Abstract
The present systematic review is aimed at evaluating the diuretic effects determined according to the natural mineral water consumption on healthy individuals. This systematic review has been performed following the guidelines of the PRISMA (preferred reporting items for systematic reviews and meta-analyses) Statement, investigating PubMed, Scopus, Web of Science and Cochrane Library from inception to November 2022. Studies performed both on animals and on humans were considered. After screening, a total of 12 studies have been identified. Of these, 11 studies were performed in Italy and 1 in Bulgaria. The time range of publication is very wide, ranging from 1962 to 2019 for human studies and from 1967 to 2001 for animal studies. All the included studies found an increase in diuresis determined according to the consumption of natural mineral water, in some cases after just one administration of the tested water. However, the quality of the studies is not so high, especially for the research conducted many years ago. Thus, it would be desirable to carry out new clinical studies using more appropriate methodological approaches and more refined methods of statistical data processing.
1. Introduction
Natural mineral water, according to the Directive EU 2009/54/EC, is defined as “microbiologically wholesome water, originating in an underground water table or deposit and emerging from a spring at one or more natural or bore exits” [1]. Based on this directive, mineral water can present some indications according to specific criteria, as follows: contains bicarbonate (bicarbonate content greater than 600 mg/L), contains sulphate (sulphate content greater than 200 mg/L), contains chloride (chloride content greater than 200 mg/L), contains calcium (calcium content greater than 150 mg/L), contains magnesium (magnesium content greater than 50 mg/L), contains fluoride (fluoride content greater than 1 mg/L), contains iron (bivalent iron content greater than 1 mg/L), acidic (free carbon dioxide content greater than 250 mg/L), contains sodium (sodium content greater than 200 mg/L). In addition, some mineral waters can be suitable for the preparation of infant food, suitable for a low-sodium diet (sodium content less than 20 mg/L), and they may be laxative or may be diuretic [1]. Additionally, mineral waters can be classified according to their physical and chemical characteristics, such as pH, temperature, and fixed residue at 180 °C. In particular, based on pH, mineral waters can be classified as acid water or alkaline water if they have, respectively, a pH < 7 or >7. Moreover, according to the temperature at source, mineral waters are described as cold (<20 °C), hypothermal (20–30 °C), mesothermal waters (30–40 °C) and hyperthermal waters (>40 °C). Additionally, mineral waters are classified according to the fixed residue at 180°, which indicates the mg of residual mineral salts after the evaporation of 1 L of water at 180 °C. According to the fixed residue at 180 °C, mineral waters are defined as very low mineral content water (<50 mg/L), low mineral content water (50–500 mg/L), medium mineral content water (500–1500 mg/L), and rich mineral content water (>1500 mg/L) [1,2].
The consumption of natural mineral waters can contribute to a proper hydration, even if there is no general consensus on the adequate daily total water intake because body weight, water balance, and plasma osmolality are relevant end points for determining the optimal fluid intake; however, there is a lack of scientific evidence in this field [3,4]. Additionally, natural mineral waters can present one or more properties favourable to health, both in terms of compensation for mineral deficiencies and specific positive effects on different apparatuses. Indeed, drinking natural mineral waters means quenching thirst and ensuring the intake of oligoelements such as calcium, sodium, magnesium, sulphur, bicarbonates, fluorine, which are fundamental, together with water, for the natural course of biochemical reactions and physiological processes [2]. For example, calcium-rich mineral waters are a very relevant source of highly bioavailable calcium with positive effects on bone biomarkers and densitometric parameters [5]. Additionally, an adequate magnesium intake seems to be useful for preventing atherosclerosis, eclampsia and insulin resistance, and for maintaining bone health, consequently preventing osteoporosis [6]. In addition, it is known that natural mineral water can have an essential role in digestive processes, nutrients’ absorption, the elimination of metabolic wastes, and can participate in the proper functioning of the circulatory system [7,8,9,10,11]. Additionally, drinking mineral water unchangeably, with respect to switching to tap water, is associated with a decrease in all-cause mortality in elderly people. Additionally, specific positive effects were demonstrated in older age: drinking natural water rich in calcium is useful to prevent insomnia, osteoporosis and tooth loss; drinking water rich in magnesium seems to protect against atherosclerosis, ischaemic heart disease, arrhythmias, sudden death, and cerebrovascular diseases; drinking sodium bicarbonate mineral water has been related to a significant reduction in total cholesterol, low-density lipoprotein cholesterol, and glycaemia [12].
All these properties are ensured by the stable composition over time of each natural mineral water, as requested by national authorities when recognizing a new mineral water. In addition, more recently, other possible positive effects for human health determined as being caused by natural mineral waters have been evaluated. For example, a study was performed to evaluate the potential therapeutic properties of mineral water in metabolic syndrome and the results suggested that water minimally mineralized and those rich in specific macronutrients (bicarbonate, sulphate and magnesium), in combination with a low-calorie diet, may contribute to control glucose levels and blood lipid in subjects affected by metabolic syndrome [13]. Another study carried out to investigate the possible role of mineral water in intestinal inflammation verified that calcium and magnesium sulphate, sodium chloride, carbonic, and ferruginous water or bicarbonate one present positive effects on intestinal inflammation and the mucosa-associated microbiota [14].
The consumption of mineral water is especially beneficial for the urinary tract, through the several functions pursued by water at this level, with particular consideration for the diuretic effect [15,16]. Mineral waters with specific features can elicit different diuretic effects by triggering peculiar mechanisms. Firstly, it was thought that just the consumption of waters characterized by a low or a very low mineral content were responsible for increasing diuresis; more recently, it was demonstrated that mineral waters with medium and rich degree of mineralization can also have diuretic effects as a result of their specific composition. Indeed, the increase in diuresis following water ingestion does not depend only on the water hypotonia, but also on the presence and relative concentrations of anions and cations characterizing the water itself [17]. Therefore, until a few years ago, only oligo-mineral water was recommended to increase diuresis, while, currently, the guidance is changing and medium or highly mineralized waters are also considered useful for this purpose. However, the most appropriate type of water for increasing diuresis is still under debate. The present systematic review is aimed at evaluating the diuretic effects determined by natural mineral water consumption on healthy individuals.
2. Materials and Methods
2.1. Selection Protocol
This systematic review has been performed following the guidelines of the PRISMA (preferred reporting items for systematic reviews and meta-analyses) Statement [18]. The review protocol was registered on PROSPERO database (registration number: CRD42021270968).
2.2. Strategy of Research
The following bibliographic and citation databases have been screened: PubMed (Medline), Scopus, Web of Science (Science and Social Science Citation Index) and Cochrane Library. The research has been performed using the keywords and MeSH terms using the Booleans operators AND–OR and the following words: “bottle water”, “tap water”, “thermal water”, “drinking water”, “mineral water”, “diuresis”, “diuretic”. The research considered all the articles up to 16 November 2022.
We considered the following inclusion criteria: all the original articles aimed at evaluating the diuretic effects of water consumption, published in any language (further selection including just those written in English or Italian), performed in vivo on animals or on humans. Case reports, case series, letters to editors, commentaries, editorials, critical, systematic review and/or meta-analysis and other articles reporting no new objective data have been excluded from selection. The references cited in critical, systematic review and/or meta- analysis have been screened for further eligible articles. No temporal limits have been set. According to the exclusion criteria, all the articles not responding to the review aim and to the predetermined inclusion criteria have been rejected.
All the references derived from the bibliographic research have been transferred to the reference software Zotero systematic review manager for duplicate removal and the further evaluation of the relevance of each article. Particularly, four investigators (M.V., A.D.G., D.M., C.P.) independently screened all of the potentially eligible studies through the reading of the title and abstract to evaluate the agreement to inclusion criteria. Furthermore, the same investigators independently and integrally read the articles considered potentially relevant. Any disagreement was solved through a discussion among the investigators.
2.3. Quality Evaluation of the Studies
At the end of the articles review process, the eligible full texts included seven clinical studies on human beings [19,20,21,22,23,24,25] and five on animals [26,27,28,29,30].
To date, concerning the bias risk evaluation on humans, there is not a universally accepted checklist already used for evaluating the methodological quality of clinical studies focusing on other therapeutic approaches but pharmacological ones. Therefore, following what had already been performed in other systematic reviews dedicated to not pharmacological treatments [31,32,33] in the same specific field [34,35], we used the checklist CLEAR NPT (checklist to evaluate a report of a nonpharmacological trial), for internal validation, which was specifically devised for not-pharmacological clinical trials by a group of experts using the Delphi method [36]. This checklist is built up of 10 questions for which a yes/no/not clear answer is possible. According to what was established by its authors [35], a score ranging from 10 to 8 corresponds to a low risk of bias, a score ranging from 7 to 5 means a medium risk of bias and a score lower than 5 represents a high risk of bias.
The risk of bias for studies on animals has been evaluated using the checklist SYRCLE’s RoB tool (risk of bias tool elaborated by the systematic review centre for laboratory animal experimentation) [37,38]. This checklist is made up of 10 questions investigating the following bias: selection, performance, detection, attrition, reporting and other possible sources. To the 10 answers. a yes/no/not clear answer is possible; any “yes” represents a low risk of bias, any “no” stands for a high risk of bias, whereas any “not clear” states that the reported data are scarce to evaluate the specific risk of bias. In the case of this checklist, as recommended by the researchers who elaborated it [38], an overall score for each study has not been determined in order not to assign a particular importance to a specific dominion which will be difficult to justify.
Four investigators (M.V., A.D.G., D.M., C.P.) independently evaluated the quality of the articles included in the review using the above-described checklists; any disagreement has been solved through discussion.
3. Results and Discussion
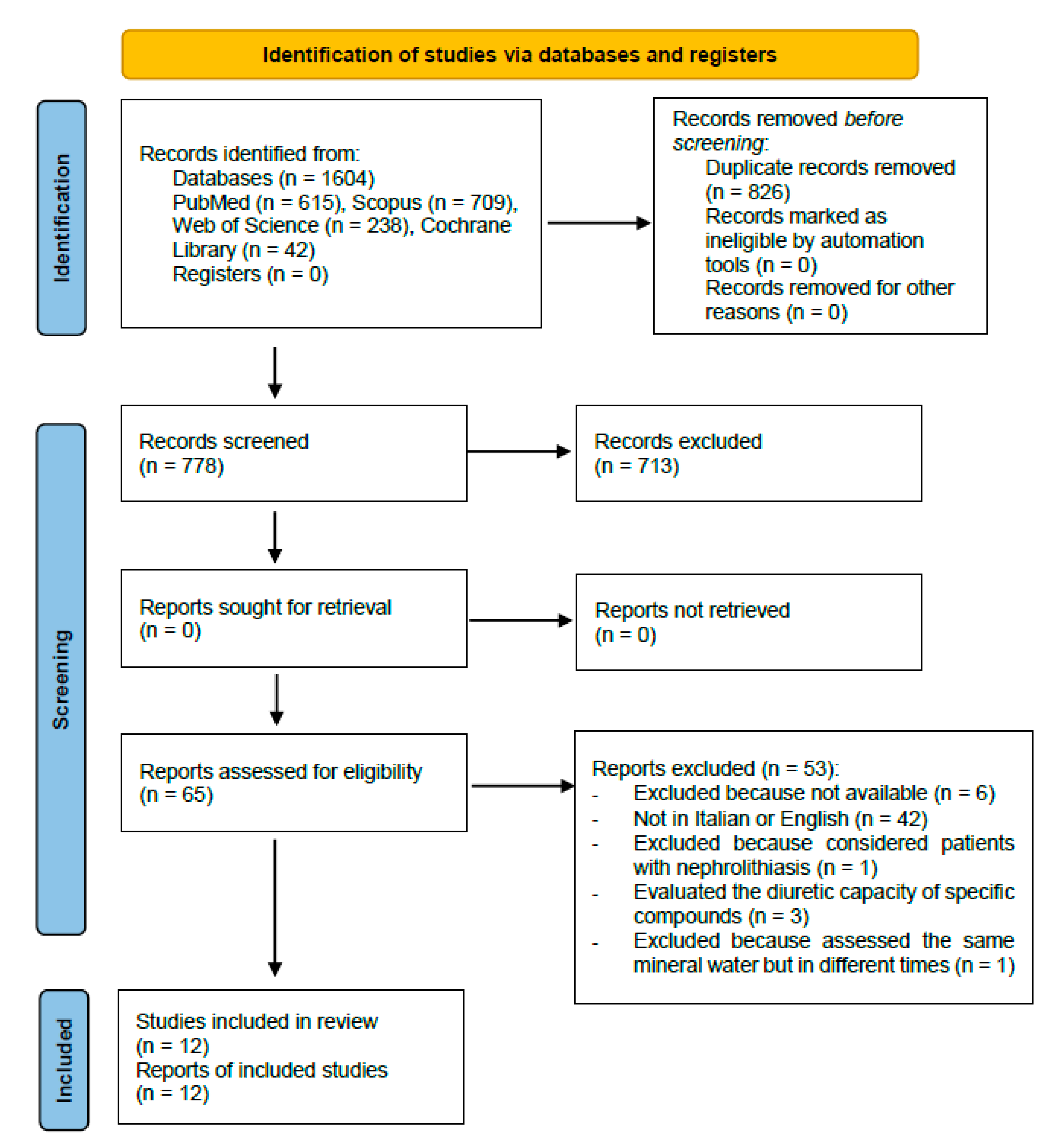
Figure 1 shows the details of the review process.
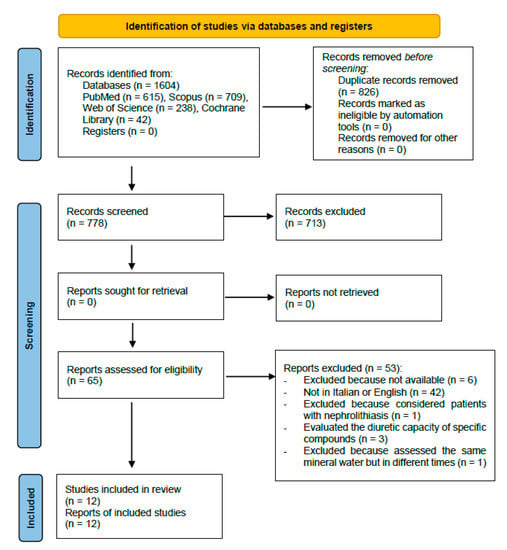
Figure 1.
Selection studies flowchart.
A total of 12 studies [19,20,21,22,23,24,25,26,27,28,29,30], to be included in the review for the qualitative synthesis, have been identified. The initial research on the Pubmed, Scopus, Web of Science and Cochrane databases has allowed us to obtain 1604 bibliographic citations, of which 778 remain after duplicate removal (of which 713 have been excluded because the title or abstract did not satisfy the eligibility criteria). The remaining 65 articles have been fully read and 42 more papers have been excluded because they had published been in different languages that were neither English nor Italian. Initially, no language limitations were set in order not to a priori exclude pertinent articles; however, during the full-text reading, just the articles published in English or Italian have been considered eligible. The former has been selected because of an international level and due to them probably having a better quality; the latter have been selected because they were performed in the country where the mineral water springs cited in the articles are located. Moreover, five more articles have been further excluded because they considered patients affected by nephrolithiasis, considered the diuretic effects of specific compounds or evaluated the same mineral water assumed in different moments. Finally, six articles were not traceable. The references of the selected articles were screened in order to find any further relevant citations; however, no other articles satisfying the inclusion criteria were found. For each article included in the qualitative synthesis of this systematic review, the main investigators extracted the following data: essential bibliographic information, the country in which the study have been set, the eventual source of funding, study design, the type of water studied, the features of the population, patients, and animals that were the object of the study (in terms of race, in case of animals, or gender and age in case of humans), description of the intervention with the studied water, and description of the control intervention (when present), main results, conclusive and original considerations, the overall risk of bias (for studies on humans). These data are summarized in Table 1 (study on human subjects) and Table 2 (study on animals).

Table 1.
Features of studies on human subjects included in the systematic review.

Table 2.
Features of animals’ studies included in the systematic review.
As shown in Table 1 and Table 2, 11 of the 12 studies were performed in Italy and 1 in Bulgaria. Indeed, Europe has a leadership position in the production and consumption of bottled water and, among the European countries, Italy is the top producer and consumer, with a per capita yearly consumption of about 200 L and more than 250 producers in 2019 [31,32]. Thus, it is understandable why Italy is the most involved country in studies in this field.
The time range of publication is very wide, from 1962 [19] to 2019 [25] for human studies, and from 1967 [26] to 2001 [30] for animal studies.
Clinical studies enrolling healthy adults and elders are reported in Table 1. The hydropinic intervention, characterized by the consumption of natural mineral water in a unique administration, usually in the morning under fasting, and/or with an administration ranging in duration from one administration [24] to 8 weeks [25]. The outcome is considered as the urinary output before and after the hydropinic treatment or compared to a control treatment performed with drinking, distilled water, isotonic solution or using a different mineral water. All the studies reported a remarkable post-treatment increase in the urinary volume. Additionally, in addition to the increase in urine volume, some studies included in the review also evidenced other indications of diuretic effects. Donnini [19], for example, reported that after the administration of the tested mineral water, the urinary excretion of electrolytes decreased progressively from the first hour onwards until reaching the lowest values at the fourth hour. This low elimination of electrolytes (and consequently of hypotonic urine) is an indirect expression of the diuretic capacity of the tested water, which induces a prolonged inhibition of the mechanism of renal concentration. Mars and Severgnini [20] recovered an increasing solid diuresis, reporting a higher level of azoturia and urinary chloride after the administration of the tested mineral water with respect to that of distilled water. Fiore and Colombrita [21] proved that the urinary-specific gravity was always increased after the administration of the water under study. This increase is probably the expression of an increase in solid diuresis. With regard to the possibility of a loss of serum electrolytes, Messina and Mammarella [22] reported that the blood electrolytes balance (Na, K, Cl, P, Ca) was not significantly modified even with prolonged treatments with a low mineral water. Additionally, Simeoni et al. [23] compared a low mineral water with a calcium bicarbonate alkaline water and found a lower excretion of urinary calcium together with an unmodified oxalate excretion following low mineral water, inducing lower CaxOx products. Nappi et al. [24] evaluated urinary density, azoturia and urate both before the acute loading of water tested and after two hours; the results showed a significant reduction in all the monitored parameters. The five studies carried out on animals, reported in Table 2, were performed on rats, administering a hydropinic treatment mineral water and drinking water, distilled water or a different mineral water as control intervention. The results of an increase in murine urinary volume following the hydropinic treatment confirmed the data obtained in human studies. Moreover, in the study of Evandri and Bolle [30], the urinary volume was not influenced by the mineralization grade of the administered water, highlighting that the low grade of water mineralization is not necessarily the reason for the diuretic effects. To explain these results, it is important to note that mineral water can increase diuresis through different mechanisms. In particular, low mineral waters increase diuresis, eliminate waste products, prevent urinary calculi and have an anti-inflammatory and anti-infective action. Rich in calcium low mineral waters present these last two actions and, according to recent studies, they prevent the formation of urinary calculi [33,34]. The increase in diuresis caused by this type of water is related to the low mineral salts content, particularly of sodium ion, which leads to an osmotic water passage at a tubular level, causing an increase in the urinary output [35]. The medium mineral bicarbonate waters have a diuretic action attributable to hypotonia and to the digesters content. Specifically, the diuretic action is attributable to the presence of alkaline earth metals, acting both on the renal parenchyma, boosting functionality and on the dynamic of urinary tract [36]. A recent study reported that medium mineral water with a high bicarbonate content produces specific urine changes, contributing to the prevention of urinary calculi. Consumption of bicarbonate medium mineral water increases the excretion of uric acid, with no risk of urinary calculi formation due to the increase in the urinary volume, urinary pH and the excretion of citrate [37]. Additionally, carbonic waters can induce an increase in diuresis. This effect is more evident for oligo-mineral or minimally mineralized water; however, carbon dioxide itself, due to the mucosa and stomach vasodilation and the following rapid absorption, presents a remarkable and proper diuretic effect [38]. Finally, rich mineral water may also present a diuretic effect, as demonstrated by a recent study reporting an increase in diuresis after the consumption of 1–2 L of a rich mineral water with a high content of bicarbonate and iron for 10 days [39].
According to the scores produced by the application of the CLEAR NTP checklist to the seven studies carried out on human subjects, these present a high risk of bias and improvable methodological aspects. However, the coherence of the outcomes reported by all the studies analysed allowed the researchers to mitigate the bias burden while strengthening the scientific evidence. Results derived from the application of the SYRCLE’s checklist highlighted some improvable methodological aspects and a certain risk of bias. Even in this case, the coherence of the results of all the studies increases the strength of the evidences.
This review presents some limitations. Firstly, we did not perform a statistical elaboration of the results of the studies included due to the wide variety of the methodological approaches used for conducting each study. However, this systematic review presents an overview of the diuretic effects derived from the consumption of natural mineral waters with different mineralization and composition. Second, the search was carried out from database inception to November 2022 and included articles published from 1962 to 2020. The analytical approach to assess both the characteristics of water and the diuretic parameters has changed over the past decades; consequently, a critical evaluation of the results of each article included should take into account the publication date. Nonetheless, this systematic review provided an overview of the scientific evidences published in this field. Finally, the quality of the studies was not so high; however, all the articles reported similar results in terms of increase in the diuresis after the consumption of tested natural mineral waters.
4. Conclusions
The main finding of the present systematic review is that all the included studies found an increase in diuresis determined by the consumption of natural mineral water after just a single administration of the tested water. This evidence emerged both in animal and human studies. Additionally, human studies also reported changes in the levels of other parameters useful for indicating an increase in diuresis, such as the decrease in urinary excretion of electrolytes, higher level of azoturia, urinary chloride and urate, and urinary specific gravity. Despite this, the blood electrolytes balance was not significantly modified even with prolonged treatments with low mineral waters.
However, the quality of the recovered studies in this field is not so high, especially for the studies that were conducted many years ago. Thus, it would be desirable to carry out new clinical studies using more appropriate methodological approaches and more refined methods of statistical data processing.
Author Contributions
Conceptualization, C.P. and M.V.; methodology, C.P., A.D.G., D.M., M.F. and M.V.; software, A.D.G., D.M., A.A. and S.C.; validation, C.P., M.V. and M.F.; investigation, C.P., A.D.G., D.M., M.F. and M.V.; data curation, C.P., M.V. and M.F.; writing—original draft preparation, A.D.G., D.M., A.A. and S.C.; writing—review and editing, C.P., M.V. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Directive 2009/54/EC of the European Parliament and of the Council of 18 June 2009 on the Exploitation and Marketing of Natural Mineral Waters. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009L0054 (accessed on 27 December 2022).
- Quattrini, S.; Pampaloni, B.; Brandi, M.L. Natural mineral waters: Chemical characteristics and health effects. Clin. Cases Miner. Bone Metab. 2016, 13, 173–180. [Google Scholar] [CrossRef]
- Valtin, H. “Drink at least eight glasses of water a day”. Really? Is there scientific evidence for “8 × 8”. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R993–R1004. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Johnson, E.C. Water Intake, Water Balance, and the Elusive Daily Water Requirement. Nutrients 2018, 10, 1928. [Google Scholar] [CrossRef]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Maraver, F.; Vitoria, I.; Ferreira-Pêgo, C.; Armijo, F.; Salas-Salvadó, J. Magnesium in tap and bottled mineral water in Spain and its contribution to nutritional recommendations. Nutr. Hosp. 2015, 31, 2297–2312. [Google Scholar] [PubMed]
- Società Italiana di Nutrizione Umana (SINU). Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana (LARN). IV Revisione; Società Italiana di Nutrizione Umana (SINU): Milano, Italy, 2014. [Google Scholar]
- Bothe, G.; Coh, A.; Auinger, A. Efficacy and safety of a natural mineral water rich in magnesium and sulphate for bowel function: A double-blind, randomized, placebo-controlled study. Eur. J. Nutr. 2017, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Bankir, L.; Perucca, J.; Norsk, P.; Bouby, N.; Damgaard, M. Relationship between sodium intake and water intake: The false and the true. Ann. Nutr. Metab. 2017, 70 (Suppl. S1), 51–61. [Google Scholar] [CrossRef] [PubMed]
- Zubkova, S.M.; Varakina, N.I.; Mikhaĭlik, L.V.; Bobkova, A.S.; Chabanenko, S.S. Experimental study of the role of essential trace elements of mineral waters in prevention of atherosclerosis risk factors. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2001, 2, 8–11. [Google Scholar]
- Toxqui, L.; Vaquero, M.P. An intervention with mineral water decreases cardiometabolic risk biomarkers. a crossover, randomised, controlled trial with two mineral waters in moderately hypercholesterolaemic adults. Nutrients 2016, 8, 400. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Y.; Ruan, H.; Li, L.; Zhao, L.; Zhang, M.; Duan, L.; He, S. Drinking natural water unchangeably is associated with reduced all-cause mortality in elderly people: A longitudinal prospective study from China. Front. Public Health 2022, 10, 981782. [Google Scholar] [CrossRef]
- Narciso, L.; Martinelli, A.; Torriani, F.; Frassanito, P.; Bernardini, R.; Chiarotti, F.; Marianelli, C. Natural Mineral Waters and Metabolic Syndrome: Insights from Obese Male and Female C57BL/6 Mice on Caloric Restriction. Front. Nutr. 2022, 24, 886078. [Google Scholar] [CrossRef]
- Barnich, N.; Rodrigues, M.; Sauvanet, P.; Chevarin, C.; Denis, S.; Le Goff, O.; Faure-Imbert, D.; Hanh, T.; Roques, C.F.; Chassaing, B.; et al. Beneficial Effects of Natural Mineral Waters on Intestinal Inflammation and the Mucosa-Associated Microbiota. Int. J. Mol. Sci. 2021, 22, 4336. [Google Scholar] [CrossRef] [PubMed]
- Mirzazadeh, M.; Nouran, M.G.; Richards, K.A.; Zare, M. Effects of drinking water quality on urinary parameters in men with and without urinary tract stones. Urology 2012, 79, 501–507. [Google Scholar] [CrossRef]
- Sulaiman, S.K.; Enakshee, J.; Traxer, O.; Somani, B.K. Which type of water is recommended for patients with stone disease (hard or soft water, or bottled water): Evidence from a systematic review over the last 3 decades. Curr. Urol. Rep. 2020, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Petraccia, L.; Liberati, G.; Masciullo, S.G.; Grassi, M.; Fraioli, A. Water, mineral waters and health. Clin. Nutr. 2006, 25, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Donnini, L. Clinical experiences on the diuretic and uricocuric actions of “Le Vallicelle” water. Minerva Urol. 1962, 14, 133–138. [Google Scholar] [PubMed]
- Mars, G.; Severgnini, B. On the diuretic and antidiuretic power of a sulfato-alkalino- silicic, medio-mineral thermal water in subjects of various ages (water of Val Màsino). G. Gerontol. 1966, 14, 1071–1079. [Google Scholar]
- Fiore, C.E.; Colombrita, G. Clinico-experimental observations on the diuretic activity of the “Currone” bicarbonate-alkaline earth medio-mineral water of Belpasso. Minerva Ecol. Idroclimatol. Fisiconucl. 1975, 15, 1–6. [Google Scholar] [PubMed]
- Messina, B.; Mammarella, A. The effects of Levissima oligomineral water on diuresis and water exchange. Minerva Med. 1983, 74, 1771–1782. [Google Scholar] [PubMed]
- Simeoni, P.G.; Barbera, G.; Battisti, A.; Battisti, P.; Frascaro, E.; Fassino, V.; Guzzetti, P.; Sardella, D.; Scuderi, A.; Vinciguerra, A.; et al. Oligomineral versus bicarbonate alkaline water on urinary excretion of solutes in normal subjects. Ital. J. Miner. Electrolyte Metab. 1998, 12, 87–89. [Google Scholar]
- Nappi, G.; De Luca, S.; Masciocchi, M.M. Diuretic action of the “Ielo” low mineral water: A case-control trial. Med. Clin. Termal. 2003, 15, 505–511. [Google Scholar]
- Roussev, B.; Sokrateva, T.; Nashar, M.; Radanova, M.; Komosinska-Vassev, K.; Olczyk, P.; Potoroko, I.; Ivanova, D. Effect of sulphur-containing mineral water on the renal function: A human interventional study. CR Acad. Bulg. Sci. 2019, 72, 1577–1584. [Google Scholar]
- Brunelli, A. Evaluation of diuresis and of urinary electrolytes (Na and K) in the rat after hydration with S. Pellegrino water, spring water or distilled water. Boll. Soc. Ital. Biol. Sper. 1967, 43, 1715–1719. [Google Scholar] [PubMed]
- Federici, P.C.; Pasqualis, A. First pharmacological studies of the properties of bottled Tesorino water. Acta Biomed. Ateneo Parm. 1984, 55, 65–84. [Google Scholar]
- Federici, P.C. Diuresis tests by fluid load in the albino rat. Critical examination of about 7000 tests. II: Test load with mineral water. G. Clin. Med. 1985, 66, 313–350. [Google Scholar]
- Federici, P.C.; Marchesi, C.; Pasqualis, A. Water loading diuresis test in albino rats. Critical study of approximately 12,000 tests. 3. Loading tests using several mineral waters bottled under various experimental conditions. Acta Biomed. Ateneo Parm. 1988, 59, 147–174. [Google Scholar]
- Evandri, M.G.; Bolle, P. Pharmaco-toxicological screening of commercially available Italian natural mineral waters. Farmaco 2001, 56, 475–482. [Google Scholar] [CrossRef] [PubMed]
- EFBW: Key Statistics. Available online: http://www.efbw.org/index.php?id=90 (accessed on 30 December 2022).
- Lorenzoni, G.; Minto, C.; Temporin, M.; Fuscà, E.; Bolzon, A.; Piras, G.; Iliceto, S.; Silano, M.; Gregori, D. (Ab) use of Health Claims in Websites: The Case of Italian Bottled Waters. Int. J. Environ. Res. Public Health 2019, 16, 3077. [Google Scholar] [CrossRef]
- Karagülle, O.; Smorag, U.; Candir, F.; Gundermann, G.; Jonas, U.; Becker, A.J.; Gehrke, A.; Gutenbrunner, C. Clinical study on the effect of mineral waters containing bicarbonate on the risk of urinary stone formation in patients with multiple episodes of CaOx-urolithiasis. World J. Urol. 2007, 25, 315–323. [Google Scholar] [CrossRef]
- Coen, G.; Sardella, D.; Barbera, G.; Ferrannini, M.; Comegna, C.; Ferazzoli, F.; Dinnella, A.; D’Anello, E.; Simeoni, P. Urinary composition and lithogenic risk in normal subjects following oligomineral versus bicarbonate-alkaline high calcium mineral water intake. Urol. Int. 2001, 67, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Grantham, J.J.; Wetmore, J.B. The medicinal use of water in renal disease. Kidney Int. 2013, 84, 45–53. [Google Scholar] [CrossRef]
- Wu, C.L.; Doong, M.L.; Wang, P.S. Involvement of cholecystokinin receptor in the inhibition of gastrointestinal motility by oxytocin in ovariectomized rats. Eur. J. Pharmacol. 2008, 580, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A.; Borghesi, M. Indications for a medium mineral high bicarbonate water (Cerelia) in urology. Arch. Ital. Urol. Androl. 2009, 81, 192–194. [Google Scholar] [PubMed]
- Siener, R.; Jahnen, A.; Hesse, A. Influence of a mineral water rich in calcium, magnesium and bicarbonate on urine composition and the risk of calcium oxalate crystallization. Eur. J. Clin. Nutr. 2004, 58, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Pes, G.M.; Realdi, G. Health properties of the Italian San Martino® mineral-rich water: A self-controlled pilot study. Biomed. Pharm. 2021, 138, 111509. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).