Polypharmacy Management in a Gender Perspective: At the Heart of the Problem: Analysis of Major Cardiac Diseases, SARS-CoV-2 Affection and Gender Distribution in a Cohort of Patients in Internal Medicine Ward
Abstract
:1. Introduction
2. Aim of This Study
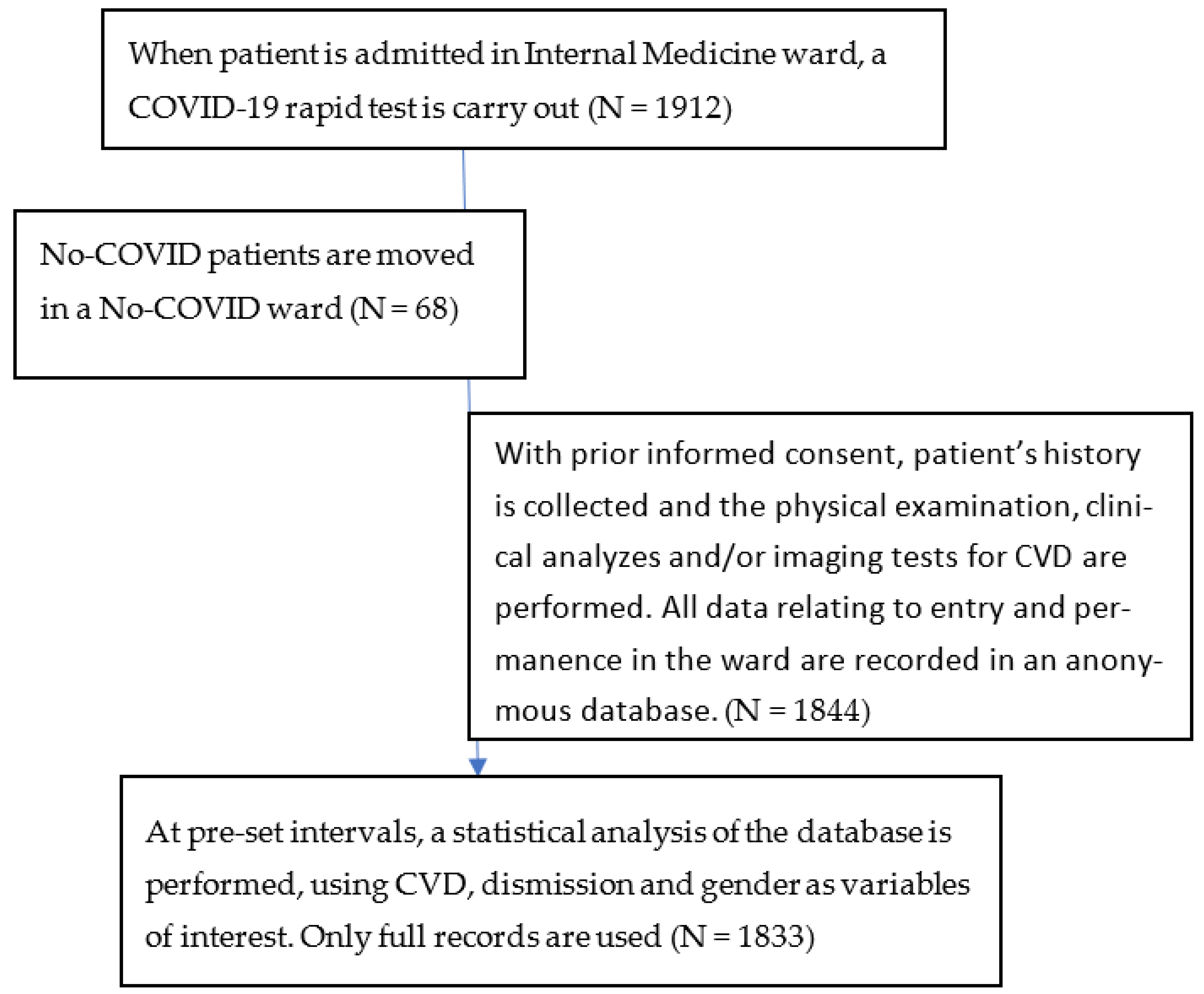
3. Materials and Methods
- ▪
- Heart failure (HF, a heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body);
- ▪
- Myocardial infarction (MI, necrosis of the myocardium caused by an obstruction of the blood supply to the heart);
- ▪
- Atrial fibrillation (AF, abnormal cardiac rhythm that is characterized by rapid, uncoordinated firing of electrical impulses in the upper chambers of the heart) and their combination.
- Patient age;
- Patient gender;
- Presence of CVD;
- Presence of other comorbidities, such as:
- ○
- Chronic kidney disease (CKD);
- ○
- Chronic obstructive pulmonary disease (COPD);
- ○
- Liver disease;
- ○
- Inflammatory bowel disease (IBD);
- ○
- Diabetes mellitus (DM);
- ○
- Connectivitis;
- ○
- Involutive encephalopathy;
- ○
- Disthyroidism;
- Active neoplasms;
- Clinical outcome, defined as either:
- ○
- Discharge
- ○
- Death
- Length of stay (LOS).
4. Results
5. Discussion
6. Limitations of the Study
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosiello, F. Sicurezza sanitaria globale e governance [Global health security and governance]. In Romina Fucà, Schengen e il Cross Border nell’Unione Europea; Aracne Editrice: Aprilia (LT), Italy, 2019; pp. 103–113. ISBN 978-88-255-2950-0. [Google Scholar]
- Gupta, A.; Pradhan, A.; Maurya, V.K.; Kumar, S.; Theengh, A.; Puri, B.; Saxena, S.K. Therapeutic approaches for SARS-CoV-2 infection. Methods 2021, 195, 29–43. [Google Scholar] [CrossRef] [PubMed]
- van Oosterhout, C.; Hall, N.; Ly, H.; Tyler, K.M. COVID-19 evolution during the pandemic—Implications of new SARS-CoV-2 variants on disease control and public health policies. Virulence 2021, 12, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Rosiello, F.; Alessi, E.; Pascucci, M.; Rainone, M.; Cipriano, E.; Di Berardino, A.; Vinci, A.; Ruggeri, M.; Ricci, S. Burden of COVID-19 on Italian Internal Medicine Wards: Delphi, SWOT, and Performance Analysis after Two Pandemic Waves in the Local Health Authority “Roma 6” Hospital Structures. Int. J. Environ. Res. Public Health 2021, 18, 5999. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zeng, H.; Jiang, H.; Yang, Y.; Yuan, Z.; Cheng, X.; Jing, Z.; Liu, B.; Chen, J.; Nie, S.; et al. CSC Expert Consensus on Principles of Clinical Management of Patients With Severe Emergent Cardiovascular Diseases During the COVID-19 Epidemic. Circulation 2020, 141, e810–e816. Available online: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.047011 (accessed on 12 March 2023). [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Ciaburri, F.; Paoli, V.D.; D’avino, M.; Campania, D.F. Gender differences in COVID-19 patients with arterial hypertension. J. Hypertens. 2021, 39, e204. [Google Scholar] [CrossRef]
- Fajar, J.K.; Ilmawan, M.; Mamada, S.; Mutiawati, E.; Husnah, M.; Yusuf, H.; Nainu, F.; Sirinam, S.; Keam, S.; Ophinni, Y.; et al. Global prevalence of persistent neuromuscular symptoms and the possible pathomechanisms in COVID-19 recovered individuals: A systematic review and meta-analysis. Narra J. 2021, 1. Available online: https://narraj.org/main/article/view/48 (accessed on 12 March 2023). [CrossRef]
- Rosiello, F. COVID-19 and Mental Health. 2021. Available online: https://zenodo.org/record/5452433 (accessed on 12 March 2023).
- Fahriani, M.; Ilmawan, M.; Fajar, J.K.; Maliga, H.A.; Frediansyah, A.; Masyeni, S.; Yusuf, H.; Nainu, F.; Rosiello, F.; Sirinam, S.; et al. Persistence of long COVID symptoms in COVID-19 survivors worldwide and its potential pathogenesis—A systematic review and meta-analysis. Narra J. 2021, 1. Available online: https://narraj.org/main/article/view/36 (accessed on 12 March 2023). [CrossRef]
- Harapan, H.; Yufika, A.; Anwar, S.; Ophinni, Y.; Yamada, C.; Sharun, K.; Gachabayov, M.; Fahriani, M.; Husnah, M.; Raad, R.; et al. Beliefs on social distancing and face mask practices during the COVID-19 pandemic in low- and middle-income countries: A cross-sectional study. F1000Research 2022, 11, 206. [Google Scholar] [CrossRef]
- Gachabayov, M.; Sharun, K.; Felsenreich, D.M.; Nainu, F.; Anwar, S.; Yufika, A.; Ophinni, Y.; Yamada, C.; Fahriani, M.; Husnah, M.; et al. Perceived risk of infection and death from COVID-19 among community members of low- and middle-income countries: A cross-sectional study. F1000Research 2022, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.-Y.; Redwood, S.; Prendergast, B.; Chen, M. Coronaviruses and the cardiovascular system: Acute and long-term implications. Eur. Heart J. 2020, 41, 1798–1800. [Google Scholar] [CrossRef]
- Sartor, G.; Del Riccio, M.; Dal Poz, I.; Bonanni, P.; Bonaccorsi, G. COVID-19 in Italy: Considerations on official data. Int. J. Infect. Dis. IJID Off Publ. Int. Soc. Infect Dis. 2020, 98, 188–190. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef]
- Matsushita, K.; Marchandot, B.; Jesel, L.; Ohlmann, P.; Morel, O. Impact of COVID-19 on the Cardiovascular System: A Review. J. Clin. Med. 2020, 9, 1407. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Mancia, G.; Dell’Oro, R.; Grassi, G. COVID-19, hypertension and cardiovascular diseases: Should we change the therapy? Pharmacol. Res. 2020, 158, 104906. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Meng, L.; Hao, M.; Zhang, Y.; Gong, T.; Guo, Z. Chronic stress: A critical risk factor for atherosclerosis. J. Int. Med. Res. 2019, 47, 1429–1440. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Orlandini, F.; Moriconi, L.; La Regina, M. Acute Complex Care Model: An organizational approach for the medical care of hospitalized acute complex patients. Eur. J Intern. Med. 2015, 26, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Aperti, P.; Tonoli, L.; Tyndall, E.; Meneghetti, O. The correct setting to improve the quality of health care process: A retrospective study in Internal Medicine Department. Ital. J. Med. 2018, 12, 285–295. [Google Scholar] [CrossRef]
- Cellini, N.; Canale, N.; Mioni, G.; Costa, S. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J. Sleep Res. 2020. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jsr.13074 (accessed on 12 March 2023).
- van der Linden, S.; Roozenbeek, J.; Compton, J. Inoculating Against Fake News About COVID-19. Front. Psychol. 2020, 11, 566790. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef]
- Ciarambino, T.; Pietrantonio, F.; Rotunno, S.; Fiorentini, A.; Cipriani, R.; Campagna, G.; Straface, G.; Pistella, E.; Giordano, M.; Rosiello, F.; et al. CO-St (COVID 19- study): Impact of the Management of Men Versus Women in the Treatment of Covid 19. A Multi-Centric Observational Study Medicine & Pharmacology. 2021. Available online: https://www.preprints.org/manuscript/202108.0119/v1 (accessed on 12 March 2023).
- Pietrantonio, F.; Vinci, A.; Rosiello, F.; Alessi, E.; Pascucci, M.; Rainone, M.; Delli Castelli, M.; Ciamei, A.; Montagnese, F.; D’Amico, R.; et al. Green Line Hospital-Territory Study: A Single-Blind Randomized Clinical Trial for Evaluation of Technological Challenges of Continuous Wireless Monitoring in Internal Medicine, Preliminary Results. Int. J. Environ. Res. Public Health 2021, 18, 10328. [Google Scholar] [CrossRef]
- Rosiello, F.; Pietrantonio, F.; Di Lorenzo, J.; Bertani, G.; Anzidei, A.; Laurelli, G.; Cipriano, E.; Di Iorio, C.; Montagnese, F.; Pascucci, M. Could the miniaturize techonologies improve patients adherence and assure better quality of life? Eur. J. Public Health 2021, 31, ckab165.159. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Rosiello, F.; Pascucci, M.; Alessi, E.; Ciamei, A.; Cipriano, E.; Di Berardino, A.; Laurelli, G.; Porzano, A.; Delli Castelli, M.; et al. Device therapy for the major complications detection and early treatment of patients with natural and iatrogenic comorbidities admitted to internal medicine wards. Eur. Heart J. 2021, 42, ehab724.3103. [Google Scholar] [CrossRef]
- Rosiello, F.; Pietrantonio, F.; Berardino, A.D.; Delli Castelli, M.; Ciamei, A.; Piccione, A.; Rainone, M.; Alessi, E.; Vinci, A.; Ruggeri, M. Is COVID 19 introducing a new model of internal medicine ward? Eur. J. Public Health 2021, 31, ckab165.035. [Google Scholar] [CrossRef]
- Joint Rapid Responce Forces (JRRFs)—Joint Integrating Concept [Internet]. Italian MInistry of Defence. Available online: https://www.difesa.it/SMD_/Staff/Reparti/III/Trasformazione/Pagine/Joint_Rapid_Responce_Forces.aspx (accessed on 12 March 2023).
- Manian, P. Chronic obstructive pulmonary disease classification, phenotypes and risk assessment. J. Thorac. Dis. 2019, 11, S1761–S1766. [Google Scholar] [CrossRef]
- Ruiz De Oña, J.M.; Gómez Fernández, M.; Celdrán, J.; Puente-Maestu, L. Neumonía en el paciente con enfermedad pulmonar obstructiva crónica. Niveles de gravedad y clases de riesgo. Arch. Bronconeumol. 2003, 39, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bianciardi, C.; Roveta, A.; Maconi, A.; Virto, N.; Bollano, A.; Barooty, S.; Volpini; Centini, G.; Kozel, D.; Ferrario, L.; et al. Dalla Gestione Del Paziente Covid-19 All’assorbimento di Risorse: Evidenze da una Valutazione Pilota [From the Management of the Covid-19 Patient to the Absorption of Resources: Evidence from a Pilot Evaluation] Impresa Sanità; Editore Temi Srl: Monza, Italy, 2020. [Google Scholar]
- Cicchetti, A.; Di Bidino, R. Interim analysis sull’impatto economico per l’Ssn del Covid-19 (DRG ospedalieri e costo terapie intensive) [Interim analysis on economic impact of COVID-19 on National Italian Healthcare]. Available online: https://altems.unicatt.it/altems-flash%20report%20altems.pdf (accessed on 12 March 2023).
- Pietrantonio, F.; Piasini, L.; Spandonaro, F. Internal Medicine and emergency admissions: From a national Hospital Discharge Records (SDO) study to a regional analysis. Ital. J. Med. 2016, 10. Available online: http://www.italjmed.org/index.php/ijm/article/view/itjm.2016.674 (accessed on 12 March 2023). [CrossRef]
- Ruggeri, M.; Signorini, A.; Drago, C.; Rosiello, F.; Marchetti, M. Modello di stima dei costi sanitari e della capacity delle terapie intensive in Italia nel trattamento di pazienti affetti da COVID-19: Valutazione dell’impatto di remdesivir. AboutOpen 2020, 7, 95–102. [Google Scholar] [CrossRef]
- Rosiello, F.; D’Oca, E. Vaccinations and the movement of antivaccers. Eur. J. Public Health. 2020, 3. Available online: https://academic.oup.com/eurpub/article/doi/10.1093/eurpub/ckaa166.700/5916042 (accessed on 12 March 2023).
- Rosiello, F.; Desideri, E.; Vinci, A.; Zelinotti, L. Adequacy of hospitals in Rome to an unconventional event (CBRNe): TTX simulation and HTA. Eur. J. Public Health 2020, 30 (Suppl. 5), ckaa166–ckaa593. Available online: https://academic.oup.com/eurpub/article/doi/10.1093/eurpub/ckaa166.593/5914901 (accessed on 12 March 2023). [CrossRef]
- Bianco, A.; Licata, F.; Zucco, R.; Papadopoli, R.; Pavia, M. Knowledge and practices regarding antibiotics use: Findings from a cross-sectional survey among Italian adults. Evol. Med. Public Health 2020, 2020, 129–138. [Google Scholar] [CrossRef]
- Vinci, A.; Ingravalle, F.; Mancinelli, S.; D’Ercole, M.; Lucaroni, F.; Palombi, L. Impact of Asbestos-Related Toxicity on Italian Working Population: Preliminary Incidence Data from the Last 5 Years across the Whole Country. In Toxic Chemical and Biological Agents; Sindona, G., Banoub, J.H., Di Gioia, M.L., Eds.; NATO Science for Peace and Security Series A: Chemistry and Biology; Springer: Dordrecht, The Netherlands, 2020; pp. 271–275. Available online: http://link.springer.com/10.1007/978-94-024-2041-8_29 (accessed on 12 March 2023).
- Appelman, Y.; van Rijn, B.B.; Ten Haaf, M.E.; Boersma, E.; Peters, S.A.E. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015, 241, 211–218. [Google Scholar] [CrossRef]
- Polverino, F.; Stern, D.A.; Ruocco, G.; Balestro, E.; Bassetti, M.; Candelli, M.; Cirillo, B.; Contoli, M.; Corsico, A.; D’Amico, F.; et al. Comorbidities, Cardiovascular Therapies, and COVID-19 Mortality: A Nationwide, Italian Observational Study (ItaliCO). Front. Cardiovasc. Med. 2020, 7, 170. [Google Scholar] [CrossRef]
- Cantini, F.; Goletti, D.; Petrone, L.; Najafi Fard, S.; Niccoli, L.; Foti, R. Immune Therapy, or Antiviral Therapy, or Both for COVID-19: A Systematic Review. Drugs 2020, 80, 1929–1946. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Bussi, A.R.; Amadasi, S.; Bresciani, E.; Caldonazzo, A.; Colombini, P.; Giovannini, M.S.; Grifi, G.; Lanzini, L.; Migliorati, P.; et al. Technological Challenges set up by Continuous Wireless Monitoring designed to Improve Management of Critically Ill Patients in an Internal Medicine Unit (LIMS study): Study Design and Preliminary Results. J. Community Prev. Med. 2019, 2, 1–7. Available online: https://asclepiusopen.com/journal-of-community-and-preventive-medicine/volume-2-issue-1/4.pdf (accessed on 12 March 2023). [CrossRef]
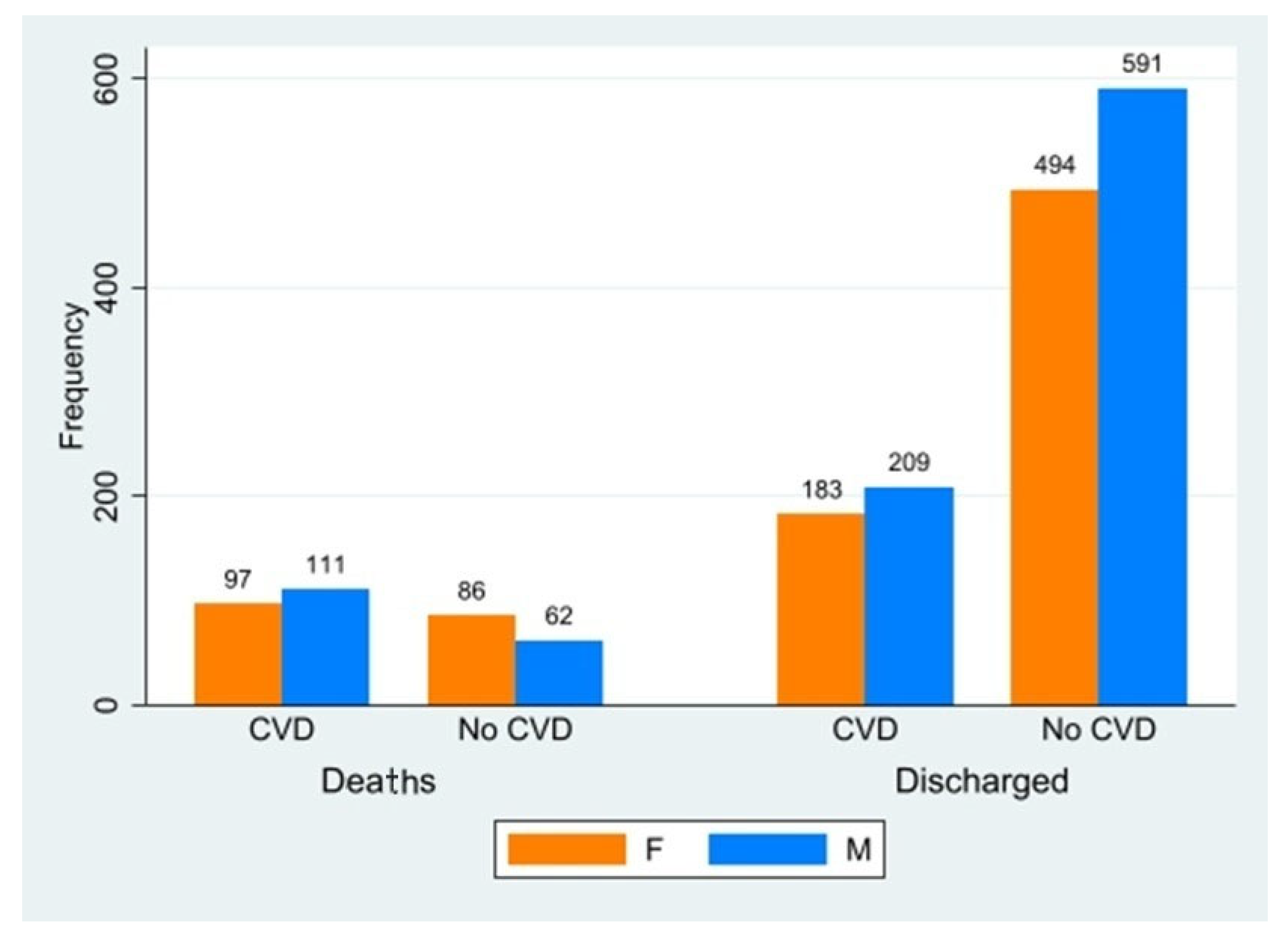
| Deaths | Discharged | Avg. LOS (SD) | Avg. Age (SD) | |
|---|---|---|---|---|
| CVD | ||||
| F | 97 | 183 | 21 (2.2) | 78.6 (1.6) |
| M | 111 | 209 | 22 (2.1) | 75.1 (1.8) |
| No-CVD | ||||
| F | 86 | 494 | 23 (2.8) | 79 (2.0) |
| M | 62 | 591 | 23 (2.5) | 77.8 (2.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrantonio, F.; Ciamei, A.; Vinci, A.; Ciarambino, T.; Alessi, E.; Pascucci, M.; Delli Castelli, M.; Zito, S.; Sanguedolce, S.; Rainone, M.; et al. Polypharmacy Management in a Gender Perspective: At the Heart of the Problem: Analysis of Major Cardiac Diseases, SARS-CoV-2 Affection and Gender Distribution in a Cohort of Patients in Internal Medicine Ward. Int. J. Environ. Res. Public Health 2023, 20, 5711. https://doi.org/10.3390/ijerph20095711
Pietrantonio F, Ciamei A, Vinci A, Ciarambino T, Alessi E, Pascucci M, Delli Castelli M, Zito S, Sanguedolce S, Rainone M, et al. Polypharmacy Management in a Gender Perspective: At the Heart of the Problem: Analysis of Major Cardiac Diseases, SARS-CoV-2 Affection and Gender Distribution in a Cohort of Patients in Internal Medicine Ward. International Journal of Environmental Research and Public Health. 2023; 20(9):5711. https://doi.org/10.3390/ijerph20095711
Chicago/Turabian StylePietrantonio, Filomena, Angela Ciamei, Antonio Vinci, Tiziana Ciarambino, Elena Alessi, Matteo Pascucci, Michela Delli Castelli, Silvia Zito, Simona Sanguedolce, Marianna Rainone, and et al. 2023. "Polypharmacy Management in a Gender Perspective: At the Heart of the Problem: Analysis of Major Cardiac Diseases, SARS-CoV-2 Affection and Gender Distribution in a Cohort of Patients in Internal Medicine Ward" International Journal of Environmental Research and Public Health 20, no. 9: 5711. https://doi.org/10.3390/ijerph20095711







