Musculoskeletal, Pulmonary, and Cardiovascular COVID-19 Sequelae in the Context of Firefighter Occupational Health: A Narrative Review
Abstract
1. Background
2. Review Process
3. Firefighting as an Occupation of Specific Concern for Those with Post-COVID-19 Sequelae
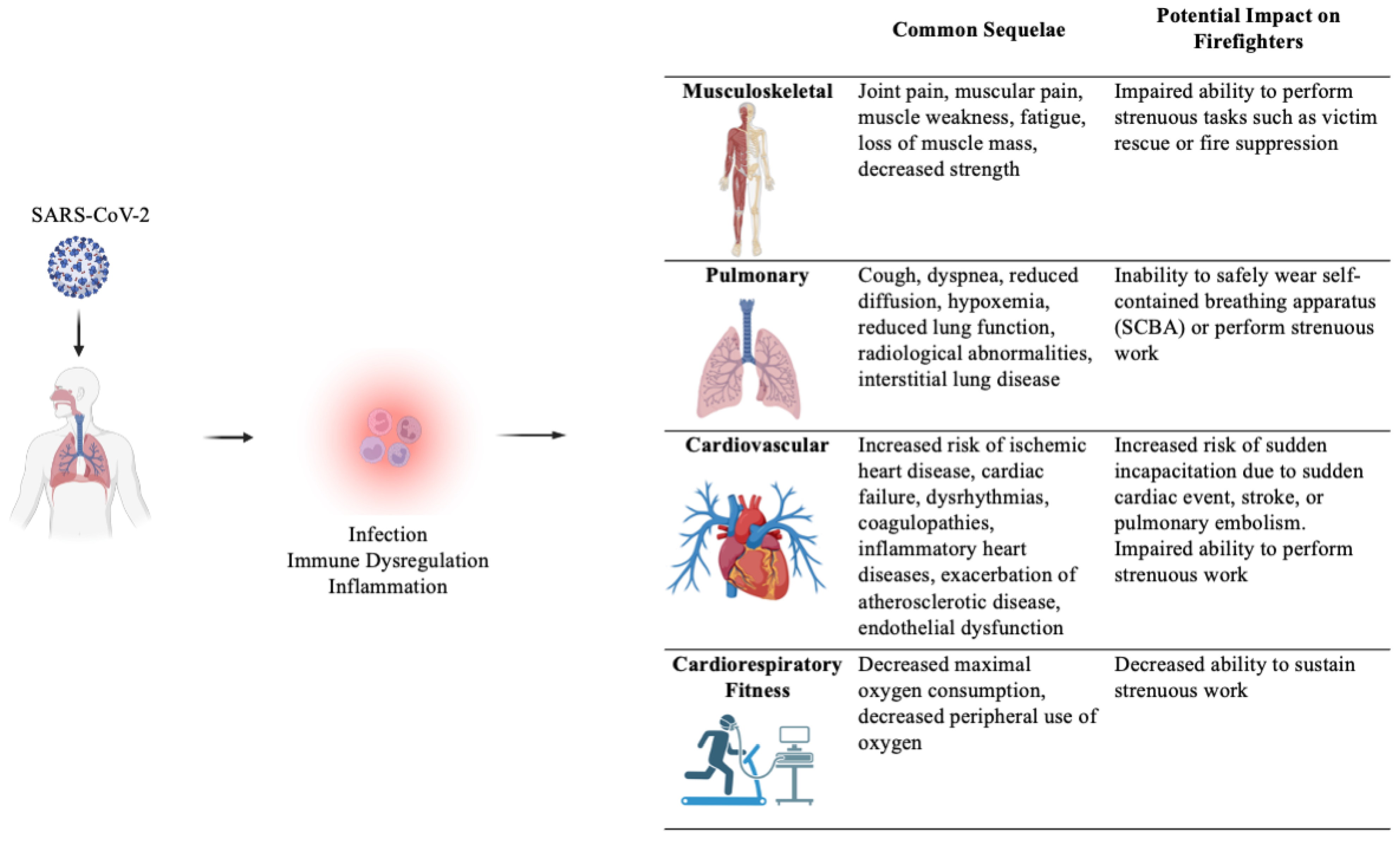
4. The Musculoskeletal System
5. The Pulmonary System
6. The Cardiovascular System
7. Cardiorespiratory Fitness
8. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IDLH | Immediately dangerous to life and health |
| SCBA | Self-contained breathing apparatus |
| DALYs | Disability adjusted life years |
| SDH | Succinate dehydrogenase |
| EMS | Emergency Medical Service |
| FEV1 | Forced expiratory volume in 1 s |
| FVC | Forced vital capacity |
| DLCO | Diffusing Lung Capacity for Carbon Monoxide |
| HRCT | High-resolution computed tomography |
| MACE | Major adverse cardiovascular events |
| FMD | Flow mediated dilation |
| TCC | Terminal complement complex |
| POTS | Postural orthostatic tachycardia |
| CRF | Cardiorespiratory fitness |
| CPET | Cardiopulmonary exercise testing |
References
- World Health Organization. COVID-19 Deaths|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 5 March 2024).
- CDC. Centers for Disease Control and Prevention. COVID Data Tracker. 2020. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 5 March 2024).
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature 2021, 600, 472–477. [CrossRef] [PubMed]
- Lofrano-Porto, A.; D’Isabel, S.; Smith, D.L. Developing a clinical-pathological framework of long COVID-related fatigue applied to public safety workers. Front. Med. 2024, 11, 1387499. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Dyer, K.; Petruzzello, S.J. Blood chemistry and immune cell changes during 1 week of intensive firefighting training. J. Therm. Biol. 2004, 29, 725–729. [Google Scholar] [CrossRef]
- Smith, D.L.; Horn, G.P.; Petruzzello, S.J.; Fahey, G.; Woods, J.; Fernhall, B. Clotting and Fibrinolytic Changes after Firefighting Activities. Med. Sci. Sports Exerc. 2014, 46, 448–454. [Google Scholar] [CrossRef]
- Smith, D.L.; DeBlois, J.P.; Kales, S.N.; Horn, G.P. Cardiovascular Strain of Firefighting and the Risk of Sudden Cardiac Events. Exerc. Sport. Sci. Rev. 2016, 44, 90–97. [Google Scholar] [CrossRef]
- Smith, D.L.; Horn, G.P.; Fernhall, B.; Kesler, R.M.; Fent, K.W.; Kerber, S.; Rowland, T.W. Electrocardiographic Responses Following Live-Fire Firefighting Drills. J. Occup. Environ. Med. 2019, 61, 1030–1035. [Google Scholar] [CrossRef]
- Khaja, S.U.; Mathias, K.C.; Bode, E.D.; Stewart, D.F.; Jack, K.; Moffatt, S.M.; Smith, D.L. Hypertension in the United States Fire Service. Int. J. Environ. Res. Public Health 2021, 18, 5432. [Google Scholar] [CrossRef]
- Moffatt, S.M.; Stewart, D.F.; Jack, K.; Dudar, M.D.; Bode, E.D.; Mathias, K.C.; Smith, D.L. Cardiometabolic health among United States firefighters by age. Prev. Med. Rep. 2021, 23, 101492. [Google Scholar] [CrossRef]
- Fahy, R.; Petrillo, J. Firefighter Fatalities in the US in 2021 [Internet]; National Fire Protection Association: Quincy, MA, USA, 2022. Available online: https://www.usfa.fema.gov/downloads/pdf/publications/firefighter-fatalities-2021.pdf (accessed on 19 February 2024).
- Retention and Recruitment for the Volunteer Emergency Services. U.S. Fire Administration. Available online: https://www.usfa.fema.gov/downloads/pdf/publications/retention-and-recruitment-for-volunteer-emergency-services.pdf (accessed on 19 February 2024).
- Hejbøl, E.K.; Harbo, T.; Agergaard, J.; Madsen, L.B.; Pedersen, T.H.; Østergaard, L.J.; Andersen, H.; Schrøder, H.D.; Tankisi, H. Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: Evidence of skeletal muscle histopathology. Eur. J. Neurol. 2022, 29, 2832–2841. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Legarra-Gorgoñon, G.; Oscoz-Ochandorena, S.; García-Alonso, Y.; García-Alonso, N.; Oteiza, J.; Ernaga Lorea, A.; Correa-Rodríguez, M.; Izquierdo, M. Reduced muscle strength in patients with long-COVID-19 syndrome is mediated by limb muscle mass. J. Appl. Physiol. 2023, 134, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Colosio, M.; Brocca, L.; Gatti, M.F.; Neri, M.; Crea, E.; Cadile, F.; Canepari, M.; Pellegrino, M.A.; Polla, B.; Porcelli, S.; et al. Structural and functional impairments of skeletal muscle in patients with postacute sequelae of SARS-CoV-2 infection. J. Appl. Physiol. 2023, 135, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; Van Weeghel, M.; Schomakers, B.V.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Paneroni, M.; Simonelli, C.; Saleri, M.; Bertacchini, L.; Venturelli, M.; Troosters, T.; Ambrosino, N.; Vitacca, M. Muscle Strength and Physical Performance in Patients Without Previous Disabilities Recovering From COVID-19 Pneumonia. Am. J. Phys. Med. Rehabil. 2021, 100, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Fanous, J.; Zero, A.M.; Rice, C.L. Muscle fatigability and post-acute COVID-19 syndrome: A case study. Physiol. Rep. 2022, 10, e15391. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; An, X.; Xiong, Y.; Shang, Y.; He, J.; Qiu, Y.; Zhang, N.; Huang, L.; Jia, J.; et al. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int. J. Infect. Dis. 2021, 112, 173–182. [Google Scholar] [CrossRef]
- Stoffels, A.A.F.; Van Voorthuizen, E.L.; Van Hees, H.W.H.; Peters, J.B.; Van Helvoort, H.A.C.; Voermans, N.C.; Doorduin, J.; Van Den Borst, B. Longitudinal Analysis of Quadriceps Muscle Strength in Patients with Previous COVID-19 Hospitalization and in Patients with Post-Acute Sequelae following Mild COVID-19. Nutrients 2022, 14, 4319. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef]
- Catalan, I.P.; Marti, C.R.; Sota, D.P.; Alvarez, A.C.; Gimeno, M.J.E.; Juana, S.F.; Rodriguez, G.H.; Bajo, E.D.; Gaya, N.T.; Blasco, J.U.; et al. Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission. J. Med. Virol. 2022, 94, 205–210. [Google Scholar] [CrossRef]
- Gamberini, L.; Mazzoli, C.A.; Gordini, G.; Prediletto, I.; Sintonen, H.; Scaramuzzo, G.; Volta, C.A.; Spadaro, S.; Allegri, D.; Colombo, D.; et al. Health-related quality of life profiles, trajectories, persistent symptoms, and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients: A prospective follow-up study. Respir. Med. 2021, 189, 106665. [Google Scholar] [CrossRef]
- Maestrini, V.; Birtolo, L.I.; Francone, M.; Galardo, G.; Galea, N.; Severino, P.; Alessandri, F.; Colaiacomo, M.C.; Cundari, G.; Chimenti, C.; et al. Cardiac involvement in consecutive unselected hospitalized COVID-19 population: In-hospital evaluation and one-year follow-up. Int. J. Cardiol. 2021, 339, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mendez, R.; Balanza-Martinez, V.; Luperdi, S.C.; Estrada, I.; Latorre, A.; Gonzalez-Jimenez, P.; Bouzas, L.; Yepez, K.; Ferrando, A.; Reyes, S.; et al. Long-term neuropsychiatric outcomes in COVID-19 survivors: A 1-year longitudinal study. J. Intern. Med. 2022, 291, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Guida, F.; Marcuzzo, A.V.; D’Alessandro, A.; Zanelli, E.; Marzolino, R.; Lazzarin, C.; Antonucci, P.; Sacchet, E.; Tofanelli, M.; et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). Int. Forum Allergy Rhinol. 2021, 59, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Hummel, T.; Hopkins, C.; Dibattista, M.; Menini, A.; Spinato, G.; Fabbris, C.; Emanuelli, E.; D’Alessandro, A.; Marzolino, R.; et al. High prevalence of long-term olfactory, gustatory, and chemesthesis dysfunction in post-COVID-19 patients: A matched case-control study with one-year follow-up using a comprehensive psychophysical evaluation. Rhinology 2021, 59, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, D.; Yan, W.; Wang, X.; Zhang, X.; Ma, K.; Chen, H.; Zeng, Z.; Wang, H.; Xing, M.; et al. Twelve-month systemic consequences of COVID-19 in patients discharged from hospital: A prospective cohort study in Wuhan, China. Clin. Infect. Dis. 2021, ciab703. [Google Scholar] [CrossRef]
- Maestre-Muniz, M.M.; Mata-Vazquez, E.; Martin-Toledano, M.; Lopez-Larramona, G.; Ruiz-Chicote, A.M.; Nieto-Sandoval, B.; Arias, A.; Lucendo, A.J. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J. Clin. Med. 2021, 10, 2945. [Google Scholar] [CrossRef]
- Rank, A.; Tzortzini, A.; Schmid, C.; Claus, R.; Kling, E.; Loll, E.; Hoffmann, R.; Dennehy, K.M.; Burger, R.; Grutzner, S.; et al. One year after mild COVID-19: The majority of patients maintain specific immunity but one in four still suffer from long-term symptoms. J. Clin. Med. 2021, 10, 3305. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Shen, Y.; Zhang, X.; Cen, Y.; Wang, B.; Zhao, S.; Zhou, Y.; Hu, B.; Wang, M.; et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw. Open 2021, 4, e2127403. [Google Scholar] [CrossRef]
- Chai, C.; Feng, X.; Lu, M.; Li, S.; Chen, K.; Wang, H.; Wang, W.; Tang, Z.; Cheng, G.; Wu, X.; et al. One-year mortality and consequences of COVID-19 in cancer patients: A cohort study. IUBMB Life 2021, 73, 1244–1256. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Baraff, A.; Fox, A.; Shahoumian, T.; Hickok, A.; O’Hare, A.M.; Bohnert, A.S.; Boyko, E.J.; Maciejewski, M.L.; Bowling, C.B.; et al. Rates and factors associated with documentation of diagnostic codes for Long COVID in the national veterans affairs health care system. JAMA Netw. Open 2022, 5, e2224359. [Google Scholar] [CrossRef] [PubMed]
- Ogungbe, O.; Gilotra, N.A.; Davidson, P.M.; Farley, J.E.; Dennison Himmelfarb, C.R.; Post, W.S.; Commodore-Mensah, Y. Cardiac postacute sequelae symptoms of SARS-CoV-2 in community-dwelling adults: A cross-sectional study. Open Heart 2022, 9, e002084. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for Long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk factors associated with post-COVID-19 condition: A systematic review and meta-analysis. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef]
- Wilk, P.; Stranges, S.; Cuschieri, S. Does sex modify the effect of pre-pandemic body mass index on the risk of Long COVID? Evidence from the longitudinal analysis of the Survey of Health Ageing and Retirement in Europe. Int. J. Obes. 2024, 48, 821–829. [Google Scholar] [CrossRef]
- Xiang, M.; Wu, X.; Jing, H.; Novakovic, V.A.; Shi, J. The intersection of obesity and (long) COVID-19: Hypoxia, thrombotic inflammation, and vascular endothelial injury. Front. Cardiovasc. Med. 2023, 10, 1062491. [Google Scholar] [CrossRef]
- Tanriverdi, A.; Savci, S.; Kahraman, B.O.; Ozpelit, E. Extrapulmonary features of post-COVID-19 patients: Muscle function, physical activity, mood, and sleep quality. Ir. J. Med. Sci. 2022, 191, 969–975. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 2023, 29, 2347–2357. [Google Scholar] [CrossRef]
- Gil, S.; De Oliveira Júnior, G.N.; Sarti, F.M.; Filho, W.J.; Longobardi, I.; Turri, J.A.O.; Shinjo, S.K.; Ferriolli, E.; Avelino-Silva, T.J.; Busse, A.L.; et al. Acute Muscle Mass Loss Predicts Long-Term Fatigue, Myalgia, and Health Care Costs in COVID-19 Survivors. J. Am. Med. Dir. Assoc. 2023, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ferrandi, P.J.; Alway, S.E.; Mohamed, J.S. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J. Appl. Physiol. 2020, 129, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Ras, J.; Kengne, A.P.; Smith, D.; Soteriades, E.S.; Leach, L. Effects of cardiovascular health, musculoskeletal health and physical fitness on occupational performance of firefighters: Protocol for a systematic review and meta-analysis. BMJ Open 2022, 12, e061435. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.; Dyer, K. United States Firefighter Injuries [Internet]; National Fire Protection Association: Quincy, MA, USA, 2023; Available online: https://www.nfpa.org/education-and-research/research/nfpa-research/fire-statistical-reports/firefighter-injuries-in-the-united-states (accessed on 19 February 2024).
- Fumagalli, A.; Misuraca, C.; Bianchi, A.; Borsa, N.; Limonta, S.; Maggiolini, S.; Bonardi, D.R.; Corsonello, A.; Di Rosa, M.; Soraci, L.; et al. Pulmonary function in patients surviving to COVID-19 pneumonia. Infection 2021, 49, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, A.; Misuraca, C.; Bianchi, A.; Borsa, N.; Limonta, S.; Maggiolini, S.; Bonardi, D.R.; Corsonello, A.; Di Rosa, M.; Soraci, L.; et al. Long-term changes in pulmonary function among patients surviving to COVID-19 pneumonia. Infection 2022, 50, 1019–1022. [Google Scholar] [CrossRef]
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef]
- Gianella, P.; Rigamonti, E.; Marando, M.; Tamburello, A.; Grazioli Gauthier, L.; Argentieri, G.; Puligheddu, C.; Pagnamenta, A.; Pons, M.; Fusi-Schmidhauser, T. Clinical, radiological and functional outcomes in patients with SARS-CoV-2 pneumonia: A prospective observational study. BMC Pulm. Med. 2021, 21, 136. [Google Scholar] [CrossRef]
- Lerum, T.V.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.M.A.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur. Respir. J. 2021, 57, 2003448. [Google Scholar] [CrossRef]
- Eizaguirre, S.; Sabater, G.; Belda, S.; Calderón, J.C.; Pineda, V.; Comas-Cufí, M.; Bonnin, M.; Orriols, R. Long-term respiratory consequences of COVID-19 related pneumonia: A cohort study. BMC Pulm. Med. 2023, 23, 439. [Google Scholar] [CrossRef]
- So, M.; Kabata, H.; Fukunaga, K.; Takagi, H.; Kuno, T. Radiological and functional lung sequelae of COVID-19: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 97. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lin, K.; Zhang, H.; Xue, Q.; Zhu, K.; Yuan, G.; Sun, Y.; Zhu, F.; Ai, J.; Wang, S.; et al. A one-year follow-up study of systematic impact of long COVID symptoms among patients post SARS-CoV-2 omicron variants infection in Shanghai, China. Emerg. Microbes Infect. 2023, 12, 2220578. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Huang, H.; Wang, C.; Jin, Z.; Zhang, Z.; He, J.; Yin, S.; Fan, M.; Huang, J.; Chen, F.; et al. Follow-up study of pulmonary function among COVID-19 survivors 1 year after recovery. J. Infect. 2021, 83, 381–412. [Google Scholar] [CrossRef]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, G.; Ronzoni, L.; Campo, G.; Priani, P.; Arena, C.; La Rosa, R.; Turrini, C.; Volta, C.A.; Papi, A.; Spadaro, S.; et al. Long-term dyspnea, regional ventilation distribution and peripheral lung function in COVID-19 survivors: A 1 year follow up study. BMC Pulm. Med. 2022, 22, 408. [Google Scholar] [CrossRef]
- Lee, J.H.; Yim, J.-J.; Park, J. Pulmonary function and chest computed tomography abnormalities 6–12 months after recovery from COVID-19: A systematic review and meta-analysis. Respir. Res. 2022, 23, 233. [Google Scholar] [CrossRef]
- Bellan, M.; Baricich, A.; Patrucco, F.; Zeppegno, P.; Gramaglia, C.; Balbo, P.E.; Carriero, A.; Amico, C.S.; Avanzi, G.C.; Barini, M.; et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci. Rep. 2021, 11, 22666. [Google Scholar] [CrossRef]
- Faverio, P.; Paciocco, G.; Tassistro, E.; Rebora, P.; Rossi, E.; Monzani, A.; Tundo, M.; Milano, C.; Messa, M.; Marocchi, R.; et al. Two-year cardio-pulmonary follow-up after severe COVID-19: A prospective study. Intern. Emerg. Med. 2023, 19, 183–190. [Google Scholar] [CrossRef]
- Soliman, S.; Soliman, H.; Crézé, M.; Brillet, P.-Y.; Montani, D.; Savale, L.; Jais, X.; Bulifon, S.; Jutant, E.-M.; Rius, E.; et al. Radiological pulmonary sequelae after COVID-19 and correlation with clinical and functional pulmonary evaluation: Results of a prospective cohort. Eur. Radiol. 2023, 34, 1037–1052. [Google Scholar] [CrossRef]
- Ballouz, T.; Menges, D.; Anagnostopoulos, A.; Domenghino, A.; Aschmann, H.E.; Frei, A.; Fehr, J.S.; Puhan, M.A. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: Population based, longitudinal cohort study. BMJ 2023, 381, e074425. [Google Scholar] [CrossRef]
- Faverio, P.; Luppi, F.; Rebora, P.; D’Andrea, G.; Stainer, A.; Busnelli, S.; Catalano, M.; Modafferi, G.; Franco, G.; Monzani, A.; et al. One-year pulmonary impairment after severe COVID-19: A prospective, multicenter follow-up study. Respir. Res. 2022, 23, 65. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Huang, L.; Gu, X.; Wang, Y.; Liu, M.; Liu, Z.; Zhang, X.; Yu, Z.; Wang, Y.; et al. Lung-function trajectories in COVID-19 survivors after discharge: A two-year longitudinal cohort study. eClinicalMedicine 2022, 54, 101668. [Google Scholar] [CrossRef]
- Guler, S.A.; Ebner, L.; Aubry-Beigelman, C.; Bridevaux, P.-O.; Brutsche, M.; Clarenbach, C.; Garzoni, C.; Geiser, T.K.; Lenoir, A.; Mancinetti, M.; et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021, 57, 2003690. [Google Scholar] [CrossRef]
- Munker, D.; Veit, T.; Barton, J.; Mertsch, P.; Mümmler, C.; Osterman, A.; Khatamzas, E.; Barnikel, M.; Hellmuth, J.C.; Münchhoff, M.; et al. Pulmonary function impairment of asymptomatic and persistently symptomatic patients 4 months after COVID-19 according to disease severity. Infection 2022, 50, 157–168. [Google Scholar] [CrossRef]
- Krueger, T.; Van Den Heuvel, J.; Van Kampen-van Den Boogaart, V.; Van Zeeland, R.; Mehagnoul-Schipper, D.J.; Barten, D.G.; Knarren, L.; Maas, A.F.G.; Wyers, C.E.; Gach, D.; et al. Pulmonary function three to five months after hospital discharge for COVID-19: A single centre cohort study. Sci. Rep. 2023, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.C.; Kemp, K.; Hayton, P.; Mudawi, D.; Wang, R.; Greaves, M.; Yioe, V.; Rivera-Ortega, P.; Avram, C.; Chaudhuri, N. Pulmonary Sequelae at 4 Months After COVID-19 Infection: A Single-Centre Experience of a COVID Follow-Up Service. Adv. Ther. 2021, 38, 4505–4519. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Huwe, M.; Hennigs, A.; Oqueka, T.; Simon, M.; Harbaum, L.; Körbelin, J.; Schmiedel, S.; Schulze Zur Wiesch, J.; Addo, M.M.; et al. Respiratory muscle dysfunction in long-COVID patients. Infection 2022, 50, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Talman, S.; Boonman-de Winter, L.J.; De Mol, M.; Hoefman, E.; Van Etten, R.W.; De Backer, I.C. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir. Med. 2021, 176, 106272. [Google Scholar] [CrossRef]
- Ekbom, E.; Frithiof, R.; Öi, E.; Larson, I.M.; Lipcsey, M.; Rubertsson, S.; Wallin, E.; Janson, C.; Hultström, M.; Malinovschi, A. Impaired diffusing capacity for carbon monoxide is common in critically ill Covid-19 patients at four months post-discharge. Respir. Med. 2021, 182, 106394. [Google Scholar] [CrossRef]
- Tarraso, J.; Safont, B.; Carbonell-Asins, J.A.; Fernandez-Fabrellas, E.; Sancho-Chust, J.N.; Naval, E.; Amat, B.; Herrera, S.; Ros, J.A.; Soler-Cataluña, J.J.; et al. Lung function and radiological findings 1 year after COVID-19: A prospective follow-up. Respir. Res. 2022, 23, 242. [Google Scholar] [CrossRef]
- Cho, J.L.; Villacreses, R.; Nagpal, P.; Guo, J.; Pezzulo, A.A.; Thurman, A.L.; Hamzeh, N.Y.; Blount, R.J.; Fortis, S.; Hoffman, E.A.; et al. Quantitative Chest CT Assessment of Small Airways Disease in Post-Acute SARS-CoV-2 Infection. Radiology 2022, 304, 185–192. [Google Scholar] [CrossRef]
- Chiner-Vives, E.; Cordovilla-Pérez, R.; de la Rosa-Carrillo, D.; García-Clemente, M.; Izquierdo-Alonso, J.L.; Otero-Candelera, R.; Pérez-de Llano, L.; Sellares-Torres, J.; de Granda-Orive, J.I. Short and Long-Term Impact of COVID-19 Infection on Previous Respiratory Diseases. Arch. Bronconeumol. 2022, 58 (Suppl. 1), 39–50. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Rodríguez-Jiménez, J.; Cancela-Cilleruelo, I.; Guerrero-Peral, A.; Martín-Guerrero, J.D.; García-Azorín, D.; Cornejo-Mazzuchelli, A.; Hernández-Barrera, V.; Pellicer-Valero, O.J. Post–COVID-19 Symptoms 2 Years After SARS-CoV-2 Infection Among Hospitalized vs Nonhospitalized Patients. JAMA Netw. Open 2022, 5, e2242106. [Google Scholar] [CrossRef]
- Mathias, K.C.; Graham, E.; Stewart, D.; Smith, D.L. Decreased Pulmonary Function Over 5 Years in US Firefighters. J. Occup. Environ. Med. 2020, 62, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Schermer, T.R.; Malbon, W.; Adams, R.; Morgan, M.; Smith, M.; Crockett, A.J. Change in Lung Function over Time in Male Metropolitan Firefighters and General Population Controls: A 3-year Follow-up Study. J. Occup. Health 2013, 55, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cone, J.; Brackbill, R.; Giesinger, I.; Yung, J.; Farfel, M. Pulmonary Fibrosis among World Trade Center Responders: Results from the WTC Health Registry Cohort. Int. J. Environ. Res. Public Health 2019, 16, 825. [Google Scholar] [CrossRef]
- Lee, C.T.; Ventura, I.B.; Phillips, E.K.; Leahy, A.; Jablonski, R.; Montner, S.; Chung, J.H.; Vij, R.; Adegunsoye, A.; Strek, M.E. Interstitial Lung Disease in Firefighters: An Emerging Occupational Hazard. Front. Med. 2022, 9, 864658. [Google Scholar] [CrossRef]
- Fabian, T.Z.; Borgerson, J.L.; Gandhi, P.D.; Baxter, C.S.; Ross, C.S.; Lockey, J.E.; Dalton, J.M. Characterization of Firefighter Smoke Exposure. Fire Technol. 2014, 50, 993–1019. [Google Scholar] [CrossRef]
- Fent, K.W.; Alexander, B.; Roberts, J.; Robertson, S.; Toennis, C.; Sammons, D.; Bertke, S.; Kerber, S.; Smith, D.; Horn, G. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J. Occup. Environ. Hyg. 2017, 14, 801–814. [Google Scholar] [CrossRef]
- Alexander, B.M.; Baxter, C.S. Plasticizer Contamination of Firefighter Personal Protective Clothing—A Potential Factor in Increased Health Risks in Firefighters. J. Occup. Environ. Hyg. 2014, 11, D43–D48. [Google Scholar] [CrossRef]
- Fent, K.W.; Toennis, C.; Sammons, D.; Robertson, S.; Bertke, S.; Calafat, A.M.; Pleil, J.D.; Wallace, M.A.G.; Kerber, S.; Smith, D.; et al. Firefighters’ absorption of PAHs and VOCs during controlled residential fires by job assignment and fire attack tactic. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Voss, R.W.; McNeel, S.; Wu, N.; Guo, T.; Wang, Y.; Israel, L.; Das, R.; Petreas, M. High Exposure of California Firefighters to Polybrominated Diphenyl Ethers. Environ. Sci. Technol. 2015, 49, 2948–2958. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.W.; Evans, D.E.; Babik, K.; Striley, C.; Bertke, S.; Kerber, S.; Smith, D.; Horn, G.P. Airborne contaminants during controlled residential fires. J. Occup. Environ. Hyg. 2018, 15, 399–412. [Google Scholar] [CrossRef]
- Horn, G.P.; Stewart, J.W.; Kesler, R.M.; DeBlois, J.P.; Kerber, S.; Fent, K.W.; Scott, W.S.; Fernhall, B.; Smith, D.L. Firefighter and fire instructor’s physiological responses and safety in various training fire environments. Saf. Sci. 2019, 116, 287–294. [Google Scholar] [CrossRef]
- Kole, C.; Stefanou, Ε.; Karvelas, N.; Schizas, D.; Toutouzas, K.P. Acute and Post-Acute COVID-19 Cardiovascular Complications: A Comprehensive Review. Cardiovasc. Drugs Ther. 2023, 38, 1017–1032. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef]
- Schnaubelt, S.; Oppenauer, J.; Tihanyi, D.; Mueller, M.; Maldonado-Gonzalez, E.; Zejnilovic, S.; Haslacher, H.; Perkmann, T.; Strassl, R.; Anders, S.; et al. Arterial stiffness in acute COVID-19 and potential associations with clinical outcome. J. Intern. Med. 2021, 290, 437–443. [Google Scholar] [CrossRef]
- Joy, G.; Artico, J.; Kurdi, H.; Seraphim, A.; Lau, C.; Thornton, G.D.; Oliveira, M.F.; Adam, R.D.; Aziminia, N.; Menacho, K.; et al. Prospective Case-Control Study of Cardiovascular Abnormalities 6 Months Following Mild COVID-19 in Healthcare Workers. JACC Cardiovasc. Imaging 2021, 14, 2155–2166. [Google Scholar] [CrossRef]
- Liao, M.-H.; Lai, Y.-C.; Lin, C.-M. Cardiovascular Risk Factors in Hospital Workers during the COVID-19 Pandemic: A Hospital-Based Repeated Measures Study. Int. J. Environ. Res. Public Health 2022, 19, 16114. [Google Scholar] [CrossRef]
- Shrestha, A.B.; Mehta, A.; Pokharel, P.; Mishra, A.; Adhikari, L.; Shrestha, S.; Yadav, R.S.; Khanal, S.; Sah, R.; Nowrouzi-Kia, B.; et al. Long COVID Syndrome and Cardiovascular Manifestations: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 491. [Google Scholar] [CrossRef]
- Tereshchenko, L.G.; Bishop, A.; Fisher-Campbell, N.; Levene, J.; Morris, C.C.; Patel, H.; Beeson, E.; Blank, J.A.; Bradner, J.N.; Coblens, M.; et al. Risk of Cardiovascular Events After COVID-19. Am. J. Cardiol. 2022, 179, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Raisi-Estabragh, Z.; Cooper, J.; Salih, A.; Raman, B.; Lee, A.M.; Neubauer, S.; Harvey, N.C.; Petersen, S.E. Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank. Heart 2023, 109, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, T.L.; McGrath, L.J.; Andersen, K.M.; Khan, F.; Malhotra, D.; Alfred, T.; Nguyen, J.L.; Puzniak, L.; Thoburn, E.; Jodar, L.; et al. Coronavirus Disease 2019 Severity and Risk of Subsequent Cardiovascular Events. Clin. Infect. Dis. 2023, 76, e42–e50. [Google Scholar] [CrossRef]
- Santoro, L.; Zaccone, V.; Falsetti, L.; Ruggieri, V.; Danese, M.; Miro, C.; Di Giorgio, A.; Nesci, A.; D’Alessandro, A.; Moroncini, G.; et al. Role of Endothelium in Cardiovascular Sequelae of Long COVID. Biomedicines 2023, 11, 2239. [Google Scholar] [CrossRef]
- Santoro, L.; Falsetti, L.; Zaccone, V.; Nesci, A.; Tosato, M.; Giupponi, B.; Savastano, M.C.; Moroncini, G.; Gasbarrini, A.; Landi, F.; et al. Impaired Endothelial Function in Convalescent Phase of COVID-19: A 3 Month Follow Up Observational Prospective Study. JCM 2022, 11, 1774. [Google Scholar] [CrossRef]
- Ratchford, S.M.; Stickford, J.L.; Province, V.M.; Stute, N.; Augenreich, M.A.; Koontz, L.K.; Bobo, L.K.; Stickford, A.S.L. Vascular alterations among young adults with SARS-CoV-2. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H404–H410. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Katogiannis, K.; Thymis, J.; Korakas, E.; Pavlidis, G.; Kazakou, P.; Panagopoulos, G.; et al. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID-19 infection. Eur. J. Heart Fail. 2022, 24, 727–729. [Google Scholar] [CrossRef]
- Podrug, M.; Koren, P.; Dražić Maras, E.; Podrug, J.; Čulić, V.; Perissiou, M.; Bruno, R.M.; Mudnić, I.; Boban, M.; Jerončić, A. Long-Term Adverse Effects of Mild COVID-19 Disease on Arterial Stiffness, and Systemic and Central Hemodynamics: A Pre-Post Study. JCM 2023, 12, 2123. [Google Scholar] [CrossRef]
- Zanoli, L.; Gaudio, A.; Mikhailidis, D.P.; Katsiki, N.; Castellino, N.; Lo Cicero, L.; Geraci, G.; Sessa, C.; Fiorito, L.; Marino, F.; et al. Vascular Dysfunction of COVID-19 Is Partially Reverted in the Long-Term. Circ. Res. 2022, 130, 1276–1285. [Google Scholar] [CrossRef]
- Szeghy, R.E.; Stute, N.L.; Province, V.M.; Augenreich, M.A.; Stickford, J.L.; Stickford, A.S.L.; Ratchford, S.M. Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. J. Appl. Physiol. 2022, 132, 1297–1309. [Google Scholar] [CrossRef]
- Eberhardt, N.; Noval, M.G.; Kaur, R.; Amadori, L.; Gildea, M.; Sajja, S.; Das, D.; Cilhoroz, B.; Stewart, O.J.; Fernandez, D.M.; et al. SARS-CoV-2 infection triggers pro-atherogenic inflammatory responses in human coronary vessels. Nat. Cardiovasc. Res. 2023, 2, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Cervia-Hasler, C.; Brüningk, S.C.; Hoch, T.; Fan, B.; Muzio, G.; Thompson, R.C.; Ceglarek, L.; Meledin, R.; Westermann, P.; Emmenegger, M.; et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science 2024, 383, eadg7942. [Google Scholar] [CrossRef] [PubMed]
- Taş, S.; Taş, Ü. Effects of COVID-19 on the Autonomic Cardiovascular System: Heart Rate Variability and Turbulence in Recovered Patients. Tex. Heart Inst. J. 2023, 50, e227952. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A.; Fanciulli, A.; Raj, S.R.; Sheldon, R.; Shibao, C.A.; Sutton, R. Cardiovascular autonomic dysfunction in post-COVID-19 syndrome: A major health-care burden. Nat. Rev. Cardiol. 2024, 21, 379–395. [Google Scholar] [CrossRef]
- Graham, E.L.; Khaja, S.; Caban-Martinez, A.J.; Smith, D.L. Firefighters and COVID-19: An Occupational Health Perspective. J. Occup. Environ. Med. 2021, 63, e556–e563. [Google Scholar] [CrossRef]
- Baratto, C.; Caravita, S.; Faini, A.; Perego, G.B.; Senni, M.; Badano, L.P.; Parati, G. Impact of COVID-19 on exercise pathophysiology: A combined cardiopulmonary and echocardiographic exercise study. J. Appl. Physiol. 2021, 130, 1470–1478. [Google Scholar] [CrossRef]
- Cassar, M.P.; Tunnicliffe, E.M.; Petousi, N.; Lewandowski, A.J.; Xie, C.; Mahmod, M.; Samat, A.H.A.; Evans, R.A.; Brightling, C.E.; Ho, L.-P.; et al. Symptom Persistence Despite Improvement in Cardiopulmonary Health—Insights from longitudinal CMR, CPET and lung function testing post-COVID-19. eClinicalMedicine 2021, 41, 101159. [Google Scholar] [CrossRef]
- Chuatrakoon, B.; Konghakote, S.; Sa-nguanmoo, P.; Nantakool, S. Long-term impact of SARS-CoV-2 infection on cardiorespiratory fitness: A meta-analysis. Front. Public Health 2023, 11, 1215486. [Google Scholar] [CrossRef]
- Kimmig, L.M.; Rako, Z.A.; Ziegler, S.; Richter, M.J.; Ashkan Tolou, G.S.; Roller, F.; Grimminger, F.; Vadász, I.; Seeger, W.; Herold, S.; et al. Long-term comprehensive cardiopulmonary phenotyping of COVID-19. Respir. Res. 2022, 23, 263. [Google Scholar] [CrossRef]
- Schwendinger, F.; Knaier, R.; Radtke, T.; Schmidt-Trucksäss, A. Low Cardiorespiratory Fitness Post-COVID-19: A Narrative Review. Sports Med. 2023, 53, 51–74. [Google Scholar] [CrossRef] [PubMed]
- Štěpánek, L.; Nakládalová, M.; Sovová, E.; Štěpánek, L.; Boriková, A.; Sovová, M.; Moravcová, K.; Ožana, J.; Jelínek, L. COVID-19 reduces cardiorespiratory fitness even months after a mild to moderate acute phase: A retrospective cohort study. Infect. Dis. 2023, 55, 684–693. [Google Scholar] [CrossRef] [PubMed]
- D’Isabel, S.; Berny, L.M.; Frost, A.; Thongphok, C.; Jack, K.; Chaudhry, S.; Arena, R.; Smith, D.L. The effect of mild to moderate COVID-19 infection on the cardiorespiratory fitness of firefighters. Front. Public Health 2023, 11, 1308605. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.A.; Wiswell, R.A. Rate and Mechanism of Maximal Oxygen Consumption Decline with Aging: Implications for Exercise Training. Sports Med. 2003, 33, 877–888. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Arena, R.; Myers, J. Reference Standards for Cardiorespiratory Fitness Measured With Cardiopulmonary Exercise Testing. Mayo Clin. Proc. 2015, 90, 1515–1523. [Google Scholar] [CrossRef]
| Days Post-Infection | |||||||
|---|---|---|---|---|---|---|---|
| Sequela | 90 | 180 | 360 | 540 | 720 | ||
| Death | NH | 2.22 | 1.17 | 1.00 | 0.96 | 0.99 | |
| H | 6.25 | 1.75 | 1.41 | 1.42 | 1.29 | ||
| Hospitalization | NH | 1.45 | 1.18 | 1.06 | 1.06 | 1.04 | |
| H | 6.83 | 3.14 | 2.66 | 2.64 | 2.57 | ||
| Ischemic Heart Disease | Acute Coronary Disease | NH | 1.73 | 1.07 | 0.93 | 1.09 | 0.93 |
| H | 18.32 | 4.31 | 1.83 | 1.91 | 1.45 | ||
| Angina | NH | 1.47 | 1.20 | 1.25 | 1.31 | 1.15 | |
| H | 5.20 | 3.09 | 2.03 | 1.79 | 2.32 | ||
| Myocardial Infarction | NH | 1.62 | 1.05 | 1.01 | 1.11 | 0.97 | |
| H | 15.89 | 4.32 | 1.78 | 2.13 | 1.56 | ||
| Ischemic Cardiomyopathy | NH | 1.31 | 1.23 | 1.05 | 1.03 | 1.10 | |
| H | 6.19 | 2.43 | 2.05 | 1.97 | 1.18 | ||
| Cardiac Failure | Cardiac Arrest | NH | 1.46 | 0.79 | 0.85 | 0.73 | 1.01 |
| H | 34.78 | 14.30 | 1.17 | 1.94 | 1.39 | ||
| Cardiogenic Shock | NH | 0.77 | 1.45 | 0.72 | 0.98 | 0.97 | |
| H | 19.41 | 6.51 | 2.15 | 1.89 | 1.84 | ||
| Heart Failure | NH | 1.91 | 1.41 | 1.22 | 1.04 | 1.06 | |
| H | 13.13 | 3.00 | 2.00 | 1.95 | 1.47 | ||
| Nonischemic Cardiomyopathy | NH | 1.72 | 1.44 | 1.13 | 1.06 | 1.00 | |
| H | 7.89 | 4.08 | 2.10 | 2.63 | 1.15 | ||
| Dysrhythmias | Atrial Fibrillation | NH | 2.14 | 1.24 | 1.20 | 1.12 | 0.95 |
| H | 16.34 | 3.22 | 1.79 | 1.49 | 1.63 | ||
| Atrial Flutter | NH | 1.58 | 1.32 | 1.16 | 0.95 | 0.96 | |
| H | 10.67 | 3.42 | 1.48 | 1.27 | 0.79 | ||
| Bradycardia | NH | 1.45 | 1.27 | 1.19 | 1.29 | 1.18 | |
| H | 8.72 | 2.24 | 1.75 | 1.81 | 1.51 | ||
| Tachycardia | NH | 2.01 | 1.26 | 1.06 | 1.03 | 1.14 | |
| H | 19.83 | 4.08 | 2.34 | 2.36 | 2.66 | ||
| Ventricular Arrhythmia | NH | 1.89 | 1.42 | 1.01 | 1.11 | 1.04 | |
| H | 16.99 | 4.67 | 1.58 | 1.95 | 2.22 | ||
| Coagulation | Pericarditis | NH | 1.87 | 1.23 | 1.13 | 1.05 | 1.15 |
| H | 17.54 | 5.47 | 2.38 | 1.55 | 1.19 | ||
| Anemia | NH | 1.82 | 1.15 | 1.01 | 1.02 | 0.94 | |
| H | 11.16 | 2.74 | 1.58 | 1.57 | 1.50 | ||
| Coagulopathy | NH | 2.00 | 1.44 | 1.15 | 1.13 | 1.23 | |
| H | 12.86 | 3.57 | 2.62 | 2.62 | 1.84 | ||
| Deep Vein Thrombosis | NH | 3.30 | 1.71 | 1.14 | 0.96 | 1.08 | |
| H | 17.63 | 2.88 | 1.95 | 2.52 | 2.13 | ||
| Pulmonary Embolism | NH | 4.99 | 1.94 | 0.87 | 1.11 | 0.95 | |
| H | 45.55 | 6.66 | 2.16 | 1.54 | 1.65 | ||
| Venous Thromboembolism | NH | 2.90 | 1.41 | 1.07 | 1.07 | 0.86 | |
| H | 20.36 | 5.69 | 1.97 | 1.78 | 1.23 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graham, E.L.; D’Isabel, S.; Lofrano-Porto, A.; Smith, D.L. Musculoskeletal, Pulmonary, and Cardiovascular COVID-19 Sequelae in the Context of Firefighter Occupational Health: A Narrative Review. Int. J. Environ. Res. Public Health 2024, 21, 1383. https://doi.org/10.3390/ijerph21101383
Graham EL, D’Isabel S, Lofrano-Porto A, Smith DL. Musculoskeletal, Pulmonary, and Cardiovascular COVID-19 Sequelae in the Context of Firefighter Occupational Health: A Narrative Review. International Journal of Environmental Research and Public Health. 2024; 21(10):1383. https://doi.org/10.3390/ijerph21101383
Chicago/Turabian StyleGraham, Elliot L., Susanne D’Isabel, Adriana Lofrano-Porto, and Denise L. Smith. 2024. "Musculoskeletal, Pulmonary, and Cardiovascular COVID-19 Sequelae in the Context of Firefighter Occupational Health: A Narrative Review" International Journal of Environmental Research and Public Health 21, no. 10: 1383. https://doi.org/10.3390/ijerph21101383
APA StyleGraham, E. L., D’Isabel, S., Lofrano-Porto, A., & Smith, D. L. (2024). Musculoskeletal, Pulmonary, and Cardiovascular COVID-19 Sequelae in the Context of Firefighter Occupational Health: A Narrative Review. International Journal of Environmental Research and Public Health, 21(10), 1383. https://doi.org/10.3390/ijerph21101383






