The Effects of 12-Week Dual-Task Physical–Cognitive Training on Gait, Balance, Lower Extremity Muscle Strength, and Cognition in Older Adult Women: A Randomized Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Participants and Eligibility
2.3. Intervention
2.3.1. Dual-Task Training
2.3.2. Education Control Group
2.4. Control of Procedures and Adherence
2.5. Outcome Measures
2.5.1. Primary Outcomes
2.5.2. Secondary Outcomes
2.6. Covariates
2.7. Statistical Analysis
3. Results
3.1. Sample Characteristic
3.2. Descriptive Statistics
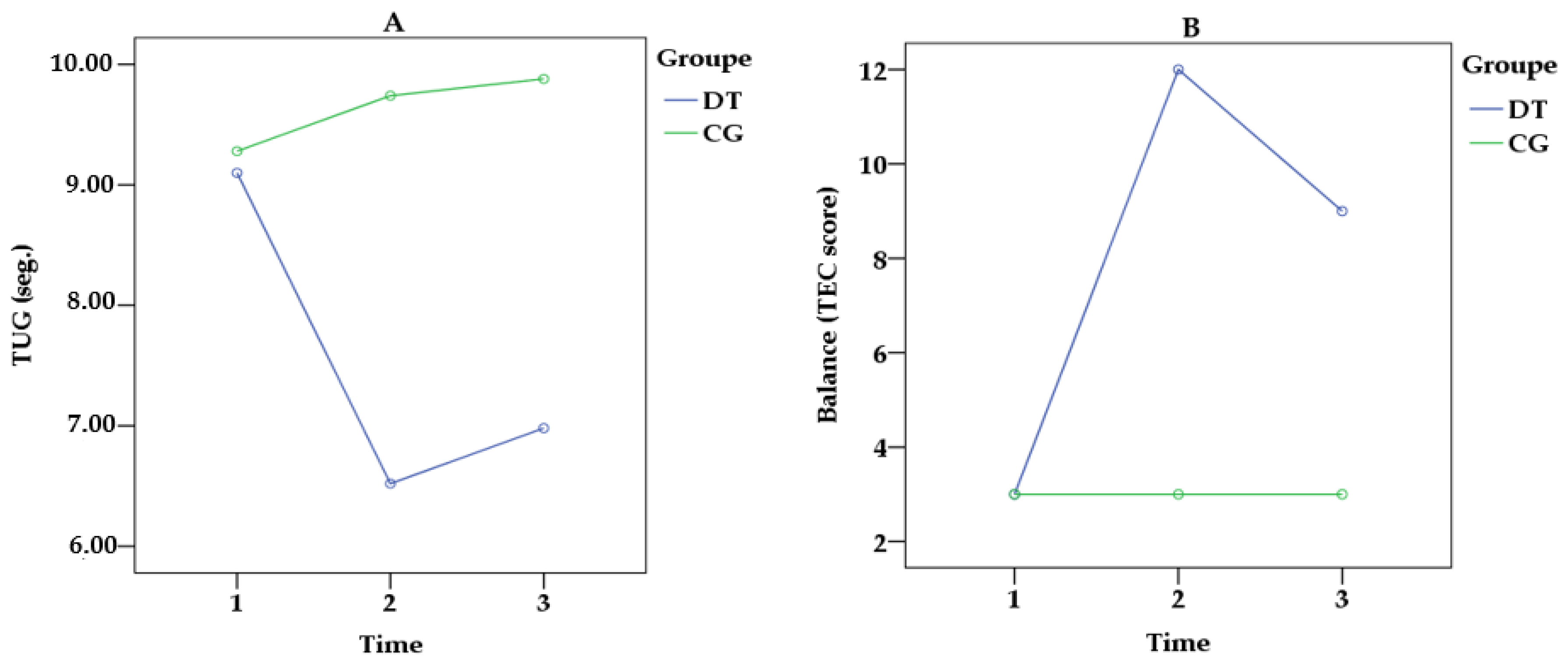
3.2.1. Gait, Body Balance, and Lower Extremity Muscle Strength
3.2.2. Cognition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dziechciaż, M.; Filip, R. Biological psychological and social determinants of old age: Bio-psycho-social aspects of human aging. Ann. Agric. Environ. Med. 2014, 21, 835–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayeni, A.; Sharples, A.; Hewson, D. The association between social vulnerability and frailty in community dwelling older people: A systematic review. Geriatrics 2022, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidet, B.; de Lange, D.W.; Boumendil, A.; Leaver, S.; Watson, X.; Boulanger, C.; Szczeklik, W.; Artigas, A.; Morandi, A.; Andersen, F.; et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: The VIP2 study. Intensive Care Med. 2020, 46, 57–69. [Google Scholar] [CrossRef]
- Costenoble, A.; Knoop, V.; Vermeiren, S.; Vella, R.A.; Debain, A.; Rossi, G.; Bautmans, I.; Verté, D.; Gorus, E.; De Vriendt, P. A comprehensive overview of activities of daily living in existing frailty instruments: A systematic literature search. Gerontologist 2021, 61, e12–e22. [Google Scholar] [CrossRef]
- Hirono, T.; Ikezoe, T.; Yamagata, M.; Kato, T.; Umehara, J.; Yanase, K.; Nakao, S.; Tsuboyama, T.; Tabara, Y.; Matsuda, F.; et al. Age-related changes in gait speeds and asymmetry during circular gait and straight-line gait in older individuals aged 60–79 years. Geriatr. Gerontol. Int. 2021, 21, 404–410. [Google Scholar] [CrossRef]
- Hamacher, D.; Liebl, D.; Hödl, C.; Heßler, V.; Kniewasser, C.K.; Thönnessen, T.; Zech, A. Gait stability and its influencing factors in older adults. Front. Physiol. 2019, 9, 1955. [Google Scholar] [CrossRef]
- Fasano, A.; Plotnik, M.; Bove, F.; Berardelli, A. The neurobiology of falls. Neurol. Sci. 2012, 33, 1215–1223. [Google Scholar] [CrossRef]
- Forbes, P.A.; Chen, A.; Blouin, J.-S. Sensorimotor control of standing balance. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 61–83. ISBN 9780444639165. [Google Scholar]
- Hu, K.; Zhou, Q.; Jiang, Y.; Shang, Z.; Mei, F.; Gao, Q.; Chen, F.; Zhao, L.; Jiang, M.; Ma, B. Association between frailty and mortality, falls, and hospitalization among patients with hypertension: A systematic review and meta-analysis. Biomed Res. Int. 2021, 2021, 2690296. [Google Scholar] [CrossRef]
- Amboni, M.; Barone, P.; Hausdorff, J.M. Cognitive contributions to gait and falls: Evidence and implications. Mov. Disord. 2013, 28, 1520–1533. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Almeida, Q.J.; Burhan, A.M.; Camicioli, R.; Doyon, J.; Fraser, S.; Li, K.; Liu-Ambrose, T.; Middleton, L.; Muir-Hunter, S.; et al. SYNERGIC TRIAL (SYNchronizing Exercises, Remedies in Gait and Cognition) a multi-Centre randomized controlled double blind trial to improve gait and cognition in mild cognitive impairment. BMC Geriatr. 2018, 18, 93. [Google Scholar] [CrossRef] [Green Version]
- Clark, B.C.; Manini, T.M.; Wages, N.P.; Simon, J.E.; Clark, L.A. Voluntary vs electrically stimulated activation in age-related muscle weakness. JAMA Netw. Open 2019, 2, e1912052. [Google Scholar] [CrossRef]
- Shaughnessy, K.A.; Hackney, K.J.; Clark, B.C.; Kraemer, W.J.; Terbizan, D.J.; Bailey, R.R.; McGrath, R. A narrative review of handgrip strength and cognitive functioning: Bringing a new characteristic to muscle memory. J. Alzheimer’s Dis. 2020, 73, 1265–1278. [Google Scholar] [CrossRef]
- Raz, N. Ageing and the Brain. In ELS; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Rabinovici, G.D.; Stephens, M.L.; Possin, K.L. Executive Dysfunction. Contin. Lifelong Learn. Neurol. 2015, 21, 646–659. [Google Scholar] [CrossRef] [Green Version]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [Green Version]
- Oschwald, J.; Guye, S.; Liem, F.; Rast, P.; Willis, S.; Röcke, C.; Jäncke, L.; Martin, M.; Mérillat, S. Brain structure and cognitive ability in healthy aging: A review on longitudinal correlated change. Rev. Neurosci. 2019, 31, 1–57. [Google Scholar] [CrossRef] [Green Version]
- Kolb, B.; Gibb, R. Principles of Neuroplasticity and Behavior. In Cognitive Neurorehabilitation; Stuss, D.T., Winocur, G., Robertson, I.H., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 6–21. ISBN 9781316529898. [Google Scholar]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Toots, A.T.M.; Taylor, M.E.; Lord, S.R.; Close, J.C.T. Associations between gait speed and cognitive domains in older people with cognitive impairment. J. Alzheimer’s Dis. 2019, 71, S15–S21. [Google Scholar] [CrossRef]
- Buracchio, T.J.; Mattek, N.C.; Dodge, H.H.; Hayes, T.L.; Pavel, M.; Howieson, D.B.; Kaye, J.A. Executive function predicts risk of falls in older adults without balance impairment. BMC Geriatr. 2011, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.K.; Lo, J.C.; Lim, J.K.W.; Chee, M.W.L.; Zhou, J. Reduced functional segregation between the default mode network and the executive control network in healthy older adults: A longitudinal study. Neuroimage 2016, 133, 321–330. [Google Scholar] [CrossRef]
- Watson, N.L.; Rosano, C.; Boudreau, R.M.; Simonsick, E.M.; Ferrucci, L.; Sutton-Tyrrell, K.; Hardy, S.E.; Atkinson, H.H.; Yaffe, K.; Satterfield, S.; et al. Executive function, memory, and gait speed decline in well-functioning older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanenko, Y.P.; Cappellini, G.; Dominici, N.; Poppele, R.E.; Lacquaniti, F. Modular control of limb movements during human locomotion. J. Neurosci. 2007, 27, 11149–11161. [Google Scholar] [CrossRef] [Green Version]
- Baggen, R.J.; van Dieën, J.H.; Van Roie, E.; Verschueren, S.M.; Giarmatzis, G.; Delecluse, C.; Dominici, N. Age-related differences in muscle synergy organization during step ascent at different heights and directions. Appl. Sci. 2020, 10, 1987. [Google Scholar] [CrossRef] [Green Version]
- Alizadehsaravi, L.; Bruijn, S.M.; Muijres, W.; Koster, R.A.J.; van Dieën, J.H. Improvement in gait stability in older adults after ten sessions of standing balance training. PLoS ONE 2022, 17, e0242115. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, C.A.; Fukuchi, R.K.; Duarte, M. Effects of walking speed on gait biomechanics in healthy participants: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Phinyomark, A.; Petri, G.; Ibáñez-Marcelo, E.; Osis, S.T.; Ferber, R. Analysis of big data in gait biomechanics: Current trends and future directions. J. Med. Biol. Eng. 2018, 38, 244–260. [Google Scholar] [CrossRef] [Green Version]
- Ruffieux, J.; Keller, M.; Lauber, B.; Taube, W. Changes in standing and walking performance under dual-task conditions across the lifespan. Sport. Med. 2015, 45, 1739–1758. [Google Scholar] [CrossRef] [Green Version]
- Beurskens, R.; Bock, O. Age-related deficits of dual-task walking: A review. Neural Plast. 2012, 2012, 131608. [Google Scholar] [CrossRef] [Green Version]
- McIsaac, T.L.; Lamberg, E.M.; Muratori, L.M. Building a framework for a dual task taxonomy. Biomed Res. Int. 2015, 2015, 591475. [Google Scholar] [CrossRef] [Green Version]
- Bayot, M.; Dujardin, K.; Tard, C.; Defebvre, L.; Bonnet, C.T.; Allart, E.; Delval, A. The interaction between cognition and motor control: A theoretical framework for dual-task interference effects on posture, gait initiation, gait and turning. Neurophysiol. Clin. 2018, 48, 361–375. [Google Scholar] [CrossRef]
- Holtzer, R.; Epstein, N.; Mahoney, J.R.; Izzetoglu, M.; Blumen, H.M. Neuroimaging of mobility in aging: A targeted review. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 1375–1388. [Google Scholar] [CrossRef]
- Kueper, J.K.; Lizotte, D.J.; Montero-Odasso, M.; Speechley, M. Cognition and motor function: The gait and cognition pooled index. PLoS ONE 2020, 15, e0238690. [Google Scholar] [CrossRef]
- Viswanathan, A.; Sudarsky, L. Balance and gait problems in the elderly. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherland, 2012; Volume 103, pp. 623–634. [Google Scholar]
- Bayot, M.; Dujardin, K.; Dissaux, L.; Tard, C.; Defebvre, L.; Bonnet, C.T.; Allart, E.; Allali, G.; Delval, A. Can dual-task paradigms predict Falls better than single task?—A systematic literature review. Neurophysiol. Clin. 2020, 50, 401–440. [Google Scholar] [CrossRef]
- Lee, H.; Sullivan, S.J.; Schneiders, A.G. The use of the dual-task paradigm in detecting gait performance deficits following a sports-related concussion: A systematic review and meta-analysis. J. Sci. Med. Sport 2013, 16, 2–7. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Li, K.Z.H.; Roudaia, E.; Lussier, M.; Bherer, L.; Leroux, A.; McKinley, P.A. Benefits of cognitive dual-task training on balance performance in healthy older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Muir-Hunter, S.W.; Wittwer, J.E. Dual-task testing to predict falls in community-dwelling older adults: A systematic review. Physiother 2016, 102, 29–40. [Google Scholar] [CrossRef]
- Wollesen, B.; Wildbredt, A.; van Schooten, K.S.; Lim, M.L.; Delbaere, K. The effects of cognitive-motor training interventions on executive functions in older people: A systematic review and meta-analysis. Eur. Rev. Aging Phys. Act. 2020, 17, 9. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- Ishigaki, E.Y.; Ramos, L.G.; Carvalho, E.S.; Lunardi, A.C. Effectiveness of muscle strengthening and description of protocols for preventing falls in the elderly: A systematic review. Braz. J. Phys. Ther. 2014, 18, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, A.; Kressig, R.W.; Muehlbauer, T.; Gschwind, Y.J.; Pfenninger, B.; Bruegger, O.; Granacher, U. Effects of a supervised versus an unsupervised combined balance and strength training program on balance and muscle power in healthy older adults: A randomized controlled trial. Gerontology 2016, 62, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Muehlbauer, T.; Gollhofer, A.; Granacher, U. Associations between measures of balance and lower-extremity muscle strength/power in healthy individuals across the lifespan: A systematic review and meta-analysis. Sport. Med. 2015, 45, 1671–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Valentine, R.J.; Evans, E.M.; Sosnoff, J.J. Lower extremity muscle quality and gait variability in older adults. Age Ageing 2012, 41, 595–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrose, A.F.; Paul, G.; Hausdorff, J.M. Risk factors for falls among older adults: A review of the literature. Maturitas 2013, 75, 51–61. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Speechley, M.; Muir-Hunter, S.W.; Sarquis-Adamson, Y.; Sposato, L.A.; Hachinski, V.; Borrie, M.; Wells, J.; Black, A.; Sejdić, E.; et al. Motor and cognitive trajectories before dementia: Results from gait and brain study. J. Am. Geriatr. Soc. 2018, 66, 1676–1683. [Google Scholar] [CrossRef]
- Pieruccini-Faria, F.; Lord, S.R.; Toson, B.; Kemmler, W.; Schoene, D. Mental flexibility influences the association between poor balance and falls in older people—A secondary analysis. Front. Aging Neurosci. 2019, 11, 133. [Google Scholar] [CrossRef] [Green Version]
- Morrell, M.; Saadi, Z.; Lam, L.; Talving, P.; Demetriades, D. Falls in the elderly: A modern look at an old problem. Am. J. Surg. 2014, 208, 249–253. [Google Scholar]
- Crenshaw, J.R.; Bernhardt, K.A.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Kaufman, K.R.; Amin, S. The circumstances, orientations, and impact locations of falls in community-dwelling older women. Arch. Gerontol. Geriatr. 2017, 73, 240–247. [Google Scholar] [CrossRef]
- Alamgir, H.; Muazzam, S.; Nasrullah, M. Unintentional falls mortality among elderly in the United States: Time for action. Injury 2020, 43, 2065–2071. [Google Scholar] [CrossRef]
- Stevens, J.A.; Sogolow, E.D. Gender differences for non-fatal unintentional fall related injuries among older adults. Inj. Prev. 2005, 11, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Teraz, K.; Šlosar, L.; Paravlić, A.H.; de Bruin, E.D.; Marusic, U. Impact of motor-cognitive interventions on selected gait and balance outcomes in older adults: A systematic review and meta-analysis of randomized controlled trials. Front. Psychol. 2022, 13, 837710. [Google Scholar] [CrossRef]
- Silsupadol, P.; Shumway-Cook, A.; Lugade, V.; van Donkelaar, P.; Chou, L.S.; Mayr, U.; Woollacott, M.H. Effects of single-task versus dual-task training on balance performance in older adults: A double-blind, randomized controlled. Arch. Phys. Med. Rehabil. 2009, 90, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Liebherr, M.; Schubert, P.; Schiebener, J.; Kersten, S.; Haas, C.T. Dual-tasking and aging-About multiple perspectives and possible implementations in interventions for the elderly. Cogent Psychol. 2016, 3, 1261440. [Google Scholar] [CrossRef]
- Falbo, S.; Condello, G.; Capranica, L.; Forte, R.; Pesce, C. Effects of physical-cognitive dual task training on executive function and gait performance in older adults: A randomized controlled trial. Biomed Res. Int. 2016, 2016, 5812092. [Google Scholar] [CrossRef] [Green Version]
- Plummer, P.; Zukowski, L.A.; Giuliani, C.; Hall, A.M.; Zurakowski, D. Effects of physical exercise interventions on gait-related dual-task interference in older adults: A systematic review and meta-analysis. Gerontology 2016, 62, 94–117. [Google Scholar] [CrossRef]
- Cepellos, V.M. Feminization of aging: A multifaceted phenomenon beyond the numbers. Rev. Adm. Empres. 2021, 61, e20190861. [Google Scholar] [CrossRef]
- IBGE-Brazilian Institute of Geography and Statistics. Projection of the Population of Brazil by Sex and Age: 2000–2060. Available online: https://ww2.ibge.gov.br/home/estatistica/populacao/projecao_da_populacao/2013/default.shtm (accessed on 23 January 2023).
- Noce Kirkwood, R.; de Souza Moreira, B.; Mingoti, S.A.; Faria, B.F.; Sampaio, R.F.; Alves Resende, R. The slowing down phenomenon: What is the age of major gait velocity decline? Maturitas 2018, 115, 31–36. [Google Scholar] [CrossRef]
- IBGE-Brazilian Institute of Geography and Statistics IBGE Releases Estimate of the Population of Municipalities for 2020. Available online: https://agenciadenoticias.ibge.gov.br/agencia-sala-de-imprensa/2013-agencia-de-noticias/releases/28668-ibge-divulga-estimativa-da-populacao-dos-municipios-para-2020 (accessed on 10 March 2023).
- Silsupadol, P.; Lugade, V.; Shumway-Cook, A.; van Donkelaar, P.; Chou, L.-S.; Mayr, U.; Woollacott, M.H. Training-related changes in dual-task walking performance of elderly persons with balance impairment: A double-blind, randomized controlled trial. Gait Posture 2009, 29, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Brucki, S.; Nitrini, R.; Caramelli, P.; Bertolucci, P.H.; Okamoto, I.H. Suggestions for utilization of the mini-mental state examination in Brazil. Arq. Neuropsiquiatr. 2003, 61, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, M.d.M.; Maduro, P.A.; Rios, P.M.B.; Nascimento, L.d.S.; Silva, C.N.; Kliegel, M.; Ihle, A. Effects of 12 weeks of physical-cognitive dual-task training on executive functions, depression, sleep quality, and quality of life in older adult women: A randomized pilot study. Sustainability 2022, 15, 97. [Google Scholar] [CrossRef]
- Nascimento, M.d.M.; Ramos, L.S.; Gomes, A.V.T.M.; Maia, N.J.S. Health education at a university open to the elderly: The experience of medical students. REVASF 2020, 10, 55–83. [Google Scholar]
- Sherrington, C.; Tiedemann, A.; Fairhall, N.; Close, J.C.T.; Lord, S.R. Exercise to prevent falls in older adults: An updated meta-analysis and best practice recommendations. NSW Public Health Bull. 2011, 22, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soubra, R.; Chkeir, A.; Novella, J.-L. A systematic review of thirty-one assessment tests to evaluate mobility in older adults. Biomed Res. Int. 2019, 2019, 1354362. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.D.M.; Silva, P.S.T. Sensory assessment of balance and estimation of the risk of falling in old women practicing Pilates mate. Arq. Ciências Saúde 2020, 27, 11. [Google Scholar] [CrossRef]
- Martins, N.I.M.; Caldas, P.R.; Cabral, E.D.; Lins, C.C.D.S.A.; Coriolano, M.d.G.W.d.S. Cognitive assessment instruments used in elderly Brazilians in the last five years. Cien. Saude Colet. 2019, 24, 2513–2530. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Wydra, G. Bedeutung, diagnose und therapie von gleichgewichtstörung. Motorik 1993, 16, 100–107. [Google Scholar]
- Nascimento, M.; Coriolano Appell, H.-J. Teste de equilíbrio corporal (TEC) para idosos independentes. Rev. Port. Cienc. Desp. 2012, 12, 72–82. [Google Scholar]
- Brucki, S.M.D.; Rocha, M.S.G. Category fluency test: Effects of age, gender and education on total scores, clustering and switching in Brazilian Portuguese-speaking subjects. Braz. J. Med. Biol. Res. 2004, 37, 1771–1777. [Google Scholar] [CrossRef] [Green Version]
- Rikli, R.E.; Jones, C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef]
- Khow, K.S.; Visvanathan, R. Falls in the aging population. Clin. Geriatr. Med. 2017, 33, 357–368. [Google Scholar] [CrossRef]
- Öhlin, J.; Ahlgren, A.; Folkesson, R.; Gustafson, Y.; Littbrand, H.; Olofsson, B.; Toots, A. The association between cognition and gait in a representative sample of very old people-the influence of dementia and walking aid use. BMC Geriatr. 2020, 20, 34. [Google Scholar] [CrossRef]
- Hsu, C.L.; Nagamatsu, L.S.; Davis, J.C.; Liu-Ambrose, T. Examining the relationship between specific cognitive processes and falls risk in older adults: A systematic review. Osteoporos. Int. 2012, 23, 2409–2424. [Google Scholar] [CrossRef] [Green Version]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. Quantitative gait markers and incident fall risk in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 896–901. [Google Scholar] [CrossRef] [Green Version]
- Gillain, S.; Boutaayamou, M.; Schwartz, C.; Dardenne, N.; Bruyère, O.; Brüls, O.; Croisier, J.-L.; Salmon, E.; Reginster, J.-Y.; Garraux, G.; et al. Gait symmetry in the dual task condition as a predictor of future falls among independent older adults: A 2-year longitudinal study. Aging Clin. Exp. Res. 2019, 31, 1057–1067. [Google Scholar] [CrossRef] [Green Version]
- Gandelman, J.; Ureste, P. Meta-analysis of the Impact of Nine Medication Classes on Falls in Elderly Persons. In Essential Reviews in Geriatric Psychiatry; Springer International Publishing: Cham, Switzerland, 2022; Volume 169, pp. 15–19. [Google Scholar]
- Helgadóttir, B.; Laflamme, L.; Monárrez-Espino, J.; Möller, J. Medication and fall injury in the elderly population; do individual demographics, health status and lifestyle matter? BMC Geriatr. 2014, 14, 92. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Hu, D.; Shi, X.; Sun, L.; Zhu, X.; Yuan, H.; Yang, Y.; Zhang, Y.; Zhao, Y.; Hu, C.; et al. Comorbidity increased the risk of falls in chinese older adults: A cross-sectional study. Int. J. Clin. Exp. Med. 2017, 10, 10753–10763. [Google Scholar]
- Bherer, L. Cognitive plasticity in older adults: Effects of cognitive training and physical exercise. Ann. N. Y. Acad. Sci. 2015, 1337, 1–6. [Google Scholar] [CrossRef]
- Cohen, J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Amato, P.P.; Kyvelidou, A.; Sternad, D.; Najafi, B.; Villalobos, R.M.; Zurakowski, D. Training dual-task walking in community-dwelling adults within 1 year of stroke: A protocol for a single-blind randomized controlled trial. BMC Neurol. 2012, 12, 129. [Google Scholar]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-H.; Tang, P.-F.; Peng, Y.-C.; Chen, H.-Y. Meta-analysis of type and complexity of a secondary task during walking on the prediction of elderly falls. Geriatr. Gerontol. Int. 2013, 13, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Cusack, T.; Blake, C. The effect of a dual task on gait speed in community dwelling older adults: A systematic review and meta-analysis. Gait Posture 2016, 44, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Tasvuran Horata, E.; Cetin, S.Y.; Erel, S. Effects of individual progressive single- and dual-task training on gait and cognition among older healthy adults: A randomized-controlled comparison study. Eur. Geriatr. Med. 2021, 12, 363–370. [Google Scholar] [CrossRef]
- Agmon, M.; Belza, B.; Nguyen, H.Q.; Logsdon, R.; Kelly, V.E. A systematic review of interventions conducted in clinical or community settings to improve dual-task postural control in older adults. Clin. Interv. Aging 2014, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, P.L.; Solomont, J.; Kowall, N.; Hausdorff, J.M. Influence of executive function on locomotor function: Divided attention increases gait variability in Alzheimer’s disease. J. Am. Geriatr. Soc. 2003, 51, 1633–1637. [Google Scholar] [CrossRef] [Green Version]
- Wollesen, B.; Voelcker-Rehage, C. Differences in cognitive-motor interference in older adults while walking and performing a visual-verbal stroop task. Front. Aging Neurosci. 2019, 10, 426. [Google Scholar] [CrossRef] [Green Version]
- Smith-Ray, R.L.; Hughes, S.L.; Prohaska, T.R.; Little, D.M.; Jurivich, D.A.; Hedeker, D. Impact of cognitive training on balance and gait in older adults. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2015, 70, 357–366. [Google Scholar] [CrossRef]
- Gobbo, S.; Bergamin, M.; Sieverdes, J.C.; Ermolao, A.; Zaccaria, M. Effects of exercise on dual-task ability and balance in older adults: A systematic review. Arch. Gerontol. Geriatr. 2014, 58, 177–187. [Google Scholar] [CrossRef]
- Nematollahi, A.; Kamali, F.; Ghanbari, A.; Etminan, Z.; Sobhani, S. Improving balance in older people: A double-blind randomized clinical trial of three modes of balance training. J. Aging Phys. Act. 2016, 24, 189–195. [Google Scholar] [CrossRef]
- Varela-Vásquez, L.A.; Minobes-Molina, E.; Jerez-Roig, J. Dual-task exercises in older adults: A structured review of current literature. J. Frailty Sarcopenia Falls 2020, 5, 31–37. [Google Scholar] [CrossRef]
- Goble, D.J.; Brar, H.; Brown, E.C.; Marks, C.R.; Baweja, H.S. Normative data for the balance tracking system modified clinical test of sensory integration and balance protocol. Med. Devices Evid. Res. 2019, 12, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Zhang, X.; Mao, M.; Sun, W.; Zhang, C.; Chen, Y.; Li, L. Relationship of proprioception, cutaneous sensitivity, and muscle strength with the balance control among older adults. J. Sport Health Sci. 2021, 10, 585–593. [Google Scholar] [CrossRef]
- Osoba, M.Y.; Rao, A.K.; Agrawal, S.K.; Lalwani, A.K. Balance and gait in the elderly: A contemporary review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Chang, R. Anatomy of the vestibular system: A review. NeuroRehabilitation 2013, 32, 437–443. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, C.-C.; Zhao, C.-G.; Hwang, I.-S. Visual effect on brain connectome that scales feedforward and feedback processes of aged postural system during unstable stance. Front. Aging Neurosci. 2021, 13, 679412. [Google Scholar] [CrossRef]
- Oswald, F.; Wahl, H.-W.; Schilling, O.; Nygren, C.; Fange, A.; Sixsmith, A.; Sixsmith, J.; Szeman, Z.; Tomsone, S.; Iwarsson, S. Relationships between housing and healthy aging in very old age. Gerontologist 2007, 47, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Xia, J.; Zhang, X.I.; Gathirua-Mwangi, W.G.; Guo, J.; Li, Y.; McKenzie, S.; Song, Y. Associations of muscle mass and strength with all-cause mortality among us older adults. Med. Sci. Sport. Exerc. 2018, 50, 458–467. [Google Scholar] [CrossRef]
- Liu, C.; Shiroy, D.M.; Jones, L.Y.; Clark, D.O. Systematic review of functional training on muscle strength, physical functioning, and activities of daily living in older adults. Eur. Rev. Aging Phys. Act. 2014, 11, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, D.K.; Banducci, S.E.; Daugherty, A.M.; Fanning, J.; Awick, E.A.; Porter, G.C.; Burzynska, A.; Shen, S.; Kramer, A.F.; McAuley, E. Effects of gait self-efficacy and lower-extremity physical function on dual-task performance in older adults. Biomed Res. Int. 2017, 2017, 8570960. [Google Scholar] [CrossRef] [Green Version]
- Hairi, N.N.; Cumming, R.G.; Naganathan, V.; Handelsman, D.J.; Le Couteur, D.G.; Creasey, H.; Waite, L.M.; Seibel, M.J.; Sambrook, P.N. Loss of muscle strength, mass (Sarcopenia), and quality (Specific Force) and its relationship with functional limitation and physical disability: The concord health and ageing in men project. J. Am. Geriatr. Soc. 2010, 58, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Koch, I.; Poljac, E.; Müller, H.; Kiesel, A. Cognitive structure, flexibility, and plasticity in human multitasking—An integrative review of dual-task and task-switching research. Psychol. Bull. 2018, 144, 557–583. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, A.; Tyburski, E.; Sołtys, A.; Karabanowicz, E. Sex differences in verbal fluency among young adults. Adv. Cogn. Psychol. 2020, 16, 92–102. [Google Scholar] [CrossRef]
- Varjacic, A.; Mantini, D.; Demeyere, N.; Gillebert, C.R. Neural signatures of Trail Making Test performance: Evidence from lesion-mapping and neuroimaging studies. Neuropsychologia 2018, 115, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Raz, N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 2014, 42, 180–192. [Google Scholar] [CrossRef] [Green Version]

| Focus | Training Tasks |
|---|---|
| Gait | (a) walking with short and wide steps, on the heels and tiptoes, on the back |
| Static balance | (a) biped, semi-tandem, tandem, single leg stance, weight on feet (hip and ankle postural stabilization strategies); (b) careful inclusion of switching between open and closed eyes. |
| Dynamic balance | (a) walking in different directions on a soft, unstable, or reduced surface (hip and ankle postural stabilization strategies); (b) inclusion of arm movements outside the center of pressure. |
| Cognitive task | (a) calculations and countdown (100, 97, 94, 91, 89, …); (b) verbal fluency (name fruits, people, or cities starting with different letters of the alphabet); (c) memorization (memorizing a sequence of 3–5 different words and after reproducing); (d) visual tasks and word spelling (i.e., reaction time: react as quickly as possible), talking with colleagues. |
| Focus | Strategies for Progression |
|---|---|
| Gait | (a) slow or fast, with short or wide steps, on the heels or on the tip of the feet, forward, backwards, low or high level, diagonals, overtake obstacles; (b) perform curves and/or turns (180°, 360°); (c) combine with the manipulation of objects, or take objects on the floor; (d) sensory input: impaired vision, enhancement of somatosensory integration. |
| Static balance | (a) variation in the demand of hip and/or ankle strategies; (b) variation in surfaces: soft/hard, stable/unstable, wide/reduced. |
| Dynamic balance | (a) walk combined with arm movements outside the pressure center (COP); (b) backward gait; (c) surface change: soft/hard, stable/unstable, wide/reduced. |
| Cognitive task | (a) progressive increase in the difficulty of counting/memorization tasks; (b) order/sequence of numbers/words; (c) variation in response time; (d) alternation of task length; (e) Stroop tasks (i.e., an alternating combination of incongruent and congruent tasks). |
| Variable | Dual-Task (n = 22) | Control Group (n = 22) | p-Value |
|---|---|---|---|
| Age (years) | 66.14 ± 4.15 | 66.27 ± 4.04 | 0.913 |
| BMI (kg/m2) (n) | 27.68 ± 3.93 | 28.18 ± 4.67 | 0.703 |
| Falls (12 months) (n) | 0.27 ± 0.19 | 0.185 ± 0.21 | 0.132 |
| Medication n (%) | 0.161 | ||
| 1–4 types | 20 (90.9%) | 19 (86.3%) | |
| >4 types | 2 (9.0%) | 3 (13.6%) | |
| Education n (%) | 0.574 | ||
| 1–4 years | 3 (13.6) | 4 (18.1) | |
| ≥5 years | 19 (86.3) | 18 (81.8) | |
| MMSE (n) | 25.27 ± 1.38 | 25.32 ± 3.57 | 0.688 |
| Comorbidities n (%) | |||
| Diabetes Mellitus Yes (f) | 4 (18.1) | 18 (81.8) | 0.545 |
| Hypertension Yes (f) | 9 (40.9) | 13 (59.0) | 0.680 |
| Visual acuity Yes (f) | 20 (90.9) | 2 (9.0) | 0.761 |
| Hearing | 0.550 | ||
| Yes (f) | 11 (50.0) | 12 (54.5) | |
| Labyrinthitis Yes (f) | 4 (18.1) | 2 (9.0) | 0.079 |
| Osteoporosis Yes (f) | 14 (63.6) | 8 (36.3) | 0.294 |
| Rheumatism | 0.488 | ||
| Yes (f) | 6 (27.2) | 16 (72.7) |
| Variable | Dual-Task | Control Group | Time | Time * Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Baseline) | (12 Weeks) | (24 Weeks) | (Baseline) | (12 Weeks) | (24 Weeks) | F | p | ηp2 | F | p | ηp2 | |
| n = 22 | n = 22 | n = 22 | n = 22 | n = 22 | n = 22 | |||||||
| Gait | ||||||||||||
| TUG (s) | 9.10 ± 1.88 | 6.52 ± 0.79 | 6.98 ± 1.17 | 9.28 ± 1.59 | 9.74 ± 1.59 | 9.88 ± 1.31 | 9.043 | 0.006 | 0.698 | 10.470 | <0.001 | 0.768 |
| TUGm (s) | 9.68 ± 2.14 | 6.74 ± 0.89 | 7.66 ± 1.32 | 9.55 ± 1.46 | 9.82 ± 1.26 | 9.94 ± 1.21 | 10.638 | 0.008 | 0.652 | 8.321 | <0.001 | 0.745 |
| TUGc (s) | 11.14 ± 2.43 | 9.31 ± 0.86 | 9.78 ± 1.41 | 11.18 ± 2.25 | 11.25 ± 1.53 | 11.10 ± 1.78 | 8.695 | 0.021 | 0.622 | 9.452 | 0.014 | 0.738 |
| Balance | ||||||||||||
| TEC | 3.00 ± 2.5 | 12.00 ± 2.7 | 9.00 ± 3.1 | 3.00 ± 2.0 | 3.00 ± 2.4 | 3.00 ± 2.0 | 10.143 | <0.001 | 0.939 | 11.125 | <0.001 | 0.989 |
| Muscle strength | ||||||||||||
| LEMS | 11 ± 4.2 | 20 ± 3.1 | 16 ± 4.3 | 11 ± 3.4 | 11 ± 3.5 | 11 ± 3.2 | 11.024 | <0.001 | 0.892 | 12.108 | <0.001 | 0.934 |
| Cognition | ||||||||||||
| VF-Total (n) | 15.00 ± 3.7 b,c | 20.00 ± 4.0 | 19.00 ± 3.1 | 15.00 ± 2.9 | 16.00 ± 3.2 | 15.00 ± 2.7 | 6.301 | 0.018 | 0.334 | 9.754 | <0.001 | 0.388 |
| VF-Category | 4.10 ± 0.91 | 5.14 ± 1.26 | 4.80 ± 0.80 | 4.11 ± 1.07 | 4.20 ± 0.83 | 4.00 ± 0.63 | 4.622 | 0.079 | 0.282 | 4.109 | 0.145 | 0.298 |
| VF-Grouping | 1.16 ± 1.17 b,c | 3.84 ± 1.58 | 3.21 ± 1.41 | 1.22 ± 1.20 | 1.28 ± 1.24 | 1.21 ± 1.11 | 6.482 | 0.032 | 0.196 | 6.278 | 0.038 | 0.214 |
| VF-Exchange | 0.60 ± 1.26 b,c | 1.60 ± 1.75 c | 1.30 ± 1.83 | 0.64 ± 1.44 | 0.80 ± 1.80 | 0.70 ± 1.03 | 8.781 | 0.002 | 0.189 | 8.253 | 0.012 | 0.247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, M.d.M.; Maduro, P.A.; Rios, P.M.B.; Nascimento, L.d.S.; Silva, C.N.; Kliegel, M.; Ihle, A. The Effects of 12-Week Dual-Task Physical–Cognitive Training on Gait, Balance, Lower Extremity Muscle Strength, and Cognition in Older Adult Women: A Randomized Study. Int. J. Environ. Res. Public Health 2023, 20, 5498. https://doi.org/10.3390/ijerph20085498
Nascimento MdM, Maduro PA, Rios PMB, Nascimento LdS, Silva CN, Kliegel M, Ihle A. The Effects of 12-Week Dual-Task Physical–Cognitive Training on Gait, Balance, Lower Extremity Muscle Strength, and Cognition in Older Adult Women: A Randomized Study. International Journal of Environmental Research and Public Health. 2023; 20(8):5498. https://doi.org/10.3390/ijerph20085498
Chicago/Turabian StyleNascimento, Marcelo de Maio, Paula Andreatta Maduro, Pâmala Morais Bagano Rios, Lara dos Santos Nascimento, Carolina Nascimento Silva, Matthias Kliegel, and Andreas Ihle. 2023. "The Effects of 12-Week Dual-Task Physical–Cognitive Training on Gait, Balance, Lower Extremity Muscle Strength, and Cognition in Older Adult Women: A Randomized Study" International Journal of Environmental Research and Public Health 20, no. 8: 5498. https://doi.org/10.3390/ijerph20085498






