Postmortem Biochemistry and Immunohistochemistry in Anaphylactic Death Due to Hymenoptera Sting: A Forensic Case Report
Abstract
1. Introduction
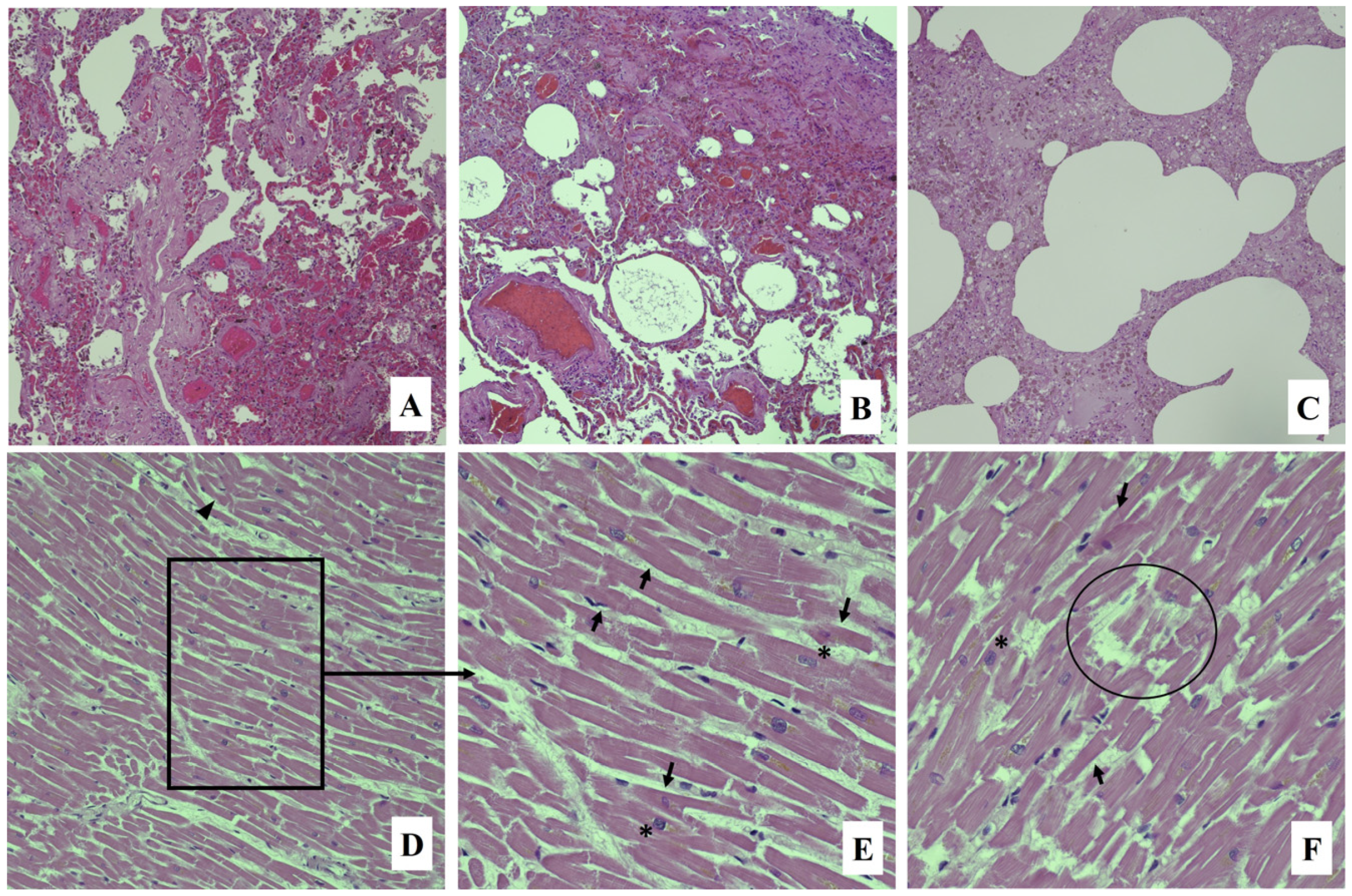
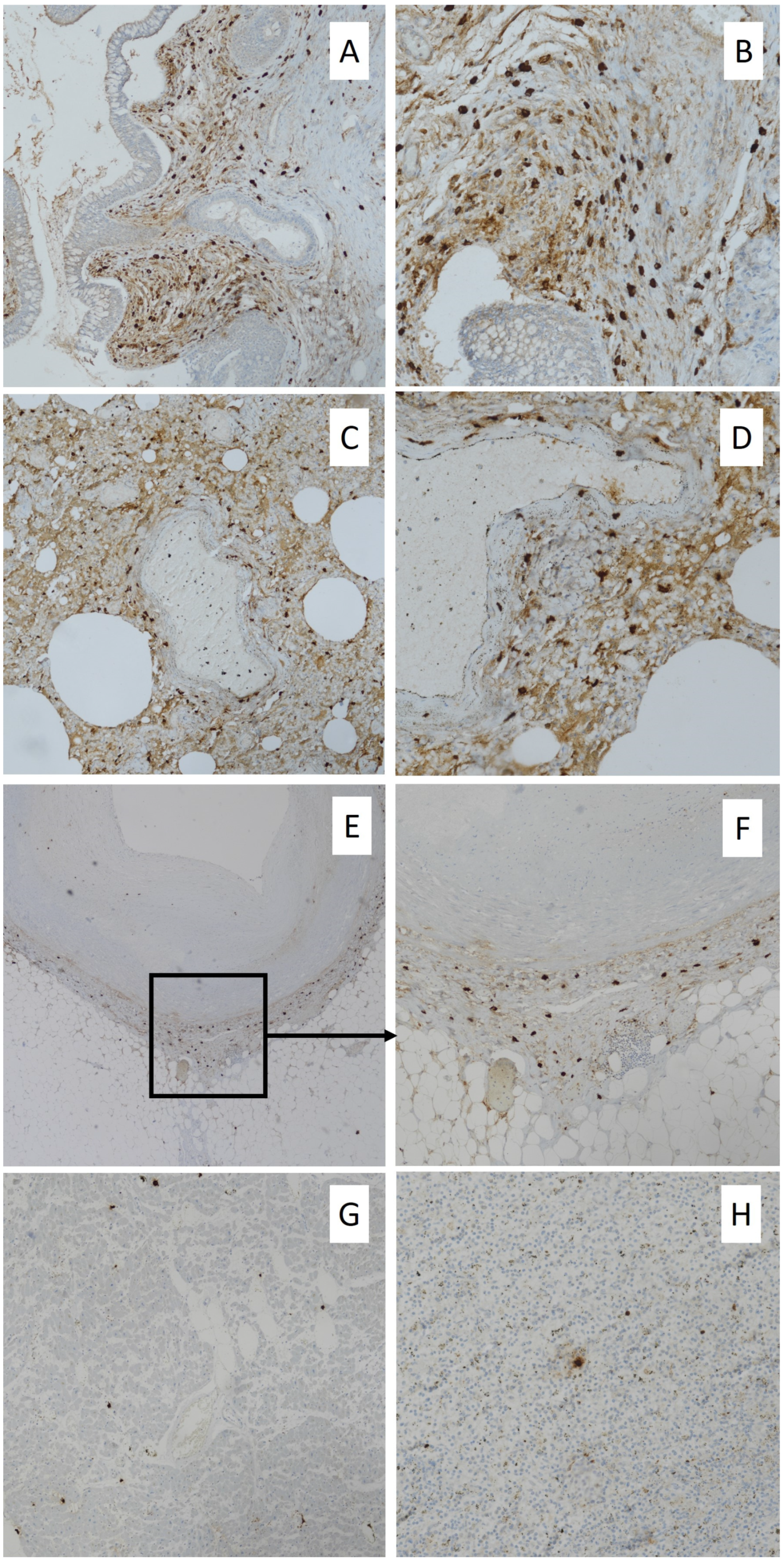
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simons, F.E.; Ardusso, L.R.; Bilò, M.B.; El-Gamal, Y.M.; Ledford, D.K.; Ring, J.; Sanchez-Borges, M.; Senna, G.E.; Sheikh, A.; Thong, B.Y.; et al. World Allergy Organization anaphylaxis guidelines: Summary. J. Allergy Clin. Immunol. 2011, 127, 587–593.e22. [Google Scholar] [CrossRef] [PubMed]
- Bagos-Estevez, A.G.; Ledford, D.K. Anaphylaxis: Definition, Epidemiology, Diagnostic Challenges, Grading System. Immunol. Allergy Clin. N. Am. 2022, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, P.A.; Rotskoff, B.D.; Lifschultz, B. Fatal anaphylaxis: Postmortem findings and associated comorbid diseases. Ann. Allergy Asthma Immunol. 2007, 98, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Bilò, M.B.; Corsi, A.; Martini, M.; Penza, E.; Grippo, F.; Bignardi, D. Fatal anaphylaxis in Italy: Analysis of cause-of-death national data, 2004–2016. Allergy 2020, 75, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Spagnolo, E.V.; Cardia, G.; Minciullo, P.L. Fatal anaphylactic shock due to a dental impression material. Int. J. Prosthodont. 2009, 22, 33–34. [Google Scholar]
- Calapai, G.; Imbesi, S.; Cafeo, V.; Ventura Spagnolo, E.; Minciullo, P.L.; Caputi, A.P.; Gangemi, S.; Milone, L. Fatal hypersensitivity reaction to an oral spray of flurbiprofen: A case report. J. Clin. Pharm. Ther. 2013, 38, 337–338. [Google Scholar] [CrossRef]
- Ventura Spagnolo, E.; Calapai, G.; Minciullo, P.L.; Mannucci, C.; Asmundo, A.; Gangemi, S. Lethal Anaphylactic Reaction to Intravenous Gelatin in the Course of Surgery. Am. J. Ther. 2016, 23, e1344–e1346. [Google Scholar] [CrossRef]
- Zirngibl, G.; Burrows, H.L. Hymenoptera stings. Pediatr. Rev. 2012, 33, 534–535; discussion 535. [Google Scholar] [CrossRef]
- Vetter, R.S.; Visscher, P.K.; Camazine, S. Mass envenomations by honey bees and wasps. West. J. Med. 1999, 170, 223–227. [Google Scholar]
- Dos Santos-Pinto, J.R.A.; Perez-Riverol, A.; Lasa, A.M.; Palma, M.S. Diversity of peptidic and proteinaceous toxins from social Hymenoptera venoms. Toxicon 2018, 148, 172–196. [Google Scholar] [CrossRef]
- Johansson, B.; Eriksson, A.; Ornehult, L. Human fatalities caused by wasp and bee stings in Sweden. Int. J. Legal Med. 1991, 104, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mosbech, H. Death caused by wasp and bee stings in Denmark 1960–1980. Allergy 1983, 38, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Riches, K.J.; Gillis, D.; James, R.A. An autopsy approach to bee sting-related deaths. Pathology 2002, 34, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- Du Toit-Prinsloo, L.; Morris, N.K.; Meyer, P.; Saayman, G. Deaths from bee stings: A report of three cases from Pretoria, South Africa. Forensic Sci. Med. Pathol. 2016, 12, 81–85. [Google Scholar] [CrossRef]
- Sravan, A.; Tse, R.; Cala, A.D. A Decline in 2 Consecutive Postmortem Serum Tryptase Levels in an Anaphylactic Death. Am. J. Forensic Med. Pathol. 2015, 36, 233–235. [Google Scholar] [CrossRef]
- Costantino, A.; Mezzetti, E.; De Matteis, A.; Volonnino, G.; De Simone, S.; Fazio, V. Comparative study between conventional and new methods in defining the cause of death from anaphylactic shock. Clin. Ter. 2021, 172, 369–371. [Google Scholar] [CrossRef]
- Cecchi, R. Diagnosis of anaphylactic death in forensics: Review and future perspectives. Leg. Med. 2016, 22, 75–81. [Google Scholar] [CrossRef]
- Shen, Y.; Li, L.; Grant, J.; Rubio, A.; Zhao, Z.; Zhang, X.; Zhou, L.; Fowler, D. Anaphylactic deaths in Maryland (United States) and Shanghai (China): A review of forensic autopsy cases from 2004 to 2006. Forensic Sci. Int. 2009, 186, 1–5. [Google Scholar] [CrossRef]
- Da Broi, U.; Moreschi, C. Post-mortem diagnosis of anaphylaxis: A difficult task in forensic medicine. Forensic Sci. Int. 2011, 204, 1–5. [Google Scholar] [CrossRef]
- Meyer, P. Confirming anaphylaxis postmortem using serodiagnostic tests. J. Clin. Pathol. 2020, 73, 781. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.E.; Krauskopf, A.; Hemmer, W.; Moritz, K.; Jarisch, R.; Reiter, C. Usefulness of post mortem determination of serum tryptase, histamine and diamine oxidase in the diagnosis of fatal anaphylaxis. Forensic Sci. Int. 2011, 212, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Tse, R.; Wong, C.X.; Kesha, K.; Garland, J.; Tran, Y.; Anne, S.; Elstub, H.; Cala, A.D.; Palmiere, C.; Patchett, K.L. Post mortem tryptase cut-off level for anaphylactic death. Forensic Sci. Int. 2018, 284, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Edston, E.; Eriksson, O.; van Hage, M. Mast cell tryptase in postmortem serum-reference values and confounders. Int. J. Legal Med. 2007, 121, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Li, D.R.; Wang, Q.; Zhang, F.; Yu, Y.G.; Wang, H.J. Postmortem Serum Tryptase Levels with Special Regard to Acute Cardiac Deaths. J. Forensic Sci. 2017, 62, 1336–1338. [Google Scholar] [CrossRef]
- McLean-Tooke, A.; Goulding, M.; Bundell, C.; White, J.; Hollingsworth, P. Postmortem serum tryptase levels in anaphylactic and non-anaphylactic deaths. J. Clin. Pathol. 2014, 67, 134–138. [Google Scholar] [CrossRef]
- Garland, J.; Philcox, W.; McCarthy, S.; Hensby-Bennet, S.; Ondruschka, B.; Woydt, L.; Da Broi, U.; Palmiere, C.; Lam, L.; Ahn, Y.; et al. The effects of different sampling techniques on peripheral post mortem tryptase levels: A recommended sampling method. Int. J. Legal Med. 2019, 133, 1477–1483. [Google Scholar] [CrossRef]
- Horn, K.D.; Halsey, J.F.; Zumwalt, R.E. Utilization of serum tryptase and immunoglobuline assay in the postmortem diagnosis of anaphylaxis. Am. J. Forensic Med. Pathol. 2004, 25, 37–43. [Google Scholar] [CrossRef]
- Pumphrey, R.S.; Roberts, I.S. Postmortem findings after fatal anaphylactic reactions. J. Clin. Pathol. 2000, 53, 273–276. [Google Scholar] [CrossRef]
- Edston, E. Accumulation of eosinophils, mast cells, and basophils in the spleen in anaphylactic deaths. Forensic Sci. Med. Pathol. 2013, 9, 496–500. [Google Scholar] [CrossRef]
- Kounis, N.G. Kounis syndrome: An update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin. Chem. Lab. Med. 2016, 54, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Navaradnam, P.; Suganthan, N.; Kumanan, T.; Sujanitha, V.; Mayorathan, U. Kounis Syndrome and Multiorgan Failure Following Multiple Wasp Stings. Cureus 2021, 13, e14606. [Google Scholar] [CrossRef] [PubMed]
- Pirasath, S.; Senthan, V.; Seneviratne, M.H. Kounis syndrome: Acute myocardial infarction following multiple bee stings. SAGE Open. Med. Case Rep. 2021, 9, 2050313X21999206. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Kahn, L.; Zilg, B. Fatal anaphylactic shock: A review of postmortem biomarkers and diagnostics. Forensic Sci. Int. 2021, 323, 110814. [Google Scholar] [CrossRef]
- Wang, X.; Yin, C.; Su, X.; Su, M. Reliable Postmortem Molecular Diagnosis of Anaphylaxis: Co-localization of Mast Cell Degranulation and Immunoglobulin E in Allergic Throat Tissues. Am. J. Forensic Med. Pathol. 2020, 41, 249–258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondello, C.; Baldino, G.; Cianci, V.; Forzese, E.; Asmundo, A.; Ieni, A.; Ventura Spagnolo, E. Postmortem Biochemistry and Immunohistochemistry in Anaphylactic Death Due to Hymenoptera Sting: A Forensic Case Report. Int. J. Environ. Res. Public Health 2023, 20, 5640. https://doi.org/10.3390/ijerph20095640
Mondello C, Baldino G, Cianci V, Forzese E, Asmundo A, Ieni A, Ventura Spagnolo E. Postmortem Biochemistry and Immunohistochemistry in Anaphylactic Death Due to Hymenoptera Sting: A Forensic Case Report. International Journal of Environmental Research and Public Health. 2023; 20(9):5640. https://doi.org/10.3390/ijerph20095640
Chicago/Turabian StyleMondello, Cristina, Gennaro Baldino, Vincenzo Cianci, Elena Forzese, Alessio Asmundo, Antonio Ieni, and Elvira Ventura Spagnolo. 2023. "Postmortem Biochemistry and Immunohistochemistry in Anaphylactic Death Due to Hymenoptera Sting: A Forensic Case Report" International Journal of Environmental Research and Public Health 20, no. 9: 5640. https://doi.org/10.3390/ijerph20095640
APA StyleMondello, C., Baldino, G., Cianci, V., Forzese, E., Asmundo, A., Ieni, A., & Ventura Spagnolo, E. (2023). Postmortem Biochemistry and Immunohistochemistry in Anaphylactic Death Due to Hymenoptera Sting: A Forensic Case Report. International Journal of Environmental Research and Public Health, 20(9), 5640. https://doi.org/10.3390/ijerph20095640








