Hypertensive Disorders of Pregnancy and Pre-Pregnancy Hypertension with Subsequent Incident Venous Thromboembolic Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
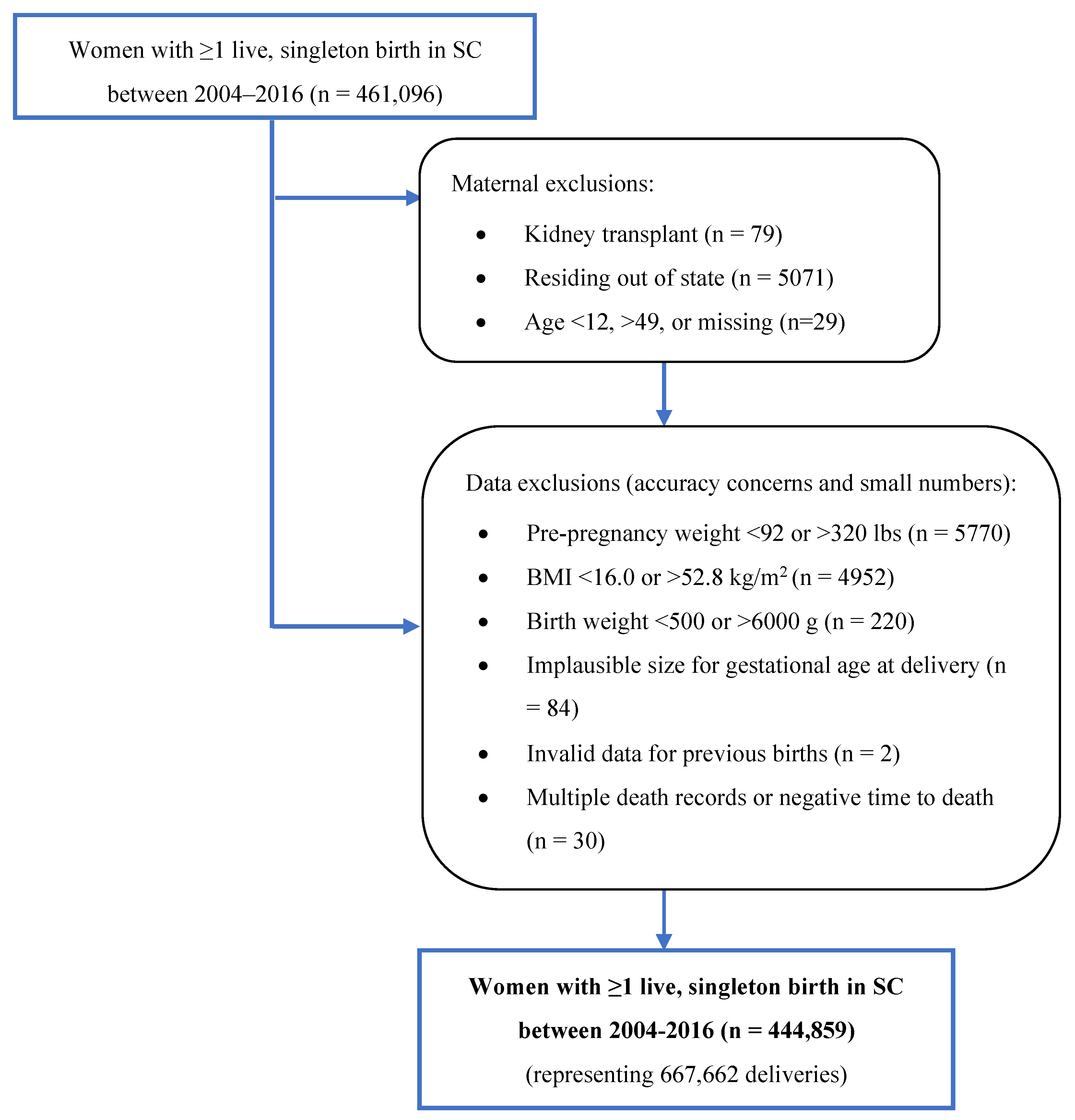
2.3. Inclusion and Exclusion Criteria
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Incident VTE Events Subsequent to HDP and/or Pre-Pregnancy Hypertension
3.2. Racial and Ethnic Differences in Incident VTE Events Following HDP and/or Pre-Pregnancy Hypertension
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heit, J.A.; Kobbervig, C.E.; James, A.H.; Petterson, T.M.; Bailey, K.R.; Melton, L.J., 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: A 30-year population-based study. Ann. Intern. Med. 2005, 143, 697–706. [Google Scholar] [CrossRef]
- Pomp, E.R.; Lenselink, A.M.; Rosendaal, F.R.; Doggen, C.J. Pregnancy, the postpartum period and prothrombotic defects: Risk of venous thrombosis in the MEGA study. J. Thromb. Haemost. 2008, 6, 632–637. [Google Scholar] [CrossRef] [PubMed]
- James, A.H.; Jamison, M.G.; Brancazio, L.R.; Myers, E.R. Venous thromboembolism during pregnancy and the postpartum period: Incidence, risk factors, and mortality. Am. J. Obstet. Gynecol. 2006, 194, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.M.; Ananth, C.V. Obstetrical venous thromboembolism: Epidemiology and strategies for prophylaxis. Semin. Perinatol. 2016, 40, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Breathett, K.; Muhlestein, D.; Foraker, R.; Gulati, M. Differences in preeclampsia rates between African American and Caucasian women: Trends from the National Hospital Discharge Survey. J. Womens Health 2014, 23, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.I.; Gonzalez, J.M.; Anderson, C.A.M.; Judd, S.E.; Rexrode, K.M.; Hlatky, M.A.; Gunderson, E.P.; Stuart, J.J.; Vaidya, D.; American Heart Association Council on Epidemiology and Prevention; et al. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e902–e916. [Google Scholar] [CrossRef]
- Johnson, J.D.; Louis, J.M. Does race or ethnicity play a role in the origin, pathophysiology, and outcomes of preeclampsia? An expert review of the literature. Am. J. Obstet. Gynecol. 2020, 226, S876–S885. [Google Scholar] [CrossRef] [PubMed]
- Grobman, W.A.; Parker, C.B.; Willinger, M.; Wing, D.A.; Silver, R.M.; Wapner, R.J.; Simhan, H.N.; Parry, S.; Mercer, B.M.; Haas, D.M.; et al. Racial Disparities in Adverse Pregnancy Outcomes and Psychosocial Stress. Obstet. Gynecol. 2018, 131, 328–335. [Google Scholar] [CrossRef]
- Ghosh, G.; Grewal, J.; Mannisto, T.; Mendola, P.; Chen, Z.; Xie, Y.; Laughon, S.K. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn. Dis. 2014, 24, 283–289. [Google Scholar]
- Miranda, M.L.; Swamy, G.K.; Edwards, S.; Maxson, P.; Gelfand, A.; James, S. Disparities in maternal hypertension and pregnancy outcomes: Evidence from North Carolina, 1994–2003. Public Health Rep. 2010, 125, 579–587. [Google Scholar] [CrossRef]
- Roberts, J.M.; Rich-Edwards, J.W.; McElrath, T.F.; Garmire, L.; Myatt, L.; Global Pregnancy Collaboration. Subtypes of Preeclampsia: Recognition and Determining Clinical Usefulness. Hypertension 2021, 77, 1430–1441. [Google Scholar] [CrossRef]
- Petersen, E.E.; Davis, N.L.; Goodman, D.; Cox, S.; Syverson, C.; Seed, K.; Shapiro-Mendoza, C.; Callaghan, W.M.; Barfield, W. Racial/Ethnic Disparities in Pregnancy-Related Deaths—United States, 2007–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.N.; Pudwell, J.; Walker, M.; Wen, S.W. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. J. Obstet. Gynaecol. Can. 2012, 34, 830–835. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Dunford, J.; Mehran, R.; Robson, S.; Kunadian, V. Pre-eclampsia and future cardiovascular risk among women: A review. J. Am. Coll. Cardiol. 2014, 63, 1815–1822. [Google Scholar] [CrossRef]
- Prandoni, P.; Bilora, F.; Marchiori, A.; Bernardi, E.; Petrobelli, F.; Lensing, A.W.; Prins, M.H.; Girolami, A. An association between atherosclerosis and venous thrombosis. N. Engl. J. Med. 2003, 348, 1435–1441. [Google Scholar] [CrossRef]
- Sorensen, H.T.; Horvath-Puho, E.; Pedersen, L.; Baron, J.A.; Prandoni, P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: A 20-year cohort study. Lancet 2007, 370, 1773–1779. [Google Scholar] [CrossRef]
- Klok, F.A.; Mos, I.C.; Broek, L.; Tamsma, J.T.; Rosendaal, F.R.; de Roos, A.; Huisman, M.V. Risk of arterial cardiovascular events in patients after pulmonary embolism. Blood 2009, 114, 1484–1488. [Google Scholar] [CrossRef]
- Roach, R.E.; Lijfering, W.M.; Flinterman, L.E.; Rosendaal, F.R.; Cannegieter, S.C. Increased risk of CVD after VT is determined by common etiologic factors. Blood 2013, 121, 4948–4954. [Google Scholar] [CrossRef]
- Lijfering, W.M.; Flinterman, L.E.; Vandenbroucke, J.P.; Rosendaal, F.R.; Cannegieter, S.C. Relationship between venous and arterial thrombosis: A review of the literature from a causal perspective. Semin. Thromb. Hemost. 2011, 37, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.; Bauersachs, R.; Scholz, U.; Zotz, R.; Bergmann, F.; Rott, H.; Linnemann, B. Prevention of Venous Thromboembolism during Pregnancy and the Puerperium with a Special Focus on Women with Hereditary Thrombophilia or Prior VTE-Position Paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Hamostaseologie 2020, 40, 572–590. [Google Scholar] [CrossRef]
- Hansen, A.T.; Schmidt, M.; Horvath-Puho, E.; Pedersen, L.; Rothman, K.J.; Hvas, A.M.; Sorensen, H.T. Preconception venous thromboembolism and placenta-mediated pregnancy complications. J. Thromb. Haemost. 2015, 13, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Mazzolai, L.; Ageno, W.; Alatri, A.; Bauersachs, R.; Becattini, C.; Brodmann, M.; Emmerich, J.; Konstantinides, S.; Meyer, G.; Middeldorp, S.; et al. Second consensus document on diagnosis and management of acute deep vein thrombosis: Updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur. J. Prev. Cardiol. 2022, 29, 1248–1263. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tuncalp, O.; Moller, A.B.; Daniels, J.; Gulmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [PubMed]
- Virkus, R.A.; Lokkegaard, E.; Lidegaard, O.; Langhoff-Roos, J.; Nielsen, A.K.; Rothman, K.J.; Bergholt, T. Risk factors for venous thromboembolism in 1.3 million pregnancies: A nationwide prospective cohort. PLoS ONE 2014, 9, e96495. [Google Scholar] [CrossRef]
- Rodger, M.A.; Langlois, N.J.; de Vries, J.I.; Rey, E.; Gris, J.C.; Martinelli, I.; Schleussner, E.; Ramsay, T.; Mallick, R.; Skidmore, B.; et al. Low-molecular-weight heparin for prevention of placenta-mediated pregnancy complications: Protocol for a systematic review and individual patient data meta-analysis (AFFIRM). Syst. Rev. 2014, 3, 69. [Google Scholar] [CrossRef][Green Version]
- Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA 2011, 306, 2459–2468. [Google Scholar] [CrossRef]
- Havers-Borgersen, E.; Butt, J.H.; Johansen, M.; Petersen, O.B.; Ekelund, C.K.; Rode, L.; Olesen, J.B.; Kober, L.; Fosbol, E.L. Preeclampsia and Long-Term Risk of Venous Thromboembolism. JAMA Netw. Open 2023, 6, e2343804. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.F.; Skjeldestad, F.E.; Sandset, P.M. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium—A register-based case-control study. Am. J. Obstet. Gynecol. 2008, 233, e231–e237. [Google Scholar] [CrossRef]
- Van Walraven, C.; Mamdani, M.; Cohn, A.; Katib, Y.; Walker, M.; Rodger, M.A. Risk of subsequent thromboembolism for patients with pre-eclampsia. BMJ 2003, 326, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.; Dahlback, B.; Marsal, K. Thrombotic risk during pregnancy: A population study. Obstet. Gynecol. 1999, 94, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Scheres, L.J.J.; Lijfering, W.M.; Groenewegen, N.F.M.; Koole, S.; de Groot, C.J.M.; Middeldorp, S.; Cannegieter, S.C. Hypertensive Complications of Pregnancy and Risk of Venous Thromboembolism. Hypertension 2020, 75, 781–787. [Google Scholar] [CrossRef]
- Alexander, G.R.; Himes, J.H.; Kaufman, R.B.; Mor, J.; Kogan, M. A United States national reference for fetal growth. Obstet. Gynecol. 1996, 87, 163–168. [Google Scholar] [CrossRef]
- Alexander, G.R.; Kotelchuck, M. Quantifying the adequacy of prenatal care: A comparison of indices. Public. Health Rep. 1996, 111, 408–418, discussion 419. [Google Scholar]
- Tayebi, T.; Hamzehgardeshi, Z.; Ahmad Shirvani, M.; Dayhimi, M.; Danesh, M. Relationship between Revised Graduated Index (R-GINDEX) of prenatal care utilization & preterm labor and low birth weight. Glob. J. Health Sci. 2014, 6, 131–137. [Google Scholar] [CrossRef]
- SAS Institute, Statistical Analytical Software, version 9.4; SAS Institute Inc.: Cary, NC, USA, 2012.
- StataCorp. Stata Statistical Software; release 13.1; StataCorp LP: College Station, TX, USA, 2013. [Google Scholar]
- Honigberg, M.C.; Zekavat, S.M.; Aragam, K.; Klarin, D.; Bhatt, D.L.; Scott, N.S.; Peloso, G.M.; Natarajan, P. Long-Term Cardiovascular Risk in Women with Hypertension During Pregnancy. J. Am. Coll. Cardiol. 2019, 74, 2743–2754. [Google Scholar] [CrossRef]
- Wen, T.; Wright, J.D.; Goffman, D.; D’Alton, M.E.; Mack, W.J.; Attenello, F.J.; Friedman, A.M. Postpartum venous thromboembolism readmissions in the United States. Am. J. Obstet. Gynecol. 2018, 219, 401.e1–401.e14. [Google Scholar] [CrossRef]
- Simpson, E.L.; Lawrenson, R.A.; Nightingale, A.L.; Farmer, R.D. Venous thromboembolism in pregnancy and the puerperium: Incidence and additional risk factors from a London perinatal database. BJOG Int. J. Obstet. Gynaecol. 2001, 108, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Garovic, V.D.; Dechend, R.; Easterling, T.; Karumanchi, S.A.; McMurtry Baird, S.; Magee, L.A.; Rana, S.; Vermunt, J.V.; August, P.; American Heart Association Council on Hypertension; et al. Hypertension in Pregnancy: Diagnosis, Blood Pressure Goals, and Pharmacotherapy: A Scientific Statement from the American Heart Association. Hypertension 2022, 79, e21–e41. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Blondon, M.; Harrington, L.B.; Righini, M.; Boehlen, F.; Bounameaux, H.; Smith, N.L. Racial and ethnic differences in the risk of postpartum venous thromboembolism: A population-based, case-control study. J. Thromb. Haemost. 2014, 12, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Philipp, C.S.; Faiz, A.S.; Beckman, M.G.; Grant, A.; Bockenstedt, P.L.; Heit, J.A.; James, A.H.; Kulkarni, R.; Manco-Johnson, M.J.; Moll, S.; et al. Differences in thrombotic risk factors in black and white women with adverse pregnancy outcome. Thromb. Res. 2014, 133, 108–111. [Google Scholar] [CrossRef][Green Version]
- Lutsey, P.L.; Wassel, C.L.; Cushman, M.; Sale, M.M.; Divers, J.; Folsom, A.R. Genetic admixture is associated with plasma hemostatic factor levels in self-identified African Americans and Hispanics: The Multi-Ethnic Study of Atherosclerosis. J. Thromb. Haemost. 2012, 10, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Favaloro, E.J.; Franchini, M.; Guidi, G.C.; Lippi, G. The role of ethnicity, age and gender in venous thromboembolism. J. Thromb. Thrombolysis 2010, 29, 489–496. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Kroegel, C.; Reissig, A. Principle mechanisms underlying venous thromboembolism: Epidemiology, risk factors, pathophysiology and pathogenesis. Respiration 2003, 70, 7–30. [Google Scholar] [CrossRef]
- Watson, C.; Saaid, H.; Vedula, V.; Cardenas, J.C.; Henke, P.K.; Nicoud, F.; Xu, X.Y.; Hunt, B.J.; Manning, K.B. Venous Thromboembolism: Review of Clinical Challenges, Biology, Assessment, Treatment, and Modeling. Ann. Biomed. Eng. 2023, in press. [Google Scholar] [CrossRef]
- Bauer, K.A.; Lip, G.Y.H. Overview of the Causes of Venous Thrombosis. Available online: https://www.uptodate.com/contents/overview-of-the-causes-of-venous-thrombosis?search=vte%20risk%20factors&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H4 (accessed on 16 October 2023).
- Geller, S.E.; Ahmed, S.; Brown, M.L.; Cox, S.M.; Rosenberg, D.; Kilpatrick, S.J. International Classification of Diseases-9th revision coding for preeclampsia: How accurate is it? Am. J. Obstet. Gynecol. 2004, 190, 1629–1633, discussion 1633–1624. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total | Neither Pre-Pregnancy Hypertension nor HDP | Pre-Pregnancy Hypertension | HDP | Both Pre-Pregnancy Hypertension and HDP | ||
|---|---|---|---|---|---|---|---|
| % or Mean ± SD | n = 444,859 | n = 395,114 (88.8%) | n = 11,580 (2.6%) | n = 25,652 (5.8%) | n = 12,513 (2.8%) | p | |
| Maternal age at delivery, y | 27.2 ± 6.0 | 27.1 ± 6.0 | 28.9 ± 6.0 | 27.1 ± 6.2 | 30.3 ± 6.4 | <0.0001 | |
| <20 | 9.9 | 10.2 | 5.4 | 11.1 | 4.7 | <0.0001 | |
| 20–24 | 26.4 | 26.9 | 20 | 26.6 | 16.4 | ||
| 25–29 | 28.4 | 28.6 | 28.3 | 28 | 23.5 | ||
| 30–34 | 22.3 | 22 | 27 | 21.1 | 27.5 | ||
| 35–39 | 10.6 | 10.1 | 15.2 | 10.4 | 20.7 | ||
| 40+ | 2.4 | 2.2 | 4.1 | 2.8 | 7.1 | ||
| Race and ethnicity | |||||||
| Non-Hispanic White | 57.6 | 58.4 | 44.6 | 58.6 | 44.8 | <0.0001 | |
| Non-Hispanic Black | 30.9 | 29.5 | 50.4 | 33.3 | 50.4 | ||
| Hispanic | 9.1 | 9.6 | 4.2 | 6.5 | 3.5 | ||
| Other | 2.4 | 2.5 | 0.8 | 1.6 | 1.2 | ||
| Education | |||||||
| Less than HS | 18.2 | 18.6 | 17.4 | 16 | 12.7 | <0.0001 | |
| HS graduate | 24.5 | 24.3 | 26.8 | 25.1 | 27.2 | ||
| Some college | 25.2 | 24.9 | 27.7 | 27.5 | 27.7 | ||
| ≥College graduate | 32 | 32.1 | 28 | 31.5 | 32.3 | ||
| Rural residence | 26.2 | 26.3 | 29.2 | 22.4 | 27 | <0.0001 | |
| Annual household income | |||||||
| <$36,000 | 27 | 26.7 | 36.9 | 25.4 | 31.1 | <0.0001 | |
| $36,000 to <$54,000 | 47.2 | 47.1 | 43.9 | 49.5 | 47.9 | ||
| ≥$54,000 | 23.7 | 24 | 17.6 | 23.4 | 20.5 | ||
| Primary payer during pregnancy | |||||||
| Medicaid | 48.2 | 47.9 | 55.3 | 49 | 50 | <0.0001 | |
| Private | 39.8 | 39.7 | 37.8 | 41.5 | 41.7 | ||
| Self-pay | 5.3 | 5.6 | 3.3 | 3.3 | 2.4 | ||
| Other | 5.9 | 6 | 3 | 5.5 | 5 | ||
| WIC eligible in pregnancy | 51.3 | 50.9 | 58 | 51.7 | 56.6 | <0.0001 | |
| Smoking during pregnancy | 11.7 | 11.8 | 12.3 | 10.9 | 11.3 | <0.0001 | |
| Pre-pregnancy BMI (kg/m2) | 27.2 ± 6.8 | 26.7 ± 6.4 | 32.3 ± 7.9 | 30.1 ± 7.6 | 33.4 ± 7.9 | <0.0001 | |
| Gestational age at delivery | 38.4 ± 1.9 | 38.6 ± 1.8 | 37.4 ± 2.6 | 37.4 ± 2.5 | 37.4 ± 2.6 | <0.0001 | |
| Mode of delivery | |||||||
| Cesarean section | 34.1 | 32.1 | 50.6 | 47.4 | 53.3 | <0.0001 | |
| Vaginal | 65.9 | 67.9 | 49.3 | 52.6 | 46.7 | ||
| Induced labor | 14.5 | 13.5 | 15.5 | 26.8 | 20.4 | <0.0001 | |
| No. of pregnancies prior to index pregnancy | 0.8 ± 1.1 | 0.8 ± 1.1 | 0.9 ± 1.2 | 0.6 ± 1.0 | 0.9 ± 1.2 | <0.0001 | |
| Previous cesarean section | |||||||
| Yes | 5.6 | 5.4 | 12.7 | 5.2 | 6.6 | <0.0001 | |
| No | 41.1 | 40.8 | 36.5 | 47.8 | 42.1 | ||
| Unknown | 53.3 | 53.9 | 50.8 | 47 | 51.3 | ||
| Previous preterm birth | 2.6 | 2.5 | 6.4 | 2.9 | 4.2 | <0.0001 | |
| Prenatal care as measured via R-GINDEX | |||||||
| Inadequate | 17.6 | 18.2 | 13.2 | 14.7 | 11.6 | <0.0001 | |
| Intermediate | 22.5 | 23 | 18.8 | 20.5 | 16.5 | ||
| Adequate | 1.6 | 1.6 | 1.8 | 1.8 | 1.6 | ||
| Intensive | 28.8 | 27.5 | 39 | 36.4 | 44.7 | ||
| No care | 0.9 | 0.9 | 1 | 0.8 | 1 | ||
| Missing | 28.5 | 28.9 | 26.1 | 26 | 24.6 | ||
| Pre-pregnancy or gestational diabetes mellitus | 6.7 | 16.3 | <0.0001 | ||||
| Incident VTE Events | ||||
|---|---|---|---|---|
| Event | Event Rate (95% CI) 1 | HR (95% CI) 2,3 | ||
| Within 1 year of delivery | ||||
| Neither pre-pregnancy HTN nor HDP | 254 | 0.64 (0.57–0.73) | Referent | |
| Pre-pregnancy HTN | 8 | 0.69 (0.35–1.38) | 0.77 | (0.36–1.66) |
| HDP | 41 | 1.60 (1.18–2.17) | 1.62 | (1.15–2.29) |
| Both pre-pregnancy HTN and HDP | 41 | 3.28 (2.42–4.46) | 2.23 | (1.60–3.35) |
| Within 5 years of delivery | ||||
| Neither pre-pregnancy HTN nor HDP | 1283 | 0.65 (0.62–0.69) | Referent | |
| Pre-pregnancy HTN | 66 | 1.14 (0.90–1.46) | 1.21 | (0.93–1.57) |
| HDP | 152 | 1.19 (1.01–1.39) | 1.35 | (1.13–1.60) |
| Both pre-pregnancy HTN and HDP | 134 | 2.16 (1.82–2.55) | 1.82 | (1.50–2.20) |
| Within 14 years of delivery | ||||
| Neither pre-pregnancy HTN nor HDP | 3019 | 0.91 (0.88–0.94) | Referent | |
| Pre-pregnancy HTN | 202 | 1.85 (1.62–2.13) | 1.36 | (1.18–1.58) |
| HDP | 297 | 1.53 (1.37–1.71) | 1.31 | (1.16–1.48) |
| Both pre-pregnancy HTN and HDP | 244 | 2.60 (2.29–2.94) | 1.74 | (1.52–2.00) |
| Non-Hispanic White Women | Non-Hispanic Black Women | ||||||
|---|---|---|---|---|---|---|---|
| Incident VTE Events | Event | Event Rate (95% CI) 1 | HR (95% CI) 2 | Event | Event Rate (95% CI) 1 | HR (95% CI) 2,3 | p 4 |
| Within 1 year of delivery | |||||||
| Neither pre-pregnancy HTN nor HDP | 136 | 0.59 (0.50–0.70) | Referent | 111 | 0.95 (0.79–1.15) | 1.28 (0.97–1.69) | 0.72 |
| Pre-pregnancy HTN | 5 | 0.97 (0.40–2.33) | 1.18 (0.43–3.21) | <5 | 0.51 (0.17–1.60) | 0.67 (0.21–2.13) | |
| HDP | 22 | 1.46 (0.96–2.22) | 1.64 (1.02–2.63) | 17 | 1.99 (1.24–3.21) | 1.87 (1.10–3.18) | |
| Both pre-pregnancy HTN and HDP | 12 | 2.14 (1.22–3.77) | 2.01 (1.10–3.68) | 27 | 4.29 (2.94–6.26) | 2.90 (1.80–4.66) | |
| Within 5 years of delivery | |||||||
| Neither pre-pregnancy HTN nor HDP | 739 | 0.64 (0.60–0.69) | Referent | 495 | 0.85 (0.78–0.93) | 1.07 (0.94–1.21) | 0.46 |
| Pre-pregnancy HTN | 23 | 0.89 (0.59–1.34) | 1.00 (0.64–1.54) | 43 | 1.48 (1.10–1.99) | 1.50 (1.09–2.07) | |
| HDP | 81 | 1.08 (0.87–1.34) | 1.29 (1.02–1.63) | 66 | 1.55 (1.22–1.98) | 1.51 (1.16–1.96) | |
| Both pre-pregnancy HTN and HDP | 44 | 1.58 (1.17–2.12) | 1.59 (1.16–2.17) | 86 | 2.75 (2.22–3.39) | 2.08 (1.62–2.66) | |
| Study period (within 14 years of delivery) | |||||||
| Neither pre-pregnancy HTN nor HDP | 1603 | 0.84 (0.80–0.88) | Referent | 1310 | 1.31 (1.24–1.38) | 1.27 (1.17–1.38) | 0.95 |
| Pre-pregnancy HTN | 70 | 1.48 (1.17–1.87) | 1.32 (1.03–1.69) | 131 | 2.33 (1.96–2.77) | 1.78 (1.48–2.15) | |
| HDP | 149 | 1.33 (1.13–1.56) | 1.29 (1.09–1.54) | 141 | 2.11 (1.79–2.49) | 1.69 (1.41–2.02) | |
| Both pre-pregnancy HTN and HDP | 88 | 2.12 (1.72–2.61) | 1.79 (1.43–2.23) | 149 | 3.07 (2.62–3.61) | 2.13 (1.78–2.55) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malek, A.M.; Wilson, D.A.; Turan, T.N.; Mateus, J.; Lackland, D.T.; Hunt, K.J. Hypertensive Disorders of Pregnancy and Pre-Pregnancy Hypertension with Subsequent Incident Venous Thromboembolic Events. Int. J. Environ. Res. Public Health 2024, 21, 89. https://doi.org/10.3390/ijerph21010089
Malek AM, Wilson DA, Turan TN, Mateus J, Lackland DT, Hunt KJ. Hypertensive Disorders of Pregnancy and Pre-Pregnancy Hypertension with Subsequent Incident Venous Thromboembolic Events. International Journal of Environmental Research and Public Health. 2024; 21(1):89. https://doi.org/10.3390/ijerph21010089
Chicago/Turabian StyleMalek, Angela M., Dulaney A. Wilson, Tanya N. Turan, Julio Mateus, Daniel T. Lackland, and Kelly J. Hunt. 2024. "Hypertensive Disorders of Pregnancy and Pre-Pregnancy Hypertension with Subsequent Incident Venous Thromboembolic Events" International Journal of Environmental Research and Public Health 21, no. 1: 89. https://doi.org/10.3390/ijerph21010089
APA StyleMalek, A. M., Wilson, D. A., Turan, T. N., Mateus, J., Lackland, D. T., & Hunt, K. J. (2024). Hypertensive Disorders of Pregnancy and Pre-Pregnancy Hypertension with Subsequent Incident Venous Thromboembolic Events. International Journal of Environmental Research and Public Health, 21(1), 89. https://doi.org/10.3390/ijerph21010089







