Dietary Nutritional Supplements Are Associated with the Deterioration of Hepatic Fibrosis in Women and Individuals without Underlying Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Eligibility
2.2. Participants
2.3. Measurements of Clinical and Biochemical Variables
2.4. Definition of Dietary Nutritional Supplements and Hepatic Fibrosis
2.5. Statistical Analysis
3. Results
3.1. Participants’ Baseline Characteristics
3.2. Association between Dietary Nutritional Supplement Consumption and ALT, FIB-4, and APRI
3.3. Association between Hepatic Fibrosis and Dietary Nutritional Supplement Consumption Stratified by Diabetes Mellitus, Hypertriglyceridemia, and Sex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarro, V.J.; Lucena, M.I. Hepatotoxicity induced by herbal and dietary supplements. Semin. Liver Dis. 2014, 34, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse effects of nutraceuticals and dietary supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef]
- Cowan, A.E.; Tooze, J.A.; Gahche, J.J.; Eicher-Miller, H.A.; Guenther, P.M.; Dwyer, J.T.; Potischman, N.; Bhadra, A.; Carroll, R.J.; Bailey, R.L. Trends in overall and micronutrient-containing dietary supplement use in US adults and children, NHANES 2007–2018. J. Nutr. 2023, 152, 2789–2801. [Google Scholar] [CrossRef] [PubMed]
- Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Ouchene, A.; Yousfi, Y.; Amrane-Abider, M.; Sahraoui-Remini, Y.; Madani, K. A global overview of dietary supplements: Regulation, market trends, usage during the COVID-19 pandemic, and health effects. Nutrients 2023, 15, 3320. [Google Scholar] [CrossRef]
- Stickel, F.; Kessebohm, K.; Weimann, R.; Seitz, H.K. Review of liver injury associated with dietary supplements. Liver Int. 2011, 31, 595–605. [Google Scholar] [CrossRef]
- Kim, M.; Yoon, E.L.; Cho, S.; Lee, C.M.; Kang, B.K.; Park, H.; Jun, D.W.; Nah, E.H. Prevalence of advanced hepatic fibrosis and comorbidity in metabolic dysfunction-associated fatty liver disease in Korea. Liver Int. 2022, 42, 1536–1544. [Google Scholar] [CrossRef]
- Yang, H.I.; Yuen, M.F.; Chan, H.L.Y.; Han, K.H.; Chen, P.J.; Kim, D.Y.; Ahn, S.H.; Chen, C.J.; Wong, V.W.S.; Seto, W.K.; et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): Development and validation of a predictive score. Lancet Oncol. 2011, 12, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.J.; Dore, G.J.; Law, M.G.; Thorpe, M.; Von Overbeck, J.; Lloyd, A.R.; Marinos, G.; Kaldor, J.M. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001, 34, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Bhala, N.; Angulo, P.; van der Poorten, D.; Lee, E.; Hui, J.M.; Saracco, G.; Adams, L.A.; Charatcharoenwitthaya, P.; Topping, J.H.; Bugianesi, E.; et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: An international collaborative study. Hepatology 2011, 54, 1208–1216. [Google Scholar] [CrossRef]
- Friedman, S.L.; Pinzani, M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 2022, 75, 473–488. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; S Sulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Vasileiadi, S.; Papavdi, M.; Sveroni, E.; Antonakaki, P.; Dellaporta, E.; Koutli, E.; Michalea, S.; Manolakopoulos, S.; Koskinas, J.; et al. Liver fibrosis staging with combination of Apri and FIB-4 scoring systems in chronic hepatitis C as an alternative to transient elastography. Ann. Gastroenterol. 2019, 32, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG clinical guideline: Evaluation of abnormal liver chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Popa, S.L.; Ismaiel, A.; Abenavoli, L.; Padureanu, A.M.; Dita, M.O.; Bolchis, R.; Munteanu, M.A.; Brata, V.D.; Pop, C.; Bosneag, A.; et al. Diagnosis of liver fibrosis using artificial intelligence: A systematic review. Medicina 2023, 59, 992. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Jocken, J.W.E.; Blaak, E.E. Sexual dimorphism in cardiometabolic health: The role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol. 2021, 17, 47–66. [Google Scholar] [CrossRef]
- Kuo, T.C.; Lu, Y.B.; Yang, C.L.; Wang, B.; Chen, L.X.; Su, C.P. Association of insulin resistance indicators with hepatic steatosis and fibrosis in patients with metabolic syndrome. BMC Gastroenterol. 2024, 24, 26. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, Q.; Wang, S. Association between cardiometabolic index and hepatic steatosis and liver fibrosis: A population-based study. Hormones 2024, 23, 477–486. [Google Scholar] [CrossRef]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71. [Google Scholar] [CrossRef]
- Quesada-Vázquez, S.; Colom-Pellicer, M.; Navarro-Masip, È.; Aragonès, G.; Del Bas, J.M.; Caimari, A.; Escoté, X. Supplementation with a Specific Combination of Metabolic Cofactors Ameliorates Non-Alcoholic Fatty Liver Disease, Hepatic Fibrosis, and Insulin Resistance in Mice. Nutrients 2021, 13, 3532. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, Y.; Zuo, L.; Nie, D.; Li, X. Dietary fiber regulates intestinal flora and suppresses liver and systemic inflammation to alleviate liver fibrosis in mice. Nutrition 2021, 81, 110959. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Kan, J.; Zheng, N.; Li, B.; Hong, Y.; Yan, J.; Tao, X.; Wu, G.; Ma, J.; Zhu, W.; et al. A botanical dietary supplement from white peony and licorice attenuates nonalcoholic fatty liver disease by modulating gut microbiota and reducing inflammation. Phytomedicine 2021, 91, 153693. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Namkhah, Z.; Naeini, F.; Rezayat, S.M.; Yaseri, M.; Mansouri, S.; Hosseinzadeh-Attar, M.J. Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double-blind, placebo-controlled, clinical trial. Int. J. Clin. Pract. 2021, 75, e14852. [Google Scholar] [CrossRef]
- Soleimani, D.; Rezaie, M.; Rajabzadeh, F.; Gholizadeh Navashenaq, J.; Abbaspour, M.; Miryan, M.; Razmpour, F.; Ranjbar, G.; Rezvani, R.; Jarahi, L.; et al. Protective effects of propolis on hepatic steatosis and fibrosis among patients with nonalcoholic fatty liver disease (NAFLD) evaluated by real-time two-dimensional shear wave elastography: A randomized clinical trial. Phytother. Res. 2021, 35, 1669–1679. [Google Scholar] [CrossRef]

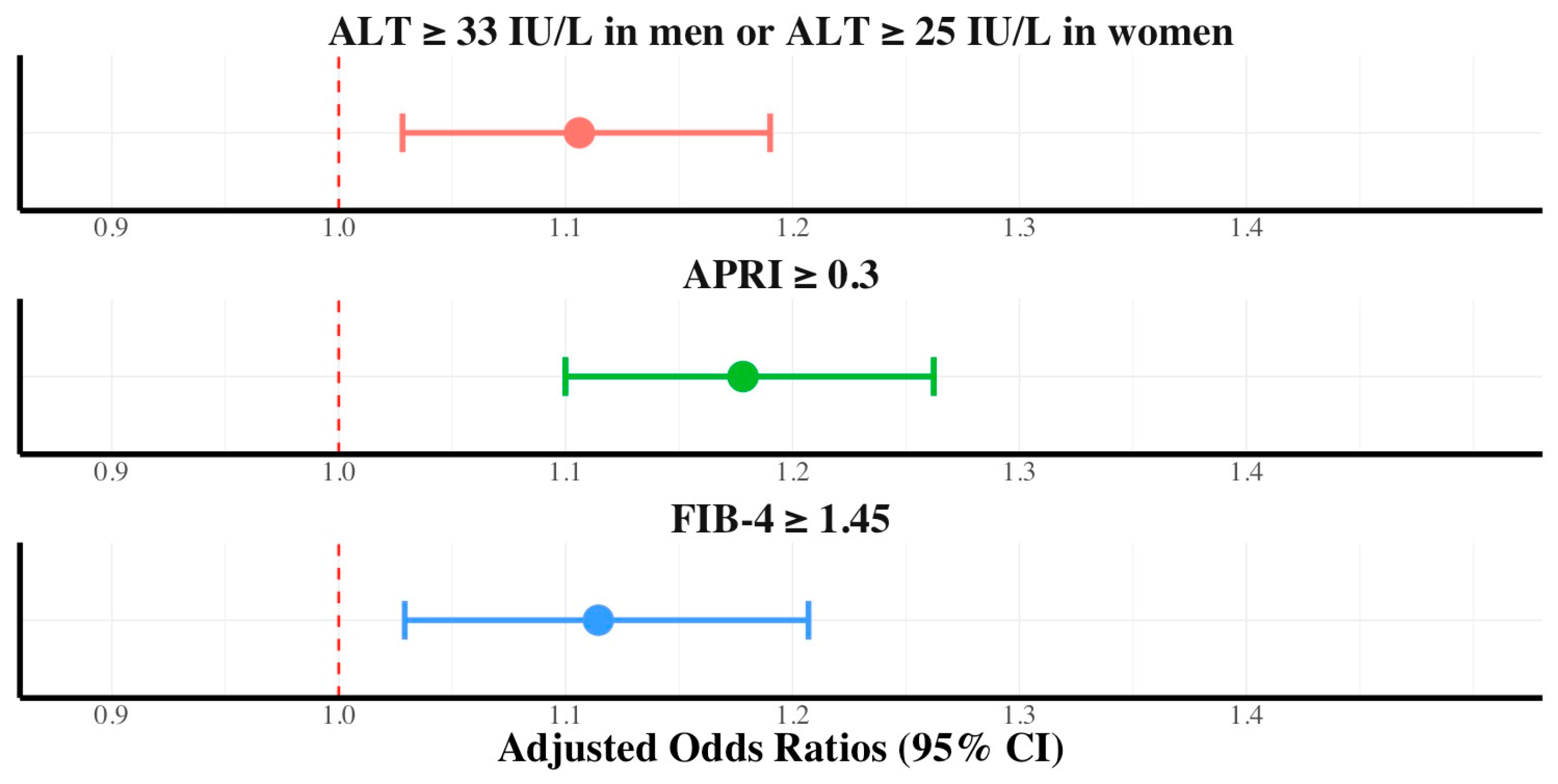
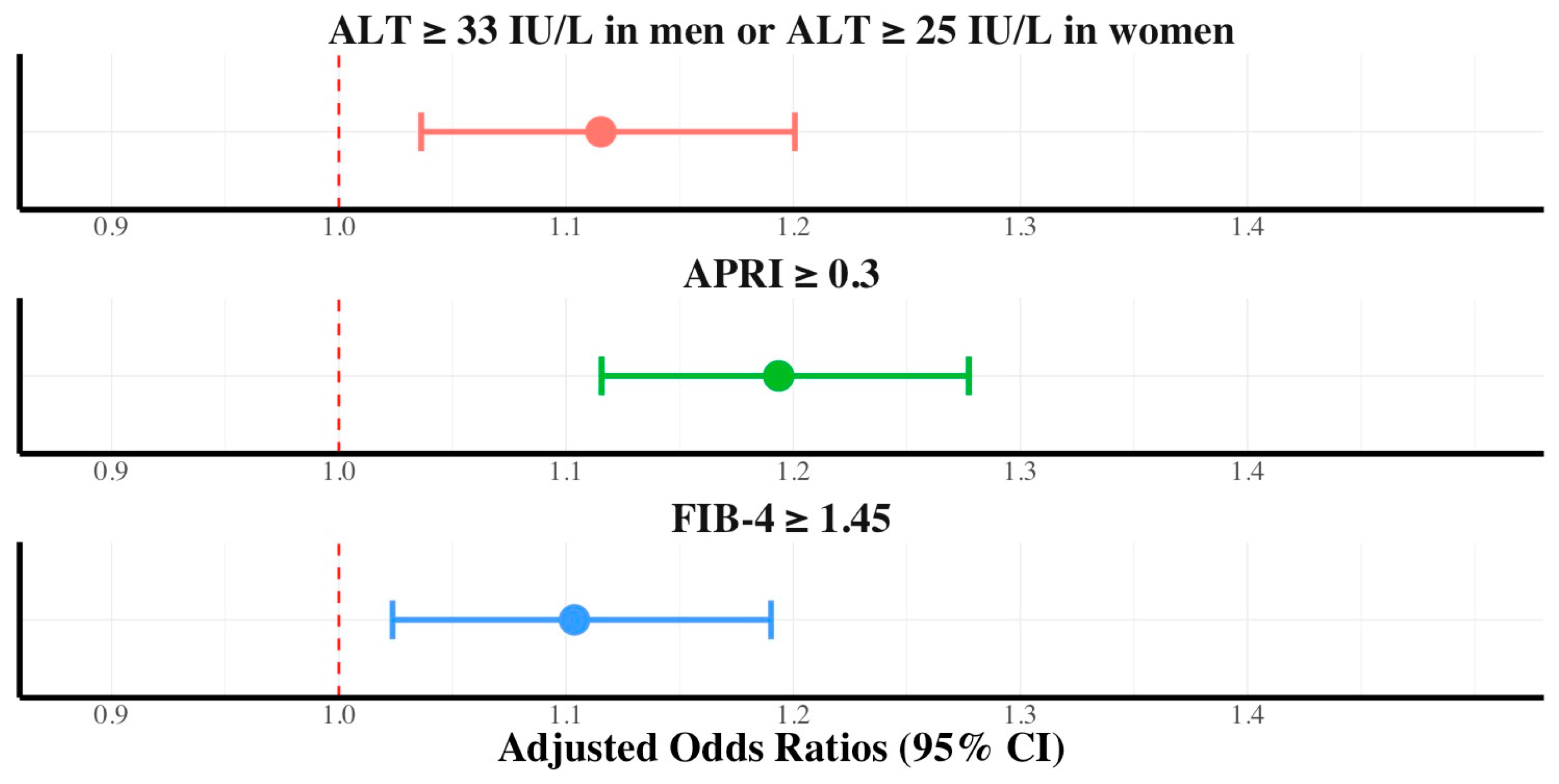
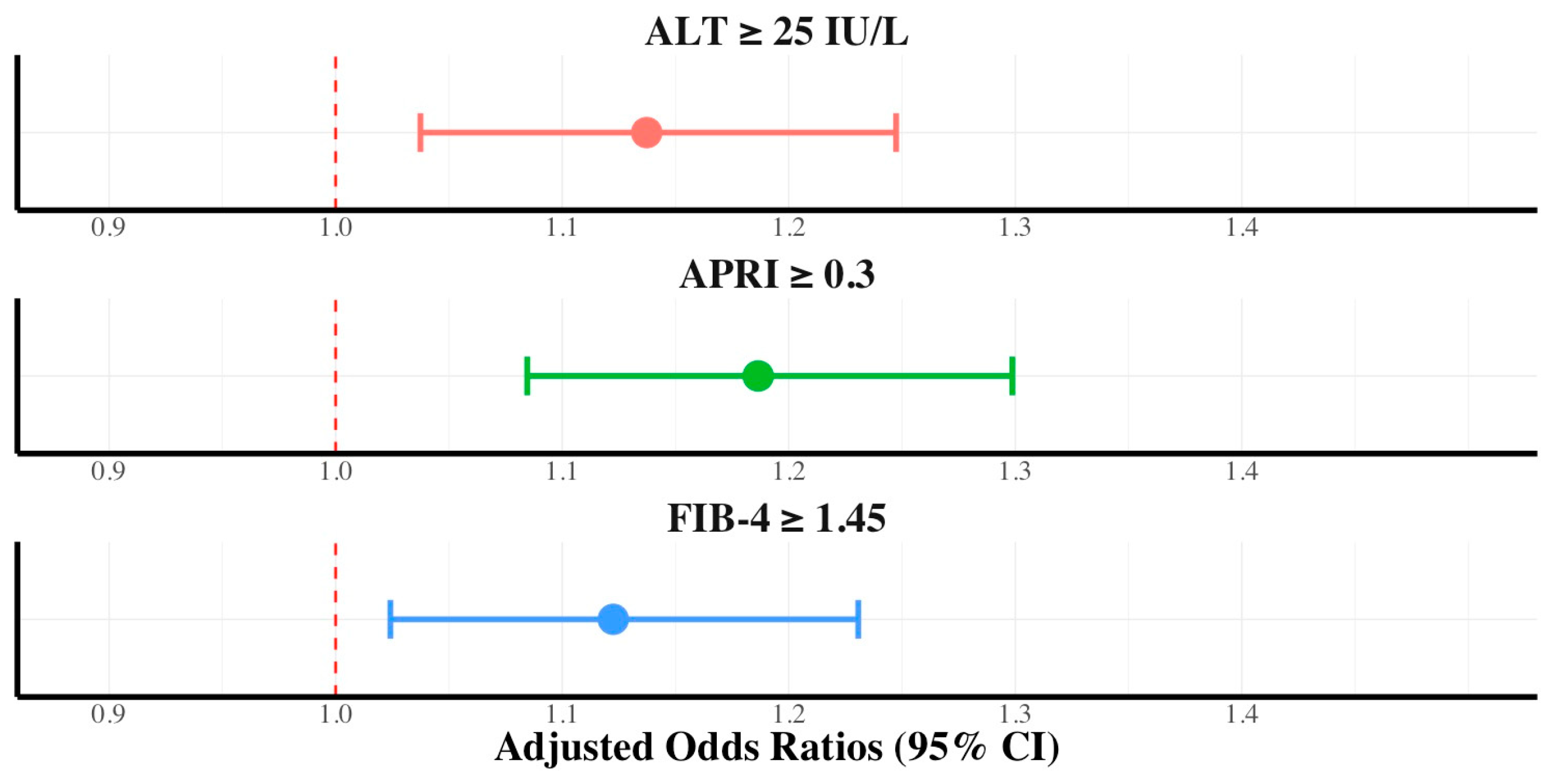
| Variables | Control (n = 6014) | Dietary Nutritional Supplement Consumer (n = 6405) | p-Value |
|---|---|---|---|
| Age (years) | 51 ± 18 | 53 ± 16 | <0.001 |
| Residential district | <0.001 | ||
| Urban | 4705 (78.2) | 5179 (80.8) | |
| Rural | 1309 (21.7) | 1226 (19.1) | |
| Education level | <0.001 | ||
| Elementary school | 1084 (18.0) | 766 (11.9) | |
| Middle school | 651 (10.8) | 568 (8.8) | |
| High school | 2188 (36.3) | 2084 (32.5) | |
| College | 2091 (34.7) | 2987 (46.6) | |
| Household income | <0.001 | ||
| Low | 1290 (21.4) | 977 (15.2) | |
| Mid-low | 1555 (25.8) | 1462 (22.8) | |
| Mid-high | 1665 (27.6) | 1768 (27.6) | |
| High | 1504 (25.0) | 2198 (34.3) | |
| Hypertension | 2190 (36.4) | 2467 (38.5) | <0.05 |
| Diabetes mellitus | 983 (16.3) | 1017 (15.8) | 0.494 |
| Hypertriglyceridemia | 1119 (18.6) | 1217 (19.0) | 0.590 |
| Smoking | <0.001 | ||
| Never smoked | 1602 (26.6) | 1658 (25.8) | |
| Ex-smoker | 2437 (40.5) | 3138 (48.9) | |
| Current smoker | 1975 (32.8) | 1609 (25.1) | |
| BMI (kg/m2) ≥ 23 | 3966 (65.9) | 4336 (67.6) | <0.05 |
| Waist circumference (cm) | 86.9 ± 9.7 | 87.4 ± 8.8 | <0.05 |
| Aerobic activity rate | 2777 (46.1) | 3230 (50.4) | <0.001 |
| Fasting glucose (mg/dL) | 103.4 ± 24.9 | 103.2 ± 22.8 | 0.780 |
| Triglycerides (mg/dL) | 147.6 ± 114.6 | 148.5 ± 107.0 | 0.672 |
| HDL-C (mg/dL) | 47.2 ± 11.4 | 47.9 ± 11.3 | <0.001 |
| LDL-C (mg/dL) | 114.5 ± 35.5 | 116.3 ± 34.6 | 0.087 |
| AST (IU/L) | 24.6 ± 13.7 | 25.2 ± 12.0 | <0.05 |
| ALT (IU/L) | 26.3 ± 20.7 | 27.1 ± 20.0 | <0.05 |
| FIB-4 ≥ 1.45 | 1713 (28.4) | 1892 (29.5) | 0.202 |
| APRI ≥ 0.30 | 1524 (25.3) | 1756 (27.4) | <0.01 |
| Variables | Control (n = 6853) | Dietary Nutritional Supplement Consumer (n = 11,367) | p-Value |
|---|---|---|---|
| Age (years) | 51 ± 18 | 53 ± 15 | <0.001 |
| Residential district | <0.001 | ||
| Urban | 5437 (79.3) | 9393 (82.6) | |
| Rural | 1416 (20.6) | 1974 (17.3) | |
| Education level | <0.001 | ||
| Elementary school | 2054 (29.9) | 2463 (21.6) | |
| Middle school | 600 (8.7) | 1163 (10.2) | |
| High school | 2006 (29.2) | 3523 (30.9) | |
| College | 2193 (32.0) | 4218 (37.1) | |
| Household income | <0.001 | ||
| Low | 1655 (24.1) | 2076 (18.2) | |
| Mid-low | 1764 (25.7) | 2714 (23.8) | |
| Mid-high | 1768 (25.7) | 3168 (27.8) | |
| High | 1666 (24.3) | 3409 (29.9) | |
| Hypertension | 2152 (31.4) | 3554 (31.2) | 0.860 |
| Diabetes mellitus | 888 (12.9) | 1270 (11.1) | <0.001 |
| Hypertriglyceridemia | 624 (9.1) | 976 (8.5) | 0.241 |
| Smoking | <0.001 | ||
| Never smoked | 6142 (89.6) | 10,368 (91.2) | |
| Ex-smoker | 418 (6.0) | 351 (3.0) | |
| Current smoker | 293 (4.2) | 648 (5.7) | |
| BMI (kg/m2) ≥ 23 | 3635 (53.0) | 5693 (50.0) | <0.001 |
| Waist circumference (cm) | 79.9 ± 10.3 | 79.7 ± 9.6 | 0.180 |
| Aerobic activity rate | 2702 (39.4) | 4968 (43.7) | <0.001 |
| Fasting glucose (mg/dL) | 98.8 ± 22.0 | 98.3 ± 19.6 | 0.074 |
| Triglycerides (mg/dL) | 113.8 ± 77.2 | 112.3 ± 71.2 | 0.196 |
| HDL-C (mg/dL) | 54.0 ± 12.8 | 56.0 ± 13.3 | <0.001 |
| LDL-C (mg/dL) | 115.8 ± 34.9 | 116.6 ± 35.4 | 0.436 |
| AST (IU/L) | 21.3 ± 11.6 | 22.1 ± 10.7 | <0.001 |
| ALT (IU/L) | 18.0 ± 14.6 | 18.8 ± 14.0 | <0.001 |
| FIB-4 ≥ 1.45 | 1663 (24.2) | 3013 (26.5) | <0.001 |
| APRI ≥ 0.30 | 933 (13.6) | 1878 (16.5) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, M.; Lee, S.; Han, K. Dietary Nutritional Supplements Are Associated with the Deterioration of Hepatic Fibrosis in Women and Individuals without Underlying Disease. Int. J. Environ. Res. Public Health 2024, 21, 1298. https://doi.org/10.3390/ijerph21101298
Cha M, Lee S, Han K. Dietary Nutritional Supplements Are Associated with the Deterioration of Hepatic Fibrosis in Women and Individuals without Underlying Disease. International Journal of Environmental Research and Public Health. 2024; 21(10):1298. https://doi.org/10.3390/ijerph21101298
Chicago/Turabian StyleCha, Minsu, Sangheun Lee, and Kijun Han. 2024. "Dietary Nutritional Supplements Are Associated with the Deterioration of Hepatic Fibrosis in Women and Individuals without Underlying Disease" International Journal of Environmental Research and Public Health 21, no. 10: 1298. https://doi.org/10.3390/ijerph21101298
APA StyleCha, M., Lee, S., & Han, K. (2024). Dietary Nutritional Supplements Are Associated with the Deterioration of Hepatic Fibrosis in Women and Individuals without Underlying Disease. International Journal of Environmental Research and Public Health, 21(10), 1298. https://doi.org/10.3390/ijerph21101298






