expression, in particular glutathione reductase, that preserve vitamin C levels, or modulating the Nrf2-Keap 1 antioxidant pathway. | Review. | Higher 25(OH)D concentrations reduce risk of infection and death from acute respiratory tract infections, including those from influenza, CoVs and pneumonia. |
| Ilie et al., 2020 [89] | ACE-2 overexpression. | Short communication, Review. | There is an association between high levels of ACE-2 and better outcomes in COVID-19. |
| Cereda et al., 2021 [101] | Protective role of calcifediol in lung damage, both modulating the sensitivity of tissue to angiotensin II and promoting overexpression of ACE-2. | Single-centre cohort study. | Very low 25(OH)D levels were highly prevalent and suggestive of deficiency among hospitalised patients with severe COVID-19. |
7. Therapeutic Perspectives
8. Novel Vitamin D3 Hydroxyderivatives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trombetta, A.C.; Smith, V.; Gotelli, E.; Ghio, M.; Paolino, S.; Pizzorni, C.; Vanhaecke, A.; Ruaro, B.; Sulli, A.; Cutolo, M. Vitamin D Deficiency and Clinical Correlations in Systemic Sclerosis Patients: A Retrospective Analysis for Possible Future Developments. PLoS ONE 2017, 12, e0179062. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Casabella, A.; Paolino, S.; Alessandri, E.; Patané, M.; Gotelli, E.; Sulli, A.; Cutolo, M. Trabecular Bone Score and Bone Quality in Systemic Lupus Erythematosus Patients. Front. Med. 2020, 7, 574842. [Google Scholar] [CrossRef] [PubMed]
- Martelli, M.; Salvio, G.; Santarelli, L.; Bracci, M. Shift Work and Serum Vitamin D Levels: A Systematic Review and Meta-Analysis. Int. J. Env. Res. Public Health 2022, 19, 8919. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic Actions of Vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efird, J.T.; Anderson, E.J.; Jindal, C.; Redding, T.S.; Thompson, A.D.; Press, A.M.; Upchurch, J.; Williams, C.D.; Choi, Y.M.; Suzuki, A. The Interaction of Vitamin D and Corticosteroids: A Mortality Analysis of 26,508 Veterans Who Tested Positive for SARS-CoV-2. Int. J. Env. Res. Public Health 2021, 19, 447. [Google Scholar] [CrossRef]
- Malabanan, A.; Veronikis, I.; Holick, M. Redefining Vitamin D Insufficiency. Lancet 1998, 351, 805–806. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current Vitamin D Status in European and Middle East Countries and Strategies to Prevent Vitamin D Deficiency: A Position Statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. MrOs Is D-Ficient. J. Clin. Endocrinol. Metab. 2009, 94, 1092–1093. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Chen, T.C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87, S1080–S1086. [Google Scholar] [CrossRef] [Green Version]
- Boulkrane, M.S.; Ilina, V.; Melchakov, R.; Fedotova, J.; Drago, F.; Gozzo, L.; Das, U.N.; Abd El-Aty, A.M.; Baranenko, D. COVID-19 Disease and Vitamin D: A Mini-Review. Front. Pharm. 2020, 11, 604579. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; de Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D Deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaia, G.; Diémoz, H.; Maluta, F.; Fountoulakis, I.; Ceccon, D.; di Sarra, A.; Facta, S.; Fedele, F.; Lorenzetto, G.; Siani, A.M.; et al. Does Solar Ultraviolet Radiation Play a Role in COVID-19 Infection and Deaths? An Environmental Ecological Study in Italy. Sci. Total Environ. 2021, 757, 143757. [Google Scholar] [CrossRef] [PubMed]
- Isaia, G.; Giorgino, R.; Rini, G.B.; Bevilacqua, M.; Maugeri, D.; Adami, S. Prevalence of Hypovitaminosis D in Elderly Women in Italy: Clinical Consequences and Risk Factors. Osteoporos. Int. 2003, 14, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Maggio, D.; Cherubini, A.; Lauretani, F.; Russo, R.C.; Bartali, B.; Pierandrei, M.; Ruggiero, C.; Macchiarulo, M.C.; Giorgino, R.; Minisola, S.; et al. 25(OH)D Serum Levels Decline with Age Earlier in Women Than in Men and Less Efficiently Prevent Compensatory Hyperparathyroidism in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Oudshoorn, C.; van der Cammen, T.J.M.; McMurdo, M.E.T.; van Leeuwen, J.P.T.M.; Colin, E.M. Ageing and Vitamin D Deficiency: Effects on Calcium Homeostasis and Considerations for Vitamin D Supplementation. Br. J. Nutr. 2009, 101, 1597–1606. [Google Scholar] [CrossRef]
- Orwoll, E.; Nielson, C.M.; Marshall, L.M.; Lambert, L.; Holton, K.F.; Hoffman, A.R.; Barrett-Connor, E.; Shikany, J.M.; Dam, T.; Cauley, J.A. Vitamin D Deficiency in Older Men. J. Clin. Endocrinol. Metab. 2009, 94, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Sempos, C.T.; Vesper, H.W.; Phinney, K.W.; Thienpont, L.M.; Coates, P.M.; Vitamin D Standardization Program (VDSP). Vitamin D Status as an International Issue: National Surveys and the Problem of Standardization. Scand. J. Clin. Lab. Invest. Suppl. 2012, 243, 32–40. [Google Scholar]
- Binkley, N.; Krueger, D.; Cowgill, C.S.; Plum, L.; Lake, E.; Hansen, K.E.; DeLuca, H.F.; Drezner, M.K. Assay Variation Confounds the Diagnosis of Hypovitaminosis D: A Call for Standardization. J. Clin. Endocrinol. Metab. 2004, 89, 3152–3157. [Google Scholar] [CrossRef] [Green Version]
- Huisman, M.; Poppelaars, J.; van der Horst, M.; Beekman, A.T.; Brug, J.; van Tilburg, T.G.; Deeg, D.J. Cohort Profile: The Longitudinal Aging Study Amsterdam. Int. J. Epidemiol. 2011, 40, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Harris, T.B.; Launer, L.J.; Eiriksdottir, G.; Kjartansson, O.; Jonsson, P.V.; Sigurdsson, G.; Thorgeirsson, G.; Aspelund, T.; Garcia, M.E.; Cotch, M.F.; et al. Age, Gene/Environment Susceptibility-Reykjavik Study: Multidisciplinary Applied Phenomics. Am. J. Epidemiol. 2007, 165, 1076–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science (1979) 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Ren, S.; Nguyen, L.; Wu, S.; Encinas, C.; Adams, J.S.; Hewison, M. Alternative Splicing of Vitamin D-24-Hydroxylase. J. Biol. Chem. 2005, 280, 20604–20611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahoor Basha, S.; Suresh, S.; Ashok Reddy, V.V.; Surya Teja, S.P. Is the Shielding Effect of Cholecalciferol in SARS CoV-2 Infection Dependable? An Evidence Based Unraveling. Clin. Epidemiol. Glob. Health 2021, 9, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T. Vitamin D and Lung Infection. Infect. Immun. 2016, 84, 3094–3096. [Google Scholar] [CrossRef] [Green Version]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral Activity and Increased Host Defense against Influenza Infection Elicited by the Human Cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef]
- de Vita, F.; Lauretani, F.; Bauer, J.; Bautmans, I.; Shardell, M.; Cherubini, A.; Bondi, G.; Zuliani, G.; Bandinelli, S.; Pedrazzoni, M.; et al. Relationship between Vitamin D and Inflammatory Markers in Older Individuals. Age 2014, 36, 9694. [Google Scholar] [CrossRef] [Green Version]
- Lei, G.-S.; Zhang, C.; Cheng, B.-H.; Lee, C.-H. Mechanisms of Action of Vitamin D as Supplemental Therapy for Pneumocystis Pneumonia. Antimicrob. Agents Chemother. 2017, 61, e01226-17. [Google Scholar] [CrossRef] [Green Version]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A. Association Between Serum 25-Hydroxyvitamin D Level and Upper Respiratory Tract Infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and Inflammaging in the Aging Process: Age-Related Diseases or Longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef]
- Gonçalves de Carvalho, C.M.R.; Ribeiro, S.M.L. Aging, Low-Grade Systemic Inflammation and Vitamin D: A Mini-Review. Eur. J. Clin. Nutr. 2017, 71, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.W.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D Deficiency Is Associated With Inflammation in Older Irish Adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruit, A.; Zanen, P. The Association between Vitamin D and C-Reactive Protein Levels in Patients with Inflammatory and Non-Inflammatory Diseases. Clin. Biochem. 2016, 49, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory Effects of 1,25-Dihydroxyvitamin D3 on Human B Cell Differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.J.; O’Garra, A. 1α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4+ T Cells to Enhance the Development of Th2 Cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive Actions of 1,25-Dihydroxyvitamin D3: Preferential Inhibition of Th1 Functions. J. Nutr. 1995, 125 (Suppl. 6), 1704S–1708S. [Google Scholar]
- Grant, W.; Lahore, H.; McDonnell, S.; Baggerly, C.; French, C.; Aliano, J.; Bhattoa, H. Evidence That Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune Modulatory Treatment of Trinitrobenzene Sulfonic Acid Colitis with Calcitriol Is Associated with a Change of a T Helper (Th) 1/Th17 to a Th2 and Regulatory T Cell Profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Penna, G.; Adorini, L. 1α,25-Dihydroxyvitamin D3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.D.; Kumar, R. Effects of 1α,25(OH)2 D3 and Its Analogs on Dendritic Cell Function. J. Cell Biochem. 2003, 88, 323–326. [Google Scholar] [CrossRef]
- Adorini, L. Intervention in Autoimmunity: The Potential of Vitamin D Receptor Agonists. Cell Immunol. 2005, 233, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Parkash, O.; Agrawal, S.; Madhan Kumar, M. T Regulatory Cells: Achilles’ Heel of Mycobacterium Tuberculosis Infection? Immunol. Res. 2015, 62, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S. Gut Microbiome, Vitamin D, ACE2 Interactions Are Critical Factors in Immune-Senescence and Inflammaging: Key for Vaccine Response and Severity of COVID-19 Infection. Inflamm. Res. 2022, 71, 13–26. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 6 March 2023).
- Shahid, Z.; Kalayanamitra, R.; McClafferty, B.; Kepko, D.; Ramgobin, D.; Patel, R.; Aggarwal, C.S.; Vunnam, R.; Sahu, N.; Bhatt, D.; et al. COVID–19 and Older Adults: What We Know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef]
- Bialek, S.; Boundy, E.; Bowen, V.; Chow, N.; Cohn, A.; Dowling, N.; Ellington, S.; Gierke, R.; Hall, A.; MacNeil, J.; et al. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 343–346. [Google Scholar] [CrossRef]
- Gold, J.A.W.; Rossen, L.M.; Ahmad, F.B.; Sutton, P.; Li, Z.; Salvatore, P.P.; Coyle, J.P.; DeCuir, J.; Baack, B.N.; Durant, T.M.; et al. Race, Ethnicity, and Age Trends in Persons Who Died from COVID-19—United States, May–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1517–1521. [Google Scholar] [CrossRef]
- WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Levin, A.T.; Hanage, W.P.; Owusu-Boaitey, N.; Cochran, K.B.; Walsh, S.P.; Meyerowitz-Katz, G. Assessing the Age Specificity of Infection Fatality Rates for COVID-19: Systematic Review, Meta-Analysis, and Public Policy Implications. Eur. J. Epidemiol. 2020, 35, 1123–1138. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. COVID-19 Integrated Surveillance: Key National Data. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Infografica_29maggio%20ITA.pdf (accessed on 6 March 2023).
- Sun, Z.-H. Clinical Outcomes of COVID-19 in Elderly Male Patients. J. Geriatr. Cardiol. 2020, 17, 243–245. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of Malnutrition and Analysis of Related Factors in Elderly Patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciacqua, A.; Pujia, R.; Arturi, F.; Hribal, M.L.; Montalcini, T. COVID-19 and Elderly: Beyond the Respiratory Drama. Intern. Emerg. Med. 2020, 15, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, Immunity and Intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, J.M.; Fulop, T.; Bryl, E. Immunosenescence and COVID-19. Mech Ageing Dev. 2022, 204, 111672. [Google Scholar] [CrossRef] [PubMed]
- Laing, A.G.; Lorenc, A.; del Molino del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A Dynamic COVID-19 Immune Signature Includes Associations with Poor Prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Zheng, J.; Wohlford-Lenane, C.; Abrahante, J.E.; Mack, M.; Sompallae, R.; McCray, P.B.; Meyerholz, D.K.; Perlman, S. IFN-I Response Timing Relative to Virus Replication Determines MERS Coronavirus Infection Outcomes. J. Clin. Investig. 2019, 129, 3625–3639. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- Qian, F.; Wang, X.; Zhang, L.; Lin, A.; Zhao, H.; Fikrig, E.; Montgomery, R.R. Impaired Interferon Signaling in Dendritic Cells from Older Donors Infected In Vitro With West Nile Virus. J. Infect Dis. 2011, 203, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Hackbart, M.; Deng, X.; Baker, S.C. Coronavirus Endoribonuclease Targets Viral Polyuridine Sequences to Evade Activating Host Sensors. Proc. Natl. Acad. Sci. 2020, 117, 8094–8103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Holle, T.A.; Moody, M.A. Influenza and Antibody-Dependent Cellular Cytotoxicity. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive Mapping of Immune Perturbations Associated with Severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 Vaccines in Development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently Neutralizing and Protective Human Antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science (1979) 2021, 371. [Google Scholar] [CrossRef]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Rodriguez-Barraquer, I.; et al. A Systematic Review of Antibody Mediated Immunity to Coronaviruses: Antibody Kinetics, Correlates of Protection, and Association of Antibody Responses with Severity of Disease. medRxiv 2020. [Google Scholar] [CrossRef]
- Adams, E.R.; Ainsworth, M.; Anand, R.; Andersson, M.I.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Bell, J.I.; Berry, T.; et al. Antibody Testing for COVID-19: A Report from the National COVID Scientific Advisory Panel. Wellcome Open Res. 2020, 5, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Foo, S.-S.; Bruzzone, R.; Vu Dinh, L.; King, N.J.C.; Mahalingam, S. Fc Receptors in Antibody-Dependent Enhancement of Viral Infections. Immunol. Rev. 2015, 268, 340–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Akondy, R.S.; Fitch, M.; Edupuganti, S.; Yang, S.; Kissick, H.T.; Li, K.W.; Youngblood, B.A.; Abdelsamed, H.A.; McGuire, D.J.; Cohen, K.W.; et al. Origin and Differentiation of Human Memory CD8 T Cells after Vaccination. Nature 2017, 552, 362–367. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn Errors of Type I IFN Immunity in Patients with Life-Threatening COVID-19. Science (1979) 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of Concomitant Immune Responses Prior to Patient Recovery: A Case Report of Non-Severe COVID-19. Nat. Med. 2020, 26, 453–455. [Google Scholar] [CrossRef] [Green Version]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Kamphuis, E.; Junt, T.; Waibler, Z.; Forster, R.; Kalinke, U. Type I Interferons Directly Regulate Lymphocyte Recirculation and Cause Transient Blood Lymphopenia. Blood 2006, 108, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Diao, B.; Wang, R.; Wang, G.; Wang, C.; Tan, Y.; Liu, L.; Wang, C.; Liu, Y.; Liu, Y.; et al. The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Directly Decimates Human Spleens and Lymph Nodes. medRxiv. 2020. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-Cells and Inflammatory Monocytes Incite Inflammatory Storms in Severe COVID-19 Patients. Nat. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Stephens, D.S.; McElrath, M.J. COVID-19 and the Path to Immunity. JAMA 2020, 324, 1279. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The Role of Vitamin D in the Prevention of Coronavirus Disease 2019 Infection and Mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Giannini, S.; Passeri, G.; Tripepi, G.; Sella, S.; Fusaro, M.; Arcidiacono, G.; Torres, M.O.; Michielin, A.; Prandini, T.; Baffa, V.; et al. Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study. Nutrients 2021, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- de Smet, D.; de Smet, K.; Herroelen, P.; Gryspeerdt, S.; Martens, G.A. Serum 25(OH)D Level on Hospital Admission Associated With COVID-19 Stage and Mortality. Am. J. Clin. Pathol. 2021, 155, 381–388. [Google Scholar] [CrossRef]
- Fasano, A.; Cereda, E.; Barichella, M.; Cassani, E.; Ferri, V.; Zecchinelli, A.L.; Pezzoli, G. COVID-19 in Parkinson’s Disease Patients Living in Lombardy, Italy. Mov. Disord. 2020, 35, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- D’Avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; de Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef] [PubMed]
- Ali, N. Role of Vitamin D in Preventing of COVID-19 Infection, Progression and Severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D Activates the Nrf2-Keap1 Antioxidant Pathway and Ameliorates Nephropathy in Diabetic Rats. Am. J. Hypertens. 2014, 27, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.Y.; Yan, S.M.; Guo, Y.M.; Zhang, B.Q.; Guo, X.Y.; Shi, B.L. Vitamin A Pretreatment Protects NO-Induced Bovine Mammary Epithelial Cells from Oxidative Stress by Modulating Nrf2 and NF-ΚB Signaling Pathways. J. Anim. Sci. 2018, 96, 1305–1316. [Google Scholar] [CrossRef]
- Dalan, R.; Bornstein, S.R.; El-Armouche, A.; Rodionov, R.N.; Markov, A.; Wielockx, B.; Beuschlein, F.; Boehm, B.O. The ACE-2 in COVID-19: Foe or Friend? Horm. Metab. Res. 2020, 52, 257–263. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, M.; Chen, H.; Chen, L. Potential Therapeutic Approaches for the Early Entry of SARS-CoV-2 by Interrupting the Interaction between the Spike Protein on SARS-CoV-2 and Angiotensin-Converting Enzyme 2 (ACE2). Biochem. Pharm. 2021, 192, 114724. [Google Scholar] [CrossRef]
- Cereda, E.; Bogliolo, L.; Klersy, C.; Lobascio, F.; Masi, S.; Crotti, S.; de Stefano, L.; Bruno, R.; Corsico, A.G.; di Sabatino, A.; et al. Vitamin D 25OH Deficiency in COVID-19 Patients Admitted to a Tertiary Referral Hospital. Clin. Nutr. 2021, 40, 2469–2472. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Bergman, P. The Link between Vitamin D and COVID-19: Distinguishing Facts from Fiction. J. Intern. Med. 2021, 289, 131–133. [Google Scholar] [CrossRef]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human Serum 25-Hydroxycholecalciferol Response to Extended Oral Dosing with Cholecalciferol. Am. J. Clin. Nutr. 2003, 77, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef] [PubMed]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19. JAMA 2021, 325, 1053. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Kim, T.-K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of Novel CYP11A1-Derived Secosteroids in the Human Epidermis and Serum and Pig Adrenal Gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef] [Green Version]
- Slominski, R.M.; Stefan, J.; Athar, M.; Holick, M.F.; Jetten, A.M.; Raman, C.; Slominski, A.T. COVID-19 and Vitamin D: A Lesson from the Skin. Exp. Derm. 2020, 29, 885–890. [Google Scholar] [CrossRef]
- Slominski, R.M.; Tuckey, R.C.; Manna, P.R.; Jetten, A.M.; Postlethwaite, A.; Raman, C.; Slominski, A.T. Extra-Adrenal Glucocorticoid Biosynthesis: Implications for Autoimmune and Inflammatory Disorders. Genes Immun. 2020, 21, 150–168. [Google Scholar] [CrossRef]
- Slominski, A.T.; Janjetovic, Z.; Kim, T.-K.; Wasilewski, P.; Rosas, S.; Hanna, S.; Sayre, R.M.; Dowdy, J.C.; Li, W.; Tuckey, R.C. Novel Non-Calcemic Secosteroids That Are Produced by Human Epidermal Keratinocytes Protect against Solar Radiation. J. Steroid Biochem. Mol. Biol. 2015, 148, 52–63. [Google Scholar] [CrossRef] [Green Version]
| Authors | Epidemiological Data | Conclusion |
|---|---|---|
| Shahid et al., 2020 [46] | China’s case fatality rate (CFR) in February 2020 was 0.7%, in contrast with individuals older than 80, whose population exhibited a CFR of 21.9%. | The SARS-CoV-2 pandemic has a much higher mortality rate in older adults. |
| Wu et al., 2020 [47] | In a report of 72,314 cases from the Chinese Center for Disease Control and Prevention, the overall case-fatality rate (CFR) was 2.3%, but it rose to 8% in individuals 70–79 years of age and 14.5% if older than 80 years of age. | CFR was elevated among those with pre-existing comorbid conditions—10.5% for cardiovascular disease, 7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension and 5.6% for cancer. |
| Bialek et al., 2020 [48] | In a report of 4226 cases from the United States, the clinical fatality rate (CFR) was 1% in adults younger than 54 years of age, from 3 to 11% in patients 65–84 years of age and 10 to 27% in those older than 85 years of age. | More than 80% of the deaths occurred in individuals older than 65 years of age. |
| Gold et al., 2020 [49] | In a report of 114,411 persons who died from COVID-19 in the United States, the percentage of decedents aged ≥65 years was 77.6%. | Persons aged ≥65 years are disproportionately represented among COVID-19–associated deaths. |
| WHO [50] | In a report of the WHO-China Joint Mission on Coronavirus Disease 2019, the clinical fatality rate was 1.4% in patients without comorbidities, 7.6% in those with cancer, 8% with chronic pulmonary disease, 8.4% with hypertension, 9.2% with diabetes and 13.2% with cardiovascular disease. | Individuals older than 60 years old have a higher risk of severe disease and death. |
| Levin et al., 2020 [51] | The infection fatality rate (IFR) in a meta-analysis of 27 studies rose from 1.4% at age 65, to 4.6% at age 75 and 15% at age 85. | COVID-19 is hazardous not only for the elderly but also for middle-aged adults. |
| ISS [52] | In an Italian report, 38.7% of infected patients were older than 70 years and 69.6% were older than 50 years; 78.4% of deaths were in patients aged between 60 to 89. | The elderly population is more exposed to COVID-19 and at higher risk of poor outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauretani, F.; Salvi, M.; Zucchini, I.; Testa, C.; Cattabiani, C.; Arisi, A.; Maggio, M. Relationship between Vitamin D and Immunity in Older People with COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 5432. https://doi.org/10.3390/ijerph20085432
Lauretani F, Salvi M, Zucchini I, Testa C, Cattabiani C, Arisi A, Maggio M. Relationship between Vitamin D and Immunity in Older People with COVID-19. International Journal of Environmental Research and Public Health. 2023; 20(8):5432. https://doi.org/10.3390/ijerph20085432
Chicago/Turabian StyleLauretani, Fulvio, Marco Salvi, Irene Zucchini, Crescenzo Testa, Chiara Cattabiani, Arianna Arisi, and Marcello Maggio. 2023. "Relationship between Vitamin D and Immunity in Older People with COVID-19" International Journal of Environmental Research and Public Health 20, no. 8: 5432. https://doi.org/10.3390/ijerph20085432
APA StyleLauretani, F., Salvi, M., Zucchini, I., Testa, C., Cattabiani, C., Arisi, A., & Maggio, M. (2023). Relationship between Vitamin D and Immunity in Older People with COVID-19. International Journal of Environmental Research and Public Health, 20(8), 5432. https://doi.org/10.3390/ijerph20085432








