Racial Differences in Blood Pressure and Autonomic Recovery Following Acute Supramaximal Exercise in Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
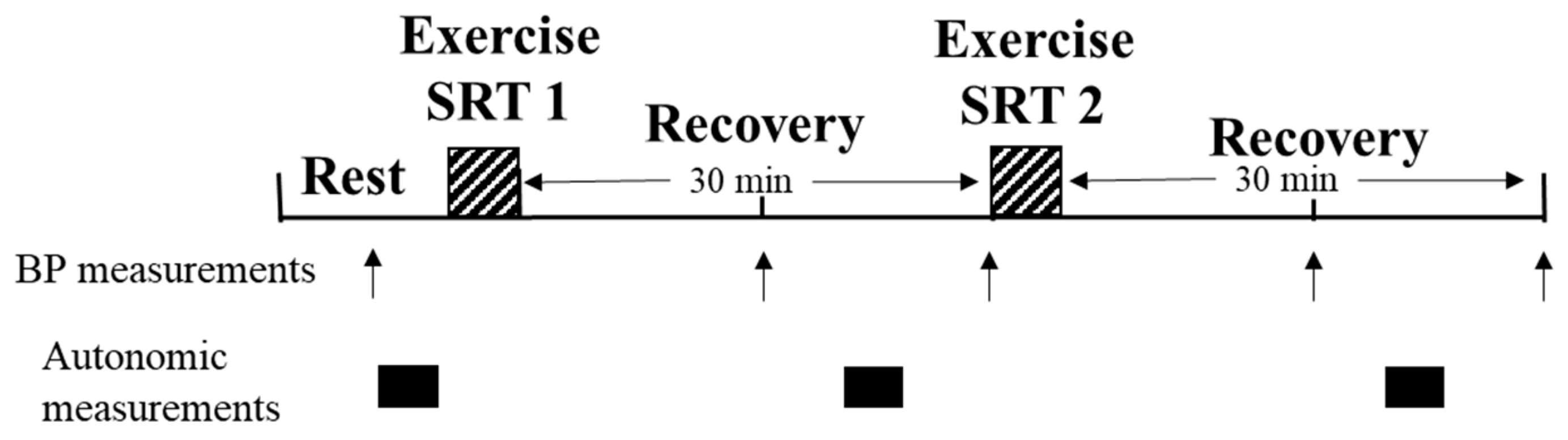
2.2. Study Design
2.3. Anthropometrics
2.4. Blood Pressure Measurement
2.5. Heart Rate Variability Recording and Analysis
2.6. Baroreflex Sensitivity Recording and Analysis
2.7. Exercise Protocol
2.8. Statistical Analyses
2.9. Power Analyses
3. Results
3.1. Participant Characteristics and Exercise Performance
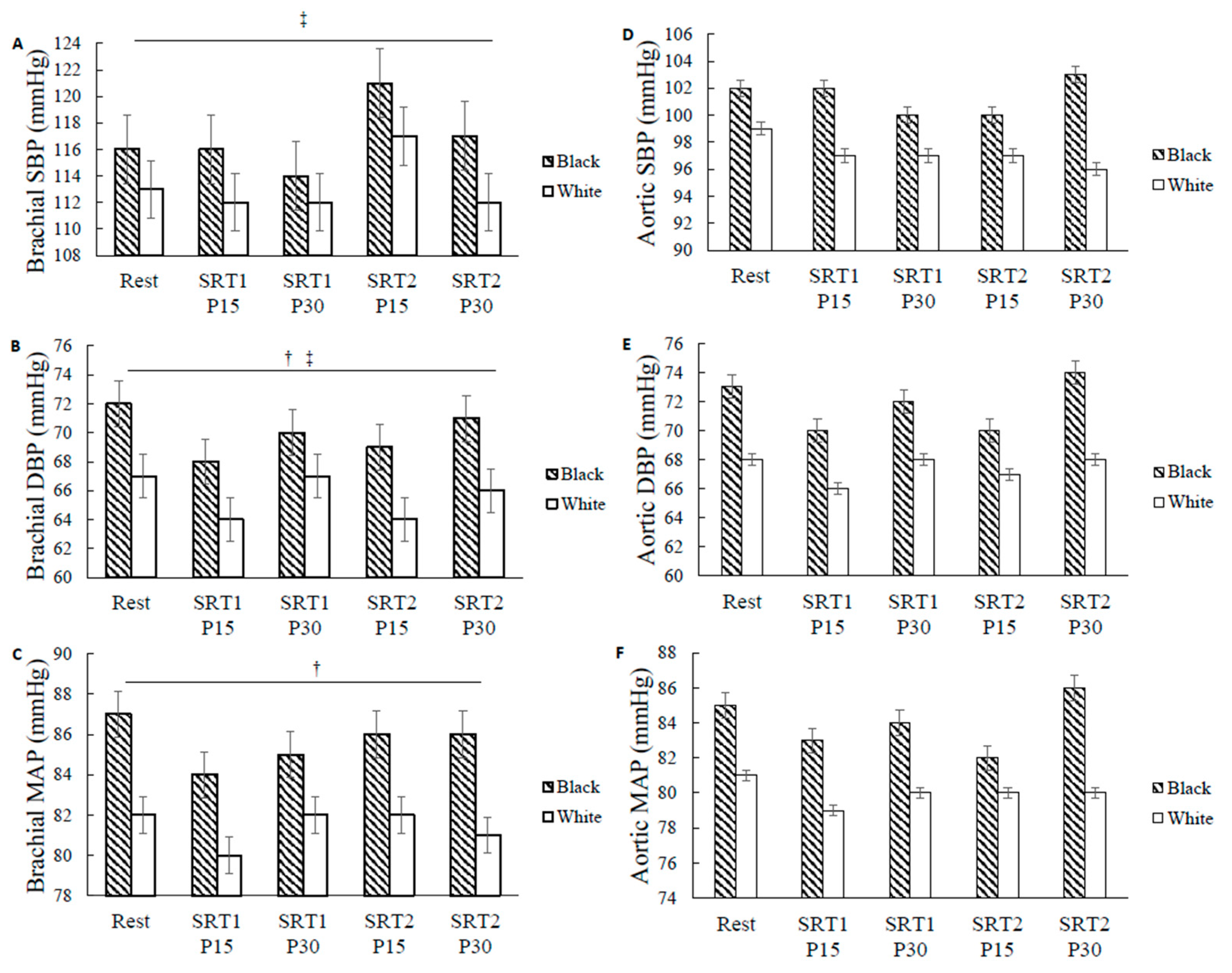
3.2. Racial Differences in BP
3.3. Racial Differences in Autonomic Recovery
4. Discussion
4.1. Racial Differences in Brachial MAP and DBP
4.2. Post-Exercise Autonomic Recovery and Racial Differences in HRV
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendes, R.; Sousa, N.; Themudo-Barata, J.L.; Reis, V.M. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Middle-Aged and Older Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial of the Acute Effects of Treadmill Walking on Glycemic Control. Int. J. Environ. Res. Public Health 2019, 16, 4163. [Google Scholar] [CrossRef] [PubMed]
- Mijwel, S.; Backman, M.; Bolam, K.A.; Olofsson, E.; Norrbom, J.; Bergh, J.; Sundberg, C.J.; Wengstrom, Y.; Rundqvist, H. Highly favorable physiological responses to concurrent resistance and high-intensity interval training during chemotherapy: The OptiTrain breast cancer trial. Breast Cancer Res. Treat. 2018, 169, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W.; Kraus, W.E.; Powell, K.E.; Haskell, W.L.; Janz, K.F.; Jakicic, J.M.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L.; et al. High-Intensity Interval Training for Cardiometabolic Disease Prevention. Med. Sci. Sport. Exerc. 2019, 51, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Kessler, H.S.; Sisson, S.B.; Short, K.R. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012, 42, 489–509. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Powers, S.K.; Howley, E.T.; Quindry, J. Exercise Physiology: Theory and Application to Fitness and Performance, 11th ed.; McGraw-Hill: Haymarket, NSW, Australia, 2020. [Google Scholar]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Farrell, M.C.; Giza, R.J.; Shibao, C.A. Race and sex differences in cardiovascular autonomic regulation. Clin. Auton. Res. 2020, 30, 371–379. [Google Scholar] [CrossRef]
- Maraboto, C.; Ferdinand, K.C. Update on hypertension in African-Americans. Prog. Cardiovasc. Dis. 2020, 63, 33–39. [Google Scholar] [CrossRef]
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief 2020, 364, 1–8. [Google Scholar]
- Bond, V.; Mills, R.M.; Caprarola, M.; Vaccaro, P.; Adams, R.G.; Blakely, R.; Roltsch, M.; Hatfield, B.; Davis, G.C.; Franks, B.D.; et al. Aerobic exercise attenuates blood pressure reactivity to cold pressor test in normotensive, young adult African-American women. Ethn. Dis. 1999, 9, 104–110. [Google Scholar]
- Yan, H.; Ranadive, S.M.; Heffernan, K.S.; Lane, A.D.; Kappus, R.M.; Cook, M.D.; Wu, P.T.; Sun, P.; Harvey, I.S.; Woods, J.A.; et al. Hemodynamic and arterial stiffness differences between African-Americans and Caucasians after maximal exercise. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H60–H68. [Google Scholar] [CrossRef] [PubMed]
- Halliwill, J.R. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc. Sport Sci. Rev. 2001, 29, 65–70. [Google Scholar] [CrossRef]
- Liu, S.; Goodman, J.; Nolan, R.; Lacombe, S.; Thomas, S.G. Blood pressure responses to acute and chronic exercise are related in prehypertension. Med. Sci. Sports Exerc. 2012, 44, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Halliwill, J.R.; Buck, T.M.; Lacewell, A.N.; Romero, S.A. Postexercise hypotension and sustained postexercise vasodilatation: What happens after we exercise? Exp. Physiol. 2013, 98, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Behun, M.A.; Cook, M.D.; Ranadive, S.M.; Lane-Cordova, A.D.; Kappus, R.M.; Woods, J.A.; Wilund, K.R.; Baynard, T.; Halliwill, J.R.; et al. Differential Post-Exercise Blood Pressure Responses between Blacks and Caucasians. PLoS ONE 2016, 11, e0153445. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ranadive, S.M.; Lane-Cordova, A.D.; Kappus, R.M.; Behun, M.A.; Cook, M.D.; Woods, J.A.; Wilund, K.R.; Baynard, T.; Halliwill, J.R.; et al. Effect of acute aerobic exercise and histamine receptor blockade on arterial stiffness in African Americans and Caucasians. J. Appl. Physiol. 2017, 122, 386–395. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018; 472p. [Google Scholar]
- Cote, A.T.; Bredin, S.S.; Phillips, A.A.; Koehle, M.S.; Warburton, D.E. Greater autonomic modulation during post-exercise hypotension following high-intensity interval exercise in endurance-trained men and women. Eur. J. Appl. Physiol. 2015, 115, 81–89. [Google Scholar] [CrossRef]
- Ketelhut, S.; Milatz, F.; Heise, W.; Ketelhut, R.G. Influence of a high-intensity interval training session on peripheral and central blood pressure at rest and during stress testing in healthy individuals. Vasa 2016, 45, 373–377. [Google Scholar] [CrossRef]
- Rossow, L.; Yan, H.; Fahs, C.A.; Ranadive, S.M.; Agiovlasitis, S.; Wilund, K.R.; Baynard, T.; Fernhall, B. Postexercise hypotension in an endurance-trained population of men and women following high-intensity interval and steady-state cycling. Am. J. Hypertens. 2010, 23, 358–367. [Google Scholar] [CrossRef]
- Drew, R.C.; Charkoudian, N.; Park, J. Neural control of cardiovascular function in black adults: Implications for racial differences in autonomic regulation. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2020, 318, R234–R244. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Pinna, G.D.; Raczak, G. Baroreflex sensitivity: Measurement and clinical implications. Ann. Noninvasive Electrocardiol. 2008, 13, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Poehling, C.P.; Llewellyn, T.L. The Effects of Submaximal and Maximal Exercise on Heart Rate Variability. Int. J. Exerc. Sci. 2019, 12, 9–14. [Google Scholar] [PubMed]
- Choi, J.B.; Hong, S.; Nelesen, R.; Bardwell, W.A.; Natarajan, L.; Schubert, C.; Dimsdale, J.E. Age and ethnicity differences in short-term heart-rate variability. Psychosom. Med. 2006, 68, 421–426. [Google Scholar] [CrossRef]
- Latchman, P.; Gates, G.; Axtell, R.; Pereira, J.; Bartels, M.; De Meersman, R.E. Spontaneous baroreflex sensitivity in young normotensive African-American women. Clin. Auton. Res. 2013, 23, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Zion, A.S.; Bond, V.; Adams, R.G.; Williams, D.; Fullilove, R.E.; Sloan, R.P.; Bartels, M.N.; Downey, J.A.; De Meersman, R.E. Low arterial compliance in young African-American males. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H457–H462. [Google Scholar] [CrossRef]
- Holwerda, S.W.; Fulton, D.; Eubank, W.L.; Keller, D.M. Carotid baroreflex responsiveness is impaired in normotensive African American men. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1639–H1645. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, R.; Bussmann, J.B.; Lesaffre, E.; Stam, H.J.; Praet, S.F. A steep ramp test is valid for estimating maximal power and oxygen uptake during a standard ramp test in type 2 diabetes. Scand. J. Med. Sci. Sport. 2015, 25, 595–602. [Google Scholar] [CrossRef]
- Bongers, B.C.; Si, D.E.V.; Helders, P.J.; Takken, T. The steep ramp test in healthy children and adolescents: Reliability and validity. Med. Sci. Sport. Exerc. 2013, 45, 366–371. [Google Scholar] [CrossRef]
- Stuiver, M.M.; Kampshoff, C.S.; Persoon, S.; Groen, W.; van Mechelen, W.; Chinapaw, M.J.M.; Brug, J.; Nollet, F.; Kersten, M.J.; Schep, G.; et al. Validation and Refinement of Prediction Models to Estimate Exercise Capacity in Cancer Survivors Using the Steep Ramp Test. Arch. Phys. Med. Rehabil. 2017, 98, 2167–2173. [Google Scholar] [CrossRef]
- Werkman, M.S.; Bongers, B.C.; Blatter, T.; Takken, T.; Wittink, H. Extended steep ramp test normative values for 19-24-year-old healthy active young adults. Eur. J. Appl. Physiol. 2020, 120, 107–115. [Google Scholar] [CrossRef]
- Weemaes, A.T.R.; Beelen, M.; Bongers, B.C.; Weijenberg, M.P.; Lenssen, A.F. Criterion Validity and Responsiveness of the Steep Ramp Test to Evaluate Aerobic Capacity in Survivors of Cancer Participating in a Supervised Exercise Rehabilitation Program. Arch. Phys. Med. Rehabil. 2021, 102, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Soc. Hypertens. 2018, 12, 579.e571–579.e573. [Google Scholar] [CrossRef]
- AMP Ltd. Operator’s Manual SphygmoCor XCEL System v1; AMP Ltd.: Sydney, NSW, Australia, 2016. [Google Scholar]
- Chen, C.H.; Nevo, E.; Fetics, B.; Pak, P.H.; Yin, F.C.; Maughan, W.L.; Kass, D.A. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 1997, 95, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Laugesen, E.; Rossen, N.B.; Peters, C.D.; Maeng, M.; Ebbehoj, E.; Knudsen, S.T.; Hansen, K.W.; Botker, H.E.; Poulsen, P.L. Assessment of central blood pressure in patients with type 2 diabetes: A comparison between SphygmoCor and invasively measured values. Am. J. Hypertens. 2014, 27, 169–176. [Google Scholar] [CrossRef]
- Weber, T.; Wassertheurer, S.; Rammer, M.; Maurer, E.; Hametner, B.; Mayer, C.C.; Kropf, J.; Eber, B. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension 2011, 58, 825–832. [Google Scholar] [CrossRef]
- Shoji, T.; Nakagomi, A.; Okada, S.; Ohno, Y.; Kobayashi, Y. Invasive validation of a novel brachial cuff-based oscillometric device (SphygmoCor XCEL) for measuring central blood pressure. J. Hypertens. 2017, 35, 69–75. [Google Scholar] [CrossRef]
- TFESCNASP Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Goulopoulou, S.; Fernhall, B.; Kanaley, J.A. Hemodynamic responses and linear and non-linear dynamics of cardiovascular autonomic regulation following supramaximal exercise. Eur. J. Appl. Physiol. 2009, 105, 525–531. [Google Scholar] [CrossRef]
- Perini, R.; Veicsteinas, A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur. J. Appl. Physiol. 2003, 90, 317–325. [Google Scholar] [CrossRef]
- Draghici, A.E.; Taylor, J.A. The physiological basis and measurement of heart rate variability in humans. J. Physiol. Anthropol. 2016, 35, 22. [Google Scholar] [CrossRef]
- Millar, P.J.; Rakobowchuk, M.; McCartney, N.; MacDonald, M.J. Heart rate variability and nonlinear analysis of heart rate dynamics following single and multiple Wingate bouts. Appl. Physiol. Nutr. Metab. 2009, 34, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Montano, N.; Ruscone, T.G.; Porta, A.; Lombardi, F.; Pagani, M.; Malliani, A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994, 90, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.; Marte, W.; Grullenberger, R.; Hacker, A.; Habenbacher, W.; Heller, A.; Wagner, C.; Wach, P.; Skrabal, F. Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput. Biol. Med. 2006, 36, 941–957. [Google Scholar] [CrossRef] [PubMed]
- Pecanha, T.; de Brito, L.C.; Fecchio, R.Y.; de Sousa, P.N.; da Silva Junior, N.D.; de Abreu, A.P.; da Silva, G.V.; Mion-Junior, D.; Forjaz, C.L. Metaboreflex activation delays heart rate recovery after aerobic exercise in never-treated hypertensive men. J. Physiol. 2016, 594, 6211–6223. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.C.; Ranadive, S.M.; Yan, H.; Lane-Cordova, A.D.; Kappus, R.M.; Cook, M.D.; Fernhall, B. Effect of acute maximal exercise on vasodilatory function and arterial stiffness in African-American and white adults. J. Hypertens. 2019, 37, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; Bassett, D.R., Jr.; Duey, W.J.; Howley, E.T.; Bond, V.; Torok, D.J.; Mancuso, P. Cardiovascular and plasma catecholamine responses to exercise in blacks and whites. Hypertension 1992, 20, 542–548. [Google Scholar] [CrossRef]
- Lemogoum, D.; Van Bortel, L.; Van den Abeele, W.; Ciarka, A.; Degaute, J.P.; van de Borne, P.; Leeman, M. Effect of beta-adrenergic stimulation on pulse wave velocity in black and white subjects. J. Hypertens. 2004, 22, 2349–2353. [Google Scholar] [CrossRef]
- Bond, V., Jr.; Thompson, G.D.; Franks, B.D.; Tearney, R.J.; Adams, R.G.; Vaccaro, P. Racial differences in minimum lower leg vascular resistance in normotensive young adults with positive and negative parental histories of hypertension. J. Cardiovasc. Risk 1996, 3, 423–426. [Google Scholar] [CrossRef]
- Ozkor, M.A.; Rahman, A.M.; Murrow, J.R.; Kavtaradze, N.; Lin, J.; Manatunga, A.; Hayek, S.; Quyyumi, A.A. Differences in vascular nitric oxide and endothelium-derived hyperpolarizing factor bioavailability in blacks and whites. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1320–1327. [Google Scholar] [CrossRef]
- Brothers, R.M.; Fadel, P.J.; Keller, D.M. Racial disparities in cardiovascular disease risk: Mechanisms of vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H777–H789. [Google Scholar] [CrossRef]
- McEniery, C.M.; Yasmin; McDonnell, B.; Munnery, M.; Wallace, S.M.; Rowe, C.V.; Cockcroft, J.R.; Wilkinson, I.B.; Anglo-Cardiff Collaborative Trial Investigators. Central pressure: Variability and impact of cardiovascular risk factors: The Anglo-Cardiff Collaborative Trial II. Hypertension 2008, 51, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, K.S.; Jae, S.Y.; Wilund, K.R.; Woods, J.A.; Fernhall, B. Racial differences in central blood pressure and vascular function in young men. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2380–H2387. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.B. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 2018, 25, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.A.; Midgley, A.W.; Soares, P.P.; Farinatti, P.T. Postexercise hypotension after maximal short-term incremental exercise depends on exercise modality. Appl. Physiol. Nutr. Metab. 2015, 40, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomski, W.; Gasiorowska, A.; Krauss, B.; Mroz, A.; Cybulski, G. Suppression of heart rate variability after supramaximal exertion. Clin. Physiol. Funct. Imaging 2007, 27, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R.; Bremner, J.D.; Su, S.; Miller, A.; Lee, F.; Cheema, F.; Goldberg, J.; Vaccarino, V. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am. Heart J. 2008, 156, 759.e1–759.e7. [Google Scholar] [CrossRef]
- Groeschel, M.; Braam, B. Connecting chronic and recurrent stress to vascular dysfunction: No relaxed role for the renin-angiotensin system. Am. J. Physiol. -Ren. Physiol. 2011, 300, F1–F10. [Google Scholar] [CrossRef]
- Heffernan, K.S.; Jae, S.Y.; Vieira, V.J.; Iwamoto, G.A.; Wilund, K.R.; Woods, J.A.; Fernhall, B. C-reactive protein and cardiac vagal activity following resistance exercise training in young African-American and white men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1098–R1105. [Google Scholar] [CrossRef]
- Djaoui, L.; Haddad, M.; Chamari, K.; Dellal, A. Monitoring training load and fatigue in soccer players with physiological markers. Physiol. Behav. 2017, 181, 86–94. [Google Scholar] [CrossRef]
- Schneider, C.; Wiewelhove, T.; Raeder, C.; Flatt, A.A.; Hoos, O.; Hottenrott, L.; Schumbera, O.; Kellmann, M.; Meyer, T.; Pfeiffer, M.; et al. Heart Rate Variability Monitoring During Strength and High-Intensity Interval Training Overload Microcycles. Front. Physiol. 2019, 10, 582. [Google Scholar] [CrossRef]
- Halliwill, J.R.; Taylor, J.A.; Eckberg, D.L. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J. Physiol. 1996, 495 Pt 1, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Grace, F.; Herbert, P.; Elliott, A.D.; Richards, J.; Beaumont, A.; Sculthorpe, N.F. High intensity interval training (HIIT) improves resting blood pressure, metabolic (MET) capacity and heart rate reserve without compromising cardiac function in sedentary aging men. Exp. Gerontol. 2018, 109, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Vasan, R.S.; Xanthakis, V. Association of Blood Pressure Responses to Submaximal Exercise in Midlife With the Incidence of Cardiovascular Outcomes and All-Cause Mortality: The Framingham Heart Study. J. Am. Heart Assoc. 2020, 9, e015554. [Google Scholar] [CrossRef]
- Brenner, J.; LeBlang, S.; Lizotte-Waniewski, M.; Schmidt, B.; Espinosa, P.S.; DeMets, D.L.; Newberg, A.; Hennekens, C.H. Mindfulness with paced breathing reduces blood pressure. Med. Hypotheses. 2020, 142, 109780. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.S.; Dunford, E.C.; MacDonald, M.J. Impact of the menstrual cycle on peripheral vascular function in premenopausal women: Systematic review and meta-analysis. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1327–H1337. [Google Scholar] [CrossRef]
- Lynn, B.M.; McCord, J.L.; Halliwill, J.R. Effects of the menstrual cycle and sex on postexercise hemodynamics. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2007, 292, R1260–R1270. [Google Scholar] [CrossRef]

| Black (n = 8) | White (n = 12) | p-Value | |
|---|---|---|---|
| Age (years) | 22 ± 1 | 22 ± 1 | 0.82 |
| Height (cm) | 167.3 ± 2.2 | 165.5 ± 1.5 | 0.48 |
| Weight (kg) | 69.2 ± 3.8 | 63.0 ± 3.2 | 0.23 |
| BMI (kg/m2) | 24.7 ± 0.7 | 22.9 ± 0.8 | 0.83 |
| MVPA volume (min/wk.) | 135.0 ± 43.3 | 179.6 ± 47.3 | 0.37 |
| Rest | SRT 1 P20 | SRT 2 P20 | p-Value | |
|---|---|---|---|---|
| BRS (ms/mmHg) ‡ | 26.3 ± 7 | 16.7 ± 4 | 13.1 ± 3 | 0.004 |
| VLF (n.u.) ‡ | 4.35 ± 0.23 | 3.18 ± 0.26 | 3.18 ± 0.34 | 0.008 |
| LF (n.u.) ‡ | 4.91 ± 0.28 | 3.65 ± 0.23 | 3.63 ± 0.30 | 0.009 |
| HF (n.u.) ‡ | 5.6 ± 0.2 | 3.5 ± 0.4 | 2.7 ± 0.5 | 0.000 |
| LF/HF ‡ | 0.8 ± 0.2 | 1.8 ± 0.3 | 3.8 ± 1.0 | 0.007 |
| RMSSD (ms) ‡ | 59.5 ± 2.7 | 26.5 ± 6.0 | 21.2 ± 4.8 | 0.000 |
| Average HR (bpm) ‡ | 63 ± 4 | 75 ± 5 | 79 ± 6 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajdek, N.; Merchant, N.; Camhi, S.M.; Yan, H. Racial Differences in Blood Pressure and Autonomic Recovery Following Acute Supramaximal Exercise in Women. Int. J. Environ. Res. Public Health 2023, 20, 5615. https://doi.org/10.3390/ijerph20095615
Bajdek N, Merchant N, Camhi SM, Yan H. Racial Differences in Blood Pressure and Autonomic Recovery Following Acute Supramaximal Exercise in Women. International Journal of Environmental Research and Public Health. 2023; 20(9):5615. https://doi.org/10.3390/ijerph20095615
Chicago/Turabian StyleBajdek, Nicole, Noelle Merchant, Sarah M. Camhi, and Huimin Yan. 2023. "Racial Differences in Blood Pressure and Autonomic Recovery Following Acute Supramaximal Exercise in Women" International Journal of Environmental Research and Public Health 20, no. 9: 5615. https://doi.org/10.3390/ijerph20095615
APA StyleBajdek, N., Merchant, N., Camhi, S. M., & Yan, H. (2023). Racial Differences in Blood Pressure and Autonomic Recovery Following Acute Supramaximal Exercise in Women. International Journal of Environmental Research and Public Health, 20(9), 5615. https://doi.org/10.3390/ijerph20095615






