Preconception Health of Indigenous Peoples in Australia, Canada, New Zealand, and the United States: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
- The association of preconception health risk factors with fertility, maternal health outcomes (i.e., during pregnancy, birth, and the postnatal period), and child health outcomes;
- Indigenous peoples’ understandings and awareness of preconception health;
- Interventions to support preconception health (including evidence on intervention development, implementation, and evaluation);
- Uptake of preconception health care and interventions, including factors that affect uptake of and access to preconception health care and interventions;
- Health professionals’ knowledge, awareness, attitudes, and behaviours relating to preconception health.
2.2. Data Extraction and Analysis
3. Results
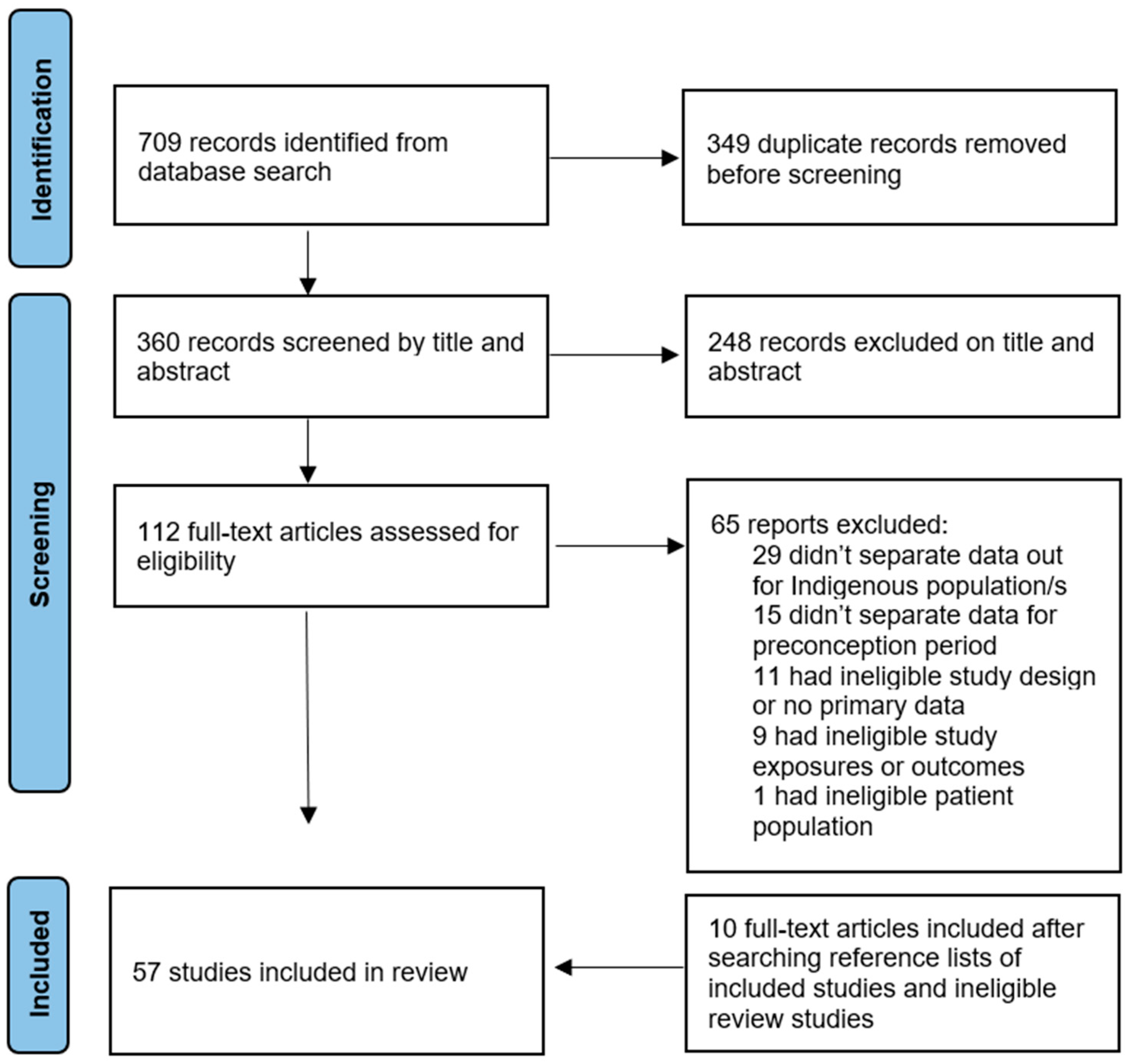
3.1. Search Results
3.2. Study Characteristics
3.3. Associations between Preconception Health Risk Factors and Maternal or Child Health Outcomes
3.4. Studies Describing Development, Implementation, or Evaluation of Preconception Health Interventions
3.5. Studies Describing Uptake of Preconception Care and Preconception Health Knowledge and Attitudes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization [WHO]. Meeting to Develop a Global Consensus on Preconception Care to Reduce Maternal and Childhood Mortality and Morbidity, 6–7 February 2012: Meeting Report; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Toivonen, K.I.; Oinonen, K.A.; Duchene, K.M. Preconception Health Behaviours: A Scoping Review. Prev. Med. 2016, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dorney, E.; Black, K.I. Preconception care. Aust. J. Gen. Pract. 2018, 47, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Hill, B.; Hall, J.; Skouteris, H.; Currie, S. Defining preconception: Exploring the concept of a preconception population. BMC Pregnancy Childbirth 2020, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- Hemsing, N.; Greaves, L.; Poole, N. Preconception health care interventions: A scoping review. Sex. Reprod. Healthc. 2017, 14, 24–32. [Google Scholar] [CrossRef]
- Harper, T.; Kuohung, W.; Sayres, L.; Willis, M.D.; Wise, L.A. Optimizing Preconception Care and Interventions for Improved Population Health. Fertil. Steril. 2022, 120, 438–448. [Google Scholar] [CrossRef]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef]
- Guy, R.; Ward, J.S.; Smith, K.S.; Su, J.Y.; Huang, R.L.; Tangey, A.; Skov, S.; Rumbold, A.; Silver, B.; Donovan, B.; et al. The impact of sexually transmissible infection programs in remote Aboriginal communities in Australia: A systematic review. Sex. Health 2012, 9, 205–212. [Google Scholar] [CrossRef]
- Campbell, S.; Lynch, J.; Esterman, A.; McDermott, R. Pre-pregnancy predictors linked to miscarriage among Aboriginal and Torres Strait Islander women in North Queensland. Aust. N. Z. J. Public Health 2011, 35, 343–351. [Google Scholar] [CrossRef]
- Gomez, G.B.; Kamb, M.L.; Newman, L.M.; Mark, J.; Broutet, N.; Hawkes, S.J. Untreated maternal syphilis and adverse outcomes of pregnancy: A systematic review and meta-analysis. Bull. World Health Organ. 2013, 91, 217–226. [Google Scholar] [CrossRef]
- Pedro, J.; Brandão, T.; Schmidt, L.; Costa, M.E.; Martins, M.V. What do people know about fertility? A systematic review on fertility awareness and its associated factors. Ups. J. Med. Sci. 2018, 123, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Witt, W.P.; Wisk, L.E.; Cheng, E.R.; Hampton, J.M.; Hagen, E.W. Preconception Mental Health Predicts Pregnancy Complications and Adverse Birth Outcomes: A National Population-Based Study. Matern. Child Health J. 2012, 16, 1525–1541. [Google Scholar] [CrossRef]
- Dorney, E.; Boyle, J.A.; Walker, R.; Hammarberg, K.; Musgrave, L.; Schoenaker, D.; Jack, B.; Black, K.I. A Systematic Review of Clinical Guidelines for Preconception Care. Semin. Reprod. Med. 2022, 40, 157–169. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs, Economic UNDo, Issues PFoI. State of the World’s Indigenous Peoples; United Nations Publications: New York, NY, USA, 2009. [Google Scholar]
- Kairuz, C.A.; Casanelia, L.M.; Bennett-Brook, K.; Coombes, J.; Yadav, U.N. Impact of racism and discrimination on physical and mental health among Aboriginal and Torres Strait islander peoples living in Australia: A systematic scoping review. BMC Public Health 2021, 21, 1302. [Google Scholar] [CrossRef]
- Anderson, I.; Robson, B.; Connolly, M.; Al-Yaman, F.; Bjertness, E.; King, A.; Tynan, M.; Madden, R.; Bang, A.; Coimbra, C.E.A.; et al. Indigenous and tribal peoples’ health (The Lancet–Lowitja Institute Global Collaboration): A population study. Lancet 2016, 388, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.S.; Lim, L.L.; Mattes, J. Prevention and Treatment of Smoking and Tobacco Use During Pregnancy in Selected Indigenous Communities in High-Income Countries of the United States, Canada, Australia, and New Zealand: An Evidence-Based Review. Chest 2017, 152, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. National Aboriginal and Torres Strait Islander Health Survey 2018–19 Financial Year 2019. Available online: https://www.abs.gov.au/statistics/people/aboriginal-and-torres-strait-islander-peoples/national-aboriginal-and-torres-strait-islander-health-survey/2018-19 (accessed on 1 November 2023).
- Statistics Canada. Health Indicators, by Aboriginal Identity, Four-Year Period Estimates. 2016. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310045701 (accessed on 1 November 2023).
- Ministry of Health. Annual Update of Key Results 2021/22: New Zealand Health Survey. 2022. Available online: https://www.health.govt.nz/publication/annual-update-key-results-2021-22-new-zealand-health-survey (accessed on 1 November 2023).
- Denny, C.H.; Floyd, R.L.; Green, P.P.; Hayes, D.K. Racial and Ethnic Disparities in Preconception Risk Factors and Preconception Care. J. Women’s Health 2012, 21, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Voaklander, B.; Rowe, S.; Sanni, O.; Campbell, S.; Eurich, D.; Ospina, M.B. Prevalence of diabetes in pregnancy among Indigenous women in Australia, Canada, New Zealand, and the USA: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e681–e698. [Google Scholar] [CrossRef]
- Carson, E.; Sharmin, S.; Maier, A.B.; Meij, J.J. Comparing indigenous mortality across urban, rural and very remote areas: A systematic review and meta-analysis. Int. Health 2018, 10, 219–227. [Google Scholar] [CrossRef]
- Jackson Pulver, L.; Haswell, M.R.; Ring, I.; Waldon, J.; Clark, W.; Whetung, V.; Kinnon, D.; Graham, D.; Chino, M.; LaValley, J.; et al. Indigenous Health: Australia, Canada, Aotearoa, New Zealand and the United States: Laying Claim to a Future that Embraces Health for Us All; World Health Organisation: Geneva, Switzerland, 2010. [Google Scholar]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews (2020 Version); JBI Manual for Evidence Synthesis; JBI: London, UK, 2020. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Daly, M.; Kipping, R.R.; Tinner, L.E.; Sanders, J.; White, J.W. Preconception exposures and adverse pregnancy, birth and postpartum outcomes: Umbrella review of systematic reviews. Paediatr. Perinat. Epidemiol. 2022, 36, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Withanage, N.N.; Botfield, J.R.; Srinivasan, S.; Black, K.I.; Mazza, D. Effectiveness of preconception care interventions in primary care: A systematic review protocol. BJGP Open 2022, 6, 0191. [Google Scholar] [CrossRef] [PubMed]
- Beks, H.; Ewing, G.; Charles, J.A.; Mitchell, F.; Paradies, Y.; Clark, R.A.; Versace, V.L. Mobile primary health care clinics for Indigenous populations in Australia, Canada, New Zealand and the United States: A systematic scoping review. Int. J. Equity Health 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Sanchez, A.; Hurd, K.; Barnabe, C. Healthcare utilization for arthritis by indigenous populations of Australia, Canada, New Zealand, and the United States: A systematic review. Semin. Arthritis Rheum. 2017, 46, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Schoenaker, D.A.; Hinton, W.; Poston, L.; Barker, M.; Alwan, N.A.; Godfrey, K.; Hanson, M.; de Lusignan, S.; The UK Preconception Partnership. A wake-up call for preconception health: A clinical review. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2021, 71, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.V.; Lassi, Z.S.; Imam, A.M.; Bhutta, Z.A. Preconception care: Promoting reproductive planning. Reprod. Health 2014, 11, S2. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Standard Geographical Classification (SGC) 2016—Volume I, The Classification. 2018. Available online: https://www.statcan.gc.ca/en/subjects/standard/sgc/2016/index (accessed on 2 November 2023).
- Australian Bureau of Statistics, 1270.0.55.005—Australian Statistical Geography Standard (ASGS): Volume 5—Remoteness Structure, July 2016. 2018. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/mf/1270.0.55.005 (accessed on 2 November 2023).
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. 2021. Available online: https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html (accessed on 2 November 2023).
- Stats, N.Z. Statistical Standard for Geographic Areas 2023. 2022. Available online: https://www.stats.govt.nz/methods/statistical-standard-for-geographic-areas-2023/ (accessed on 2 November 2023).
- Vaismoradi, M.; Turunen, H.; Bondas, T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs. Health Sci. 2013, 15, 398–405. [Google Scholar] [CrossRef]
- Bower, C.; Maxwell, S.; Hickling, S.; D’Antoine, H.; O’Leary, P. Folate status in Aboriginal people before and after mandatory fortification of flour for bread-making in Australia. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 233–237. [Google Scholar] [CrossRef]
- Campbell, S.; Lynch, J.; Esterman, A.; McDermott, R. Pre-Pregnancy Predictors of Diabetes in Pregnancy Among Aboriginal and Torres Strait Islander Women in North Queensland, Australia. Matern. Child Health J. 2012, 16, 1284–1292. [Google Scholar] [CrossRef]
- Campbell, S.K.; Lynch, J.; Esterman, A.; McDermott, R. Pre-pregnancy predictors of hypertension in pregnancy among Aboriginal and Torres Strait Islander women in north Queensland, Australia; a prospective cohort study. BMC Public Health 2013, 13, 138. [Google Scholar] [CrossRef]
- Gilbert, E.; Collins, R.; Webster, V.; Boyd, N.; Maple-Brown, L.; Boyle, J.; Smith-Vaughan, H. Using co-design to develop a culturally responsive reproductive health learning resource for Aboriginal and Torres Strait Islander youth. Health Promot. J. Austr. 2021, 32 (Suppl. S1), 179–185. [Google Scholar] [CrossRef]
- Griffiths, E.; Marley, J.V.; Atkinson, D. Preconception Care in a Remote Aboriginal Community Context: What, When and by Whom? Int. J. Environ. Res. Public Health 2020, 17, 3702. [Google Scholar] [CrossRef]
- Kennedy, M.; Kumar, R.; Ryan, N.M.; Bennett, J.; La Hera Fuentes, G.; Gould, G.S. Codeveloping a multibehavioural mobile phone app to enhance social and emotional well-being and reduce health risks among Aboriginal and Torres Strait Islander women during preconception and pregnancy: A three-phased mixed-methods study. BMJ Open 2021, 11, e052545. [Google Scholar] [CrossRef] [PubMed]
- Lucas, I.M.; Barr, E.L.M.; Barzi, F.; Longmore, D.K.; Lee, I.L.; Kirkwood, M.; Whitbread, C.; Connors, C.; Boyle, J.A.; Simon, D.; et al. Gestational diabetes is associated with postpartum hemorrhage in Indigenous Australian women in the PANDORA study: A prospective cohort. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2021, 155, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Maple-Brown, L.; Lee, I.L.; Longmore, D.; Barzi, F.; Connors, C.; Boyle, J.A.; Moore, E.; Whitbread, C.; Kirkwood, M.; Graham, S.; et al. Pregnancy And Neonatal Diabetes Outcomes in Remote Australia: The PANDORA study-an observational birth cohort. Int. J. Epidemiol. 2019, 48, 307–318. [Google Scholar] [CrossRef]
- Munns, A.; Mahony, A.; Miller, K.; Whitehead, A. The WA Goldfields Aboriginal Community Antenatal Program—A community midwifery initiative. Collegian 2016, 23, 367–372. [Google Scholar] [CrossRef][Green Version]
- Schumacher, T.L.; Weatherall, L.; Keogh, L.; Sutherland, K.; Collins, C.E.; Pringle, K.G.; Rae, K.M. Reprint of characterizing gestational weight gain in a cohort of indigenous Australian women. Midwifery 2019, 74, 148–156. [Google Scholar] [CrossRef]
- Sina, M.; Hoy, W.; Wang, Z. Anthropometric predictors of gestational hypertensive disorders in a remote aboriginal community: A nested case-control study. BMC Res. Notes 2014, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; Callaway, L.K. The effect of obesity on pregnancy outcomes among Australian Indigenous and non-Indigenous women. Med. J. Aust. 2014, 201, 592–595. [Google Scholar] [CrossRef]
- Bains, A.; Pakseresht, M.; Roache, C.; Beck, L.; Sheehy, T.; Gittelsohn, J.; Corriveau, A.; Sharma, S. Healthy Foods North improves diet among Inuit and Inuvialuit women of childbearing age in Arctic Canada. J. Hum. Nutr. Diet. 2014, 27, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, M.L.; Yuan, Y.; Shen, Y.; Toth, E.L.; Bell, R.C.; Oster, R.T. Pregnancy and development of diabetes in First Nations and non-First Nations women in Alberta, Canada. Diabet. Med. 2021, 38, e14372. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.; Lawford, K.M.; Otterman, V.; Darling, E.K. Mental health indicators among pregnant Aboriginal women in Canada: Results from the Maternity Experiences Survey. Health Promot. Chronic Dis. Prev. Can. 2018, 38, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.; Kalra, S.; Wahi, G.; McDonald, S.; Desai, D.; Wilson, J.; Jacobs, L.; Smoke, S.; Hill, P.; Hill, K.; et al. Maternal and Newborn Health Profile in a First Nations Community in Canada. J. Obstet. Gynaecol. Can. 2013, 35, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Oster, R.T.; King, M.; Morrish, D.W.; Mayan, M.; Toth, E.L. Diabetes in pregnancy among First Nations women in Alberta, Canada: A retrospective analysis. BMC Pregnancy Childbirth 2014, 14, 136. [Google Scholar] [CrossRef]
- Mallard, S.R.; Gray, A.R.; Houghton, L.A. Periconceptional bread intakes indicate New Zealand’s proposed mandatory folic acid fortification program may be outdated: Results from a postpartum survey. BMC Pregnancy Childbirth 2012, 12, 8. [Google Scholar] [CrossRef]
- Mallard, S.R.; Houghton, L.A. Public health policy to redress iodine insufficiency in pregnant women may widen sociodemographic disparities. Public Health Nutr. 2014, 17, 1421–1429. [Google Scholar] [CrossRef]
- Tromop-van Dalen, C.; Fairley, S.L.; Aitken, A.; Grace Li, W.Y. Contraception and Pre-Conception Counselling in Cardiac Patients: We Can Do Better. Experience From a Tertiary Centre in New Zealand. Heart Lung Circ. 2021, 30, 158–161. [Google Scholar] [CrossRef]
- Anderson, K.; Spicer, P.; Peercy, M. Obesity, Diabetes, and Birth Outcomes Among American Indians and Alaska Natives. Matern. Child Health J. 2016, 20, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Charron-Prochownik, D.; Moore, K.R.; Stotz, S.; Akers, A.; Beirne, S.; Brega, A.G.; Chalmers, L.; Fischl, A.; Garrow, H.; Gonzales, K.; et al. Comparing American Indian/Alaska Native Adolescent Daughters’ and Their Mothers’ Awareness, Knowledge, Attitudes, and Behaviors Regarding Risk for Gestational Diabetes: Implications for Mother-Daughter Communication on Reproductive Health. Diabetes Educ. 2023, 49, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Declercq, E.; MacDorman, M.; Osterman, M.; Belanoff, C.; Iverson, R. Prepregnancy Obesity and Primary Cesareans among Otherwise Low-Risk Mothers in 38 U.S. States in 2012. Birth Issues Perinat. Care 2015, 42, 309–318. [Google Scholar] [CrossRef]
- Deutsch, A.R.; Lustfield, R.; Hanson, J.D. Where there’s a will, there’s a way? Strategies to reduce or abstain from alcohol use developed by Northern Plains American Indian women participating in a brief, alcohol-exposed pregnancy preconceptual intervention. Alcohol. Clin. Exp. Res. 2021, 45, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, H.; Srinath, M.; Rockhill, K.; Hogue, C. The Association Between Diabetes Mellitus Among American Indian/Alaska Native Populations with Preterm Birth in Eight US States from 2004–2011. Matern. Child Health J. 2015, 19, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.R.; Clapp, J.D.; Calac, D.; Kolander, C.; Nyquist, C.; Chambers, C.D. Creating a culturally appropriate web-based behavioral intervention for American Indian/Alaska Native women in Southern California: The healthy women healthy Native nation study. Am. Indian Alsk. Nativ. Ment. Health Res. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Hadley, M.E.; Day, G.; Beans, J.A.; Groen, R.S. Postpartum hemorrhage: Moving from response to prevention for Alaska Native mothers. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2021, 155, 290–295. [Google Scholar] [CrossRef]
- Halvorson, K.L.; Vogt, H.B.; Kightlinger, L.; Stevens, D. The Impact of Maternal Diabetes, Obesity and Race on Infant Birth Weights in South Dakota. South Dak. Med. 2017, 70, 61–66. [Google Scholar]
- Hanson, J.D.; Winberg, A.; Elliott, A.J. Development of a Media Campaign on Fetal Alcohol Spectrum Disorders for Northern Plains American Indian Communities. Health Promot. Pract. 2011, 13, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.D.; Miller, A.L.; Winberg, A.; Elliott, A.J. Prevention of alcohol-exposed pregnancies among nonpregnant American Indian women. Am. J. Health Promot. 2013, 27, S66–S73. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.D.; Ingersoll, K.; Pourier, S. Development and Implementation of CHOICES Group to Reduce Drinking, Improve Contraception, and Prevent Alcohol-Exposed Pregnancies in American Indian Women. J. Subst. Abus. Treat. 2015, 59, 45–51. [Google Scholar] [CrossRef]
- Hanson, J.D.; Nelson, M.E.; Jensen, J.L.; Willman, A.; Jacobs-Knight, J.; Ingersoll, K. Impact of the CHOICES Intervention in Preventing Alcohol-Exposed Pregnancies in American Indian Women. Alcohol. Clin. Exp. Res. 2017, 41, 828–835. [Google Scholar] [CrossRef]
- Hiratsuka, V.Y.; Reid, M.; Chang, J.; Jiang, L.; Brega, A.G.; Fyfe-Johnson, A.L.; Huyser, K.R.; Johnson-Jennings, M.; Conway, C.; Steiner, J.F.; et al. Associations Between Rurality, pre-pregnancy Health Status, and Macrosomia in American Indian/Alaska Native Populations. Matern. Child Health J. 2022, 26, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Hunsberger, M.; Rosenberg, K.D.; Donatelle, R.J. Racial/Ethnic Disparities in Gestational Diabetes Mellitus: Findings from a Population-Based Survey. Womens Health Issues 2010, 20, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Kenyon, D.B.; Hanson, J.D. Preventing alcohol-exposed pregnancy among American-Indian youth. Sex Educ. 2016, 16, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Saraiva, C.; Curtis, M.; Wilson, H.G.; Troyan, J.; Sharma, A.J. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, California, 2007–2009. Am. J. Public Health 2013, 103, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lammers, C.; Hulme, P.; Wey, H.; Kerkvliet, J.; Arunachalam, S. Understanding Women’s Awareness and Access to Preconception Health Care in a Rural Population: A Cross Sectional Study. J. Community Health 2017, 42, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Lowrey, O.; Ciampaglio, K.; Messerli, J.L.; Hanson, J.D. Utilization of the Transtheoretical Model to Determine the Qualitative Impact of a Tribal FASD Prevention Program. SAGE Open 2019, 9, 215824401882236. [Google Scholar] [CrossRef] [PubMed]
- Montag, A.C.; Brodine, S.K.; Alcaraz, J.E.; Clapp, J.D.; Allison, M.A.; Calac, D.; Hull, A.D.; Gorman, J.R.; Jones, K.L.; Chambers, C.D. Preventing alcohol-exposed pregnancy among an American Indian/Alaska Native population: Effect of a screening, brief intervention, and referral to treatment intervention. Alcohol. Clin. Exp. Res. 2015, 39, 126–135. [Google Scholar] [CrossRef]
- Montag, A.C.; Dusek, M.L.; Ortega, M.L.; Camp-Mazzetti, A.; Calac, D.J.; Chambers, C.D. Tailoring an Alcohol Intervention for American Indian Alaska Native Women of Childbearing Age: Listening to the Community. Alcohol. Clin. Exp. Res. 2017, 41, 1938–1945. [Google Scholar] [CrossRef]
- Moore, K.R.; Stotz, S.; Nadeau, K.J.; Terry, M.A.; Garcia-Reyes, Y.; Gonzales, K.L.; Charron-Prochownik, D. Recommendations from American Indian and Alaska Native Adolescent Girls for a Community-Based Gestational Diabetes Risk Reduction and Reproductive Health Education Program. Res. J. Women’s Health 2019, 6, 1. [Google Scholar] [CrossRef]
- Moore, K.R.; Stotz, S.; Abujaradeh, H.; Marshall, G.; Terry, M.A.; Charron-Prochownik, D.; Stopping GDM Study Group. Reducing risk for gestational diabetes among American Indian and Alaska Native teenagers: Tribal leaders’ recommendations. Int. J. Gynecol. Obstet. 2021, 155, 195–200. [Google Scholar] [CrossRef]
- Mukherjee, S.; Fennie, K.; Coxe, S.; Madhivanan, P.; Trepka, M.J. Racial and ethnic differences in the relationship between antenatal stressful life events and postpartum depression among women in the United States: Does provider communication on perinatal depression minimize the risk? Ethn. Health 2018, 23, 542–565. [Google Scholar] [CrossRef]
- Nadeau, K.J.; Stotz, S.A.; Moore, K.; Garcia-Reyes, Y.; Sereika, S.M.; Stein, H.; Charron-Prochownik, D. Beta Testing of a Gestational Diabetes Risk Reduction Intervention for American Indian and Alaska Native Teens. J. Pediatr. Health Care 2020, 34, 418–423. [Google Scholar] [CrossRef]
- Richards, J.; Mousseau, A. Community-based participatory research to improve preconception health among Northern Plains American Indian adolescent women. Am. Indian Alsk. Nativ. Ment. Health Res. 2012, 19, 154–185. [Google Scholar] [CrossRef]
- Rockhill, K.; Dorfman, H.; Srinath, M.; Hogue, C. The Effects of Prepregnancy Body Mass Index and Gestational Weight Gain on Fetal Macrosomia Among American Indian/Alaska Native Women. Matern. Child Health J. 2015, 19, 2480–2491. [Google Scholar] [CrossRef] [PubMed]
- Seal, N.; Broome, M.E. Prepregnancy Body Mass Index and Feeding Practices in Relation to Infants’ Growth. J. Nurse Pract. 2013, 9, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, U.; Weber, T.L.; Hanson, J.D. “But Problems Dwell so the Urge Is Constant…” Qualitative Data Analysis of the OST CHOICES Program. Alcohol. Clin. Exp. Res. 2018, 42, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Stotz, S.; Charron-Prochownik, D.; Terry, M.A.; Gonzales, K.; Moore, K. Reducing Risk for Gestational Diabetes Mellitus (GDM) Through a Preconception Counseling Program for American Indian/Alaska Native Girls: Perceptions From Women With Type 2 Diabetes or a History of GDM. Diabetes Educ. 2019, 45, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Stotz, S.A.; Charron-Prochownik, D.; Terry, M.A.; Marshall, G.; Fischl, A.R.; Moore, K.R. Stopping Gestational Diabetes in American Indian and Alaska Native Girls: Nutrition as a Key Component to Gestational Diabetes Risk Reduction. Curr. Dev. Nutr. 2021, 5 (Suppl. S4), 13–21. [Google Scholar] [CrossRef]
- Stotz, S.A.; Hebert, L.E.; Charron-Prochownik, D.; Scarton, L.; Moore, K.R.; Sereika, S.M.; Stopping GDM Study Group. Relationship between food insecurity and a gestational diabetes risk reduction intervention: Outcomes among American Indian and Alaska Native adolescent and young adult females. Transl. Behav. Med. 2023, 13, 645–665. [Google Scholar] [CrossRef]
- Tabet, M.; Jakhar, S.; Williams, C.A.; Rawat, U.; Hailegiorgis, Y.D.; Flick, L.H.; Chang, J. Racial/Ethnic Differences in Correlates of Spontaneous and Medically-Indicated Late Preterm Births among Adolescents. J. Pediatr. Adolesc. Gynecol. 2017, 30, 63–70. [Google Scholar] [CrossRef]
- Terry, M.A.; Stotz, S.A.; Charron-Prochownik, D.; Beirne, S.; Gonzales, K.; Marshall, G.; Moore, K.R.; Stopping GDM Study Group. Recommendations from an expert panel of health professionals regarding a gestational diabetes risk reduction intervention for American Indian/Alaska Native Teens. Pediatr. Diabetes 2020, 21, 415–421. [Google Scholar] [CrossRef]
- Tiwari, R.; Enquobahrie, D.A.; Wander, P.L.; Painter, I.; Souter, V. A retrospective cohort study of race/ethnicity, pre-pregnancy weight, and pregnancy complications. J. Matern.-Fetal Neonatal Med. 2021, 35, 6388–6395. [Google Scholar] [CrossRef] [PubMed]
- Wojcicki, J.M.; Young, M.B.; Perham-Hester, K.A.; De Schweinitz, P.; Gessner, B.D. Risk factors for obesity at age 3 in Alaskan children, including the role of beverage consumption: Results from Alaska PRAMS 2005-2006 and Its three-year follow-up survey, CUBS, 2008–2009. PLoS ONE 2015, 10, e0118711. [Google Scholar] [CrossRef]
- Zamora-Kapoor, A.; Nelson, L.; Buchwald, D.; Walker, L.; Mueller, B. Pre-Eclampsia in American Indians/Alaska Natives and Whites: The Significance of Body Mass Index. Matern Child Health J. 2016, 20, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Mallard, S.R.; Houghton, L.A. Folate knowledge and consumer behaviour among pregnant New Zealand women prior to the potential introduction of mandatory fortification. Asia Pac. J. Clin. Nutr. 2012, 21, 440–449. [Google Scholar] [PubMed]
- Lim, S.; Harrison, C.; Callander, E.; Walker, R.; Teede, H.; Moran, L. Addressing Obesity in Preconception, Pregnancy, and Postpartum: A Review of the Literature. Curr. Obes. Rep. 2022, 11, 405–414. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies; AIHW: Canberra, Australia, 2023.
- Australian Government Department of Health. Fifth National Aboriginal and Torres Strait Islander Blood Borne Viruses and Sexually Transmissible Infections Strategy 2018–2022; Australian Government Department of Health: Canberra, Australia, 2018.
- Klein, J.; Boyle, J.; Kirkham, R.; Connors, C.; Whitbread, C.; Oats, J.; Barzi, F.; McIntyre, D.; Lee, I.; Luey, M.; et al. Preconception care for women with type 2 diabetes mellitus: A mixed-methods study of provider knowledge and practice. Diabetes Res. Clin. Pract. 2017, 129, 105–115. [Google Scholar] [CrossRef]
- Kirkham, R.; Trap-Jensen, N.; Boyle, J.A.; Barzi, F.; Barr, E.L.M.; Whitbread, C.; Van Dokkum, P.; Kirkwood, M.; Connors, C.; On behalf of the NT Diabetes in Pregnancy Partnership; et al. Diabetes care in remote Australia: The antenatal, postpartum and inter-pregnancy period. BMC Pregnancy Childbirth 2019, 19, 389. [Google Scholar] [CrossRef]
- Hussein, N.; Kai, J.; Qureshi, N. The effects of preconception interventions on improving reproductive health and pregnancy outcomes in primary care: A systematic review. Eur. J. Gen. Pract. 2016, 22, 42–52. [Google Scholar] [CrossRef]
- Osborne, K.; Baum, F.; Brown, L. What Works? A Review of Actions Addressing the Social and Economic Determinants of Indigenous Health; AIHW: Canberra, Australia, 2013.
- Kendall, E.; Barnett, L. Principles for the development of Aboriginal health interventions: Culturally appropriate methods through systemic empathy. Ethn. Health 2015, 20, 437–452. [Google Scholar] [CrossRef]
- Walters, K.L.; Johnson-Jennings, M.; Stroud, S.; Rasmus, S.; Charles, B.; John, S.; Allen, J.; Kaholokula, J.K.; Look, M.A.; de Silva, M.; et al. Growing from Our Roots: Strategies for Developing Culturally Grounded Health Promotion Interventions in American Indian, Alaska Native, and Native Hawaiian Communities. Prev Sci. 2020, 21 (Suppl. S1), 54–64. [Google Scholar] [CrossRef] [PubMed]
- Steel, A.; Carter, T. Balancing our gaze on preconception health and care to include men. Adv. Integr. Med. 2021, 8, 79–80. [Google Scholar] [CrossRef]
- Carter, T.; Schoenaker, D.; Adams, J.; Steel, A. Paternal preconception modifiable risk factors for adverse pregnancy and offspring outcomes: A review of contemporary evidence from observational studies. BMC Public Health 2023, 23, 509. [Google Scholar] [CrossRef]
- Kotelchuck, M.; Lu, M. Father’s Role in Preconception Health. Matern. Child Health J. 2017, 21, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
- OECD. Delivering Quality Education and Health Care to All; OECD Publishing: Paris, France, 2021. [Google Scholar]
- National Center for Health Statistics. National Health Interview Survey: Interactive Summary Health Statistics for Adults—2019–2021. Available online: https://wwwn.cdc.gov/NHISDataQueryTool/SHS_adult/index.html (accessed on 2 November 2023).
| Author and Date | Indigenous Population | Geographical Remoteness | Study Type | Participants | Preconception Health Areas |
|---|---|---|---|---|---|
| Australia | |||||
| Bower et al. (2016) [40] | Aboriginal | Urban and rural | Repeated cross-sectional study | Men and women | Folic acid |
| Campbell et al. (2011) [10] | Aboriginal and Torres Strait Islander | Rural | Prospective cohort | Women | Tobacco, diet, BMI, waist circumference, physical activity, alcohol, diabetes, STIs, systolic and diastolic blood pressure (SBP and DBP), cholesterol, triglycerides, red cell folate, fasting glucose, albumin/creatinine ratio, gamma-glutamyl transferase, metabolic syndrome |
| Campbell et al. (2012) [41] | Aboriginal and Torres Strait Islander | Rural | Prospective cohort | Women | Tobacco, diet, BMI, waist circumference, physical activity, alcohol, diabetes, SBP and DBP, cholesterol, triglycerides, red cell folate, fasting glucose, albumin/creatinine ratio, gamma-glutamyl transferase, hyper-triglyceridemic waist, metabolic syndrome |
| Campbell et al. (2013) [42] | Aboriginal and Torres Strait Islander | Rural | Prospective cohort | Women | Tobacco, diet, BMI, waist circumference, physical activity, alcohol, SBP and DBP, cholesterol, triglycerides, red cell folate, fasting glucose, albumin/creatinine ratio, gamma-glutamyl transferase, metabolic syndrome |
| Gilbert et al. (2021) [43] | Aboriginal and Torres Strait Islander | Rural | Mixed methods | Men and women | General preconception health and fertility |
| Griffiths et al. (2020) [44] | Aboriginal | Rural | Retrospective audit | Women | Tobacco, diet, BMI, alcohol, mental health, STIs, proteinuria, folic acid, vaccination, cervical screening, chronic disease management, fertility management, family and domestic violence |
| Kennedy et al. (2021) [45] | Aboriginal and Torres Strait Islander | Urban and rural | Mixed methods | Women | Tobacco, diet, alcohol, mental health |
| Lucas et al. (2021) [46] | Indigenous Australians | Rural | Prospective cohort | Women | BMI, diabetes |
| Maple-Brown et al. (2019) [47] | Indigenous Australians | Rural | Prospective cohort | Women | BMI, diabetes |
| Munns et al. (2016) [48] | Aboriginal | Rural | Mixed methods | Health service staff | General preconception health |
| Schumacher et al. (2019) [49] | Indigenous Australians | Rural | Prospective cohort | Women | BMI, diabetes, hypertension |
| Sina et al. (2014) [50] | Aboriginal | Rural | Nested case–control | Women | BMI, SBP and DBP, pre-existing albuminuria, waist circumference, hip circumference, waist-to-hip ratio, waist-to-height ratio |
| Thrift and Callaway (2014) [51] | Indigenous Australians | Urban and rural | Retrospective cohort | Women | BMI, diabetes, hypertension |
| Canada | |||||
| Bains et al. (2014) [52] | Inuit and Inuvialuit | Rural | Controlled pre–post | Women | Diet, physical activity |
| Mackenzie et al. (2021) [53] | First Nations | Urban and rural | Retrospective cohort | Women | BMI #, diabetes, hypertension |
| Nelson et al. (2018) [54] | First Nations, Inuit, and Métis | Urban and rural | Cross-sectional survey | Women | Mental health |
| Oliveira et al. (2013) [55] | First Nations | Urban | Retrospective cohort | Women | Tobacco, BMI, diabetes, hypertension |
| Oster et al. (2014) [56] | First Nations | Urban and rural | Retrospective cohort | Women | BMI #, diabetes, hypertension |
| New Zealand | |||||
| Mallard et al. (2012) [57] | Māori | Not stated/unclear | Cross-sectional survey | Women | Folic acid |
| Mallard and Houghton (2014) [58] | Māori | Not stated/unclear | Cross-sectional survey | Women | Iodine |
| Tromop-van Dalen et al. (2021) [59] | Māori | Not stated/unclear | Mixed methods | Health professionals and women | Congenital and rheumatic heart disease |
| United States | |||||
| Anderson et al. (2016) [60] | American Indian and Alaska Native (AI/AN) | Urban and rural | Retrospective cohort | Women | BMI |
| Charron-Prochownik et al. (2023) [61] | AI/AN | Urban and rural | Randomised controlled trial (baseline only) | Women | BMI, physical activity, diet |
| Declercq et al. (2015) [62] | AI/AN | Urban and rural | Retrospective cohort | Women | BMI |
| Deutsch et al. (2021) [63] | AI/AN | Not stated/unclear | Qualitative written responses | Women | Alcohol |
| Dorfman et al. (2015) [64] | AI/AN | Urban and rural | Retrospective cohort | Women | BMI, diabetes, hypertension |
| Gorman et al. (2013) [65] | AI/AN | Not stated/unclear | Qualitative focus groups and interviews | Women and health service staff | Alcohol |
| Hadley et al. (2021) [66] | Alaska Native | Urban and rural | Case–control | Women | BMI |
| Halvorson et al. (2017) [67] | Native American | Urban and rural | Retrospective cohort | Women | BMI, diabetes |
| Hanson et al. (2011) [68] | American Indian | Not stated/unclear | Mixed methods | Elders, community, and women | Alcohol |
| Hanson et al. (2013) [69] | American Indian | Rural | Longitudinal survey | Women | Alcohol |
| Hanson et al. (2015) [70] | American Indian | Rural | Mixed methods | Women | Alcohol |
| Hanson et al. (2017) [71] | American Indian | Urban and rural | Longitudinal survey | Women | Alcohol |
| Hiratsuka et al. (2022) [72] | AI/AN | Urban and rural | Retrospective cohort | Women | BMI, diabetes |
| Hunsberger et al. (2010) [73] | AI/AN | Urban and rural | Retrospective cohort | Women | BMI |
| Jensen et al. (2016) [74] | American Indian | Urban and rural | Qualitative interviews | Health professionals, men, and women | Alcohol |
| Kim et al. (2013) [75] | American Indian | Urban and rural | Retrospective cohort | Women | BMI |
| Lammers et al. (2017) [76] | Native American | Rural | Cross-sectional survey | Women | General preconception health |
| Lowrey et al. (2019) [77] | American Indian | Urban and rural | Qualitative written responses | Women | Alcohol |
| Montag et al. (2015) [78] | AI/AN | Not stated/unclear | Randomised controlled trial | Women | Alcohol |
| Montag et al. (2017) [79] | AI/AN | Not stated/unclear | Qualitative focus groups and interviews | Health professionals, women, community members | Alcohol |
| Moore et al. (2019) [80] | AI/AN | Urban | Qualitative focus groups | Women | BMI, diet, physical activity |
| Moore et al. (2021) [81] | AI/AN | Not stated/unclear | Qualitative focus groups | Health professionals and elders | BMI, diet, physical activity |
| Mukherjee et al. (2018) [82] | AI/AN | Urban and rural | Retrospective cohort | Women | Mental health |
| Nadeau et al. (2020) [83] | AI/AN | Urban | Pre–post | Women | BMI, diet, physical activity |
| Richards and Mousseau (2012) [84] | American Indian | Not stated/unclear | Randomised controlled trial | Women | General preconception health |
| Rockhill et al. (2015) [85] | AI/AN | Urban and rural | Retrospective cohort | Women | BMI |
| Seal and Broome (2013) [86] | American Indian | Not stated/unclear | Cross-sectional survey | Women | BMI |
| Shreshtha et al. (2018) [87] | American Indian | Urban and rural | Mixed methods | Women | Alcohol |
| Stotz et al. (2019) [88] | AI/AN | Urban | Qualitative interviews and focus groups | Women | BMI, diet, physical activity |
| Stotz et al. (2021) [89] | AI/AN | Not stated/unclear | Qualitative interviews and focus groups | Health professionals, elders, and women | BMI, diet, physical activity |
| Stotz et al. (2023) [90] | AI/AN | Not stated/unclear | Randomised controlled trial | Women | BMI, diet, physical activity |
| Tabet et al. (2017) [91] | AI/AN | Urban and rural | Retrospective cohort | Women | BMI |
| Terry et al. (2020) [92] | AI/AN | Urban and rural | Qualitative interviews and focus groups | Health professionals | BMI, diet, physical activity |
| Tiwari et al. (2021) [93] | AI/AN | Not stated/unclear | Retrospective cohort | Women | BMI |
| Wojcicki et al. (2015) [94] | Alaska Native | Urban and rural | Retrospective cohort | Women | BMI |
| Zamora-Kapoor et al. (2016) [95] | AI/AN | Urban and rural | Retrospective cohort | Women | BMI |
| Author and Date | Setting (Indigenous Population, Geographical Remoteness) | Study Description | Participants/Sample | Confounding Factors Adjusted For | Key Results |
|---|---|---|---|---|---|
| Australia | |||||
| Campbell et al. (2011) [10] | Aboriginal and Torres Strait Islander, Rural | Prospective cohort study of Indigenous women to understand predictors of miscarriage | Included 1009 Indigenous women of 15–44 years who participated in a 1999–2000 health screening program in far-north Queensland | Age and ethnicity Age, ethnicity, and risky drinking | APR of becoming pregnant:
|
| Campbell et al. (2012) [41] | Aboriginal and Torres Strait Islander, Rural | Prospective cohort study of Indigenous women to understand predictors of diabetes in pregnancy | Included 1009 Indigenous women of 15–44 years who participated in a 1998–2000 health screening program in north Queensland | Age and ethnicity | APR of diabetes in pregnancy:
|
| Campbell et al. (2013) [42] | Aboriginal and Torres Strait Islander, Rural | Prospective cohort study of Indigenous women to understand predictors of hypertension in pregnancy | Included 1009 Indigenous women of 15–44 years who participated in a 1998–2000 health screening program in north Queensland | Age and ethnicity | APR of hypertension in pregnancy:
|
| Lucas et al. (2021) [46] | Indigenous Australians, Rural | Prospective cohort study of women to understand risk factors for postpartum haemorrhage | Included 1102 women, including 525 Indigenous women in the Northern Territory, who participated in the Pregnancy And Neonatal Diabetes Outcomes in Remote Australia (PANDORA) study 2011–2017 | Age | AOR of postpartum haemorrhage:
|
| Maple-Brown et al. (2019) [47] | Indigenous Australians, Rural | Prospective cohort study of women to understand pregnancy outcomes associated with pre-pregnancy and gestational diabetes. | Included 1135 women, including 542 Indigenous women in the Northern Territory, who participated in the Pregnancy And Neonatal Diabetes Outcomes in Remote Australia (PANDORA) study 2011–2017 | Crude | Women with type 2 vs. women without type 2 diabetes:
|
| Sina et al. (2014) [50] | Aboriginal, Rural | Nested case–control study of women living in a remote Aboriginal community to understand predictors of gestational hypertensive disorders | Included 168 women living in a remote Aboriginal community who participated in a health screening program from 1992 to 1995 and were subsequently pregnant (28 women with gestational hypertensive disorders, matched at baseline with 140 women) | Age at pregnancy, alcohol consumption, and smoking | AOR of gestational hypertensive disorders:
|
| Schumacher et al. (2019) [49] | Indigenous Australians, Rural | Prospective cohort study of pregnant women who identified as Indigenous or carrying an Indigenous child to understand the relationship between pre-pregnancy BMI and gestational weight gain | Included 110 pregnant women who identified as Indigenous or carrying an Indigenous child who attended antenatal clinics in regional and remote NSW | Crude | Excessive weight gain during pregnancy by BMI category:
|
| Thrift and Callaway (2014) [51] | Indigenous Australians, Urban and rural | Retrospective cohort study of women, including Indigenous women, to understand associations between overweight/obesity, Indigenous status and perinatal outcomes | Included 265,848 women, including 13,582 Indigenous women, with singleton births from July 2007 to 2011 | Maternal age, parity, geographical location, and smoking | APR of gestational diabetes:
|
| Canada | |||||
| Mackenzie et al. (2021) [53] | First Nations, Urban and rural | Retrospective cohort study of linked data of First Nations and non-First Nations women to understand risk of developing diabetes after pregnancy | Included 370,923 women, including 21,215 First Nations women, who gave birth in Alberta (singleton pregnancy) from 1999 to 2014 | Maternal age, smoking anytime during pregnancy, pre-existing and pregnancy-induced hypertension, caesarean section, and stillbirth | HR of developing diabetes after pregnancy:
|
| Nelson et al. (2018) [54] | First Nations, Inuit, and Métis, Urban and rural | Cross-sectional survey of Canadian women, including Aboriginal women, to understand determinants of postpartum depression | Included 76,508 women (weighted), including 3130 Aboriginal women (Inuit, Metis, or First Nations living off-reserve) who had given birth to a singleton live baby from November 2005 to May 2006 | Age, marital status, education, employment, income, parity, time to prenatal care, planned pregnancy status, alcohol use, drug use, and smoking status during pregnancy | ARR of postpartum depression:
|
| Oliveira et al. (2013) [55] | First Nations, Urban | Retrospective cohort study of First Nations women compared with non-First Nations women to understand maternal profiles and birth outcomes | Included 451 First Nations women from the Six Nations Reserve, Ontario, with singleton pregnancies 2005–2010 and 622 non-First Nations women from nearby Hamilton, Ontario, with singleton pregnancies 2005–2009 | CrudeNewborn sex, gestational age, hypertension prior to or during pregnancy, gestational weight gain, and smoking during pregnancy | Mean gestational weight gain (kg):
|
| Oster et al. (2014) [56] | First Nations, Urban and rural | Retrospective cohort study of First Nations and non-First Nations women to understand diabetes in pregnancy (pre-pregnancy and gestational diabetes) | Included 427,058 women, including 28,306 First Nations women, who gave birth in Alberta 2000–2009 | Age, previous stillbirth, previous caesarean section, previous LGA infant, proteinuria, and drug dependency | AOR of gestational diabetes
|
| United States | |||||
| Anderson et al. (2016) [60] | AI/AN, Urban and rural | Retrospective cohort study of birth data from women, including AI/AN women, to understand relationship between pre-pregnancy BMI, diabetes (pre-pregnancy and gestational), and birth outcomes | Included 5,193,386 women, including 44,570 AI/AN women, with singleton first births from 2009 to 2013 | Age, education, calendar year, prenatal care, marital status, and child sex | OR of preterm birth:
|
| Declercq et al. (2015) [62] | AI/AN, Urban and rural | Retrospective cohort study of women, including AI/AN women, to understand the relationship between pre-pregnancy obesity and caesarean delivery | Included 2,233,144 women, including AI/AN women, who had a singleton, vertex birth (37–41 weeks) in 2012 and no prior caesarean | Crude | Caesarean rates by BMI category:
|
| Dorfman et al. (2015) [64] | AI/AN, Urban and rural | Retrospective cohort study of women, including AI/AN women, to understand association between diabetes (pre-pregnancy and gestational) and preterm birth | Included 12,420 AI/AN women with live singleton births who participated in the 2004–2011 Pregnancy Risk Assessment Monitoring System (PRAMS) | Crude | OR of preterm birth:
|
| Hadley et al. (2021) [66] | Alaska Native, Urban and rural | Case–control study of Alaska Native women to understand risk factors for postpartum haemorrhage | Included 384 Alaska Native women (128 cases, 256 controls) who gave birth in 2018–2019 at the Alaska Native Medical Center | Crude | OR of postpartum haemorrhage:
|
| Halvorson et al. (2017) [67] | Native American, Urban and rural | Retrospective cohort study of birth data from women, including Alaska Native women, to understand relationship between race, pre-pregnancy BMI, diabetes, and birth outcomes | Included 70,679 women, including 11,416 Native American women with singleton live births from 2006 to 2011 | Crude | Mean birthweight by diabetes status:
|
| Hiratsuka et al. (2022) [72] | AI/AN, Urban and rural | Retrospective cohort study of AI/AN women to understand the association of macrosomia with pre-pregnancy health status and social determinants of health | Included 1136 AI/AN women with singleton births from 2011–2019 receiving Indian Health Service care | Age at pregnancy, health insurance type, chronic conditions, gestation, and country-level social determinants of health indicators | AOR of macrosomia:
|
| Hunsberger et al. (2010) [73] | AI/AN, Urban and rural | Retrospective cohort study of Oregon women, including AI/AN women, to understand risk factors for gestational diabetes | Included 3883 women, including 493 AI/AN women, with live births who participated in the 2004–2011 Pregnancy Risk Assessment Monitoring System (PRAMS) | Crude | Prevalence of gestational diabetes by BMI category:
|
| Kim et al. (2013) [75] | American Indian, Urban and rural | Retrospective cohort study of women, including Native American women, to understand contribution of BMI to gestational diabetes risk across racial groups | Included 1,228,265 women, including 4134 American Indian women, with a live singleton birth in 2007–2009 | Maternal age, parity, and nativity | ARR of gestational diabetes:
|
| Mukherjee et al. (2018) [82] | AI/AN, Urban and rural | Retrospective cohort study of women, including AI/AN women, to understand risk factors for postpartum depression | Included 87,565 women, including 2757 AI/AN women, who participated in the 2009–2011 Pregnancy Risk Assessment Monitoring System (PRAMS) | Maternal education, marital status, pregnancy intention, and prenatal stressors | AOR of postpartum depression:
|
| Rockhill et al. (2015) [85] | AI/AN, Urban and rural | Retrospective cohort study of AI/AN women to understand relationship between pre-pregnancy BMI and gestational weight gain on birthweight | Included 8663 AI/AN women who participated in the Pregnancy Risk Assessment Monitoring System 2004–2011 | AI/AN race, maternal age, parity, any diabetes during pregnancy, smoking during pregnancy, gestational weight gain, and state of residence | AOR of high-birthweight baby (4000 g+):
|
| Seal and Broome (2013) [86] | American Indian, Not stated/unclear | Cross-sectional survey of American Indian mothers of full-term infants to understand relationships between pre-pregnancy BMI, feeding practices, and infant growth | Included 98 American Indian mothers of infants of 14–20 weeks who visited an outpatient clinic | Crude | No significant associations between pre-pregnancy BMI and birthweight. |
| Tabet et al. (2017) [91] | AI/AN, Urban and rural | Retrospective cohort study of women, including AI/AN women, to understand risk factors for late preterm birth (34–36 weeks’ gestation) | Included 171,573 women <20 years, including 2459 AI/AN women, with singleton first births at 34–44 weeks’ gestation in 2012 | Age, marital status, health insurance, prenatal care, infant sex, gestational weight gain, and infection | AOR of spontaneous late preterm birth:
|
| Tiwari et al. (2021) [93] | AI/AN, Not stated/unclear | Retrospective cohort study of women, including AI/AN women, using data from the Obstetrical Care Outcomes Assessment Program database to understand the relationship between pre-pregnancy overweight/obesity and pregnancy complications | Included 72,697 women, including 978 AI/AN women, with singleton live births from 2014 to 2018 in Washington State | Maternal age, parity, delivery hospital, health insurance, substance abuse, nicotine use, and alcohol use | ARR of pre-eclampsia:
|
| Wojcicki et al. (2015) [94] | Alaska Native, Urban and rural | Retrospective cohort study of Alaskan women, including Alaska Native women, to understand maternal and child factors associated with obesity at age 3 | Included 833 women, including 277 Alaska Native women, who completed the Risk Assessment Monitoring System 2005–2006 and the Childhood Understanding Behaviors Survey (CUBS) 2008–2009 | Maternal age, child witnessing abuse, geographical location, participating in WIC nutrition program antenatally, breastfeeding, child fried potato consumption, child fruit juice consumption, maternal chew/spit tobacco use, and income | AOR obese child at age 3 years:
|
| Zamora-Kapoor et al. (2016) [95] | AI/AN, Urban and rural | Retrospective cohort study of AI/AN women matched with non-Indigenous women to understand risk factors for pre-eclampsia | Included 7194 AI/AN women with singleton live first births in Washington State 2003–2013, matched with a sample of 63,886 white women | N/A | Risk of pre-eclampsia by BMI category:
|
| Author and Date | Setting (Indigenous Pop, Geographical Remoteness) | Intervention | Study Description | Participants/Sample | Key Results |
|---|---|---|---|---|---|
| Australia | |||||
| Bower et al. (2016) [40] | Aboriginal, Urban and rural | Mandatory folic acid bread fortification | Repeated cross-sectional study of Aboriginal men and non-pregnant women and analysis of WA Register of Developmental Anomalies to compare folate status and Neural Tube Defects in the Aboriginal population before and after bread fortification | Included 95 Aboriginal men and non-pregnant women age 16–44 years (subsequent measurement) | Mean red cell folate concentrations increased by 41% in males and 49% in females after mandatory fortification (mean difference before and after fortification: males 129 ng/mL, 95% CI 81–177; females 186 ng/mL, 95% CI 139–233). The prevalence ratio for Neural Tube Defects in the Aboriginal population for the post-fortification period (2010–2014) was 0.32 (95% CI 0.15–0.69) compared with 1980–2009. |
| Kennedy et al. (2021) [45] | Aboriginal and Torres Strait Islander, Urban and rural | Preconception and pregnancy multibehavioural change app for Aboriginal women (MAMA-EMPOWER) | Mixed methods study including 1. interviews with Aboriginal women, 2. workshop with Aboriginal women, 3. app trial with Aboriginal women, and 4. user Mobile Application Rating Scale survey of Aboriginal women to develop and test MAMA-EMPOWER | Phase 1 interviews: 8 urban Aboriginal women, Phase 2 workshop: 6 Aboriginal women, Phase 3 app trial: 16 urban and regional Aboriginal women, and Phase 3 survey: 5 Aboriginal women | Participants informed the content, functionality, and user experience of the app. User MARS survey ratings were highest for information (mean score 3.80 out of 5, SD 0.77) and aesthetics (mean score 3.87, SD 0.74), while functionality (mean score 3.0, SD 0.73), engagement (mean score 3.2, SD 1.08), and subjective quality (mean score 3.2, SD 0.95) had lower scores. Qualitative feedback indicated that the developed app was acceptable; however, functionality was problematic. |
| Munns et al. (2016) [48] | Aboriginal, Rural | Preconception health activities as part of a community antenatal program | Mixed methods review, including interviews and focus groups with staff and partner agencies and quantitative reporting data, to understand acceptability and satisfaction with an Aboriginal Community Antenatal Program which included preconception services | Included 22 staff and representatives from partner agencies | Participants raised a range of organisational factors, staff factors, cultural issues, and interagency issues which affected implementation. |
| Canada | |||||
| Bains et al. (2014) [52] | Inuit and Inuvialuit, Rural | Twelve-month community-based diet and lifestyle intervention aimed at Inuit and Inuvialuit women of childbearing age (Healthy Foods North) | Controlled pre–post evaluation | Included 136 women of 19–44 years from 6 Inuit and Inuvialuit communities in Arctic Canada (79 exposed and 57 in control group) | ANCOVA analysis demonstrated a positive impact of the intervention on vitamin A (pre–post change for intervention group vs. control= 558.23 mcg, 95% CI 179.86–936.59) and vitamin D (pre–post change for intervention group vs. control = 89.23 IU, 95% CI 3.86–174.60). There was no significant change in energy, sugar, or fat consumption or other nutrients (folate, calcium, or iron). |
| New Zealand | |||||
| Mallard and Houghton (2014) [58] | Māori, Not stated/unclear | Mandatory iodine bread fortification | Cross-sectional survey of postpartum women to understand iodine intake | Included 723 women who had given birth in birthing centres across New Zealand in March–April 2011, including 93 Māori women | Following fortification, an estimated 5% of Māori women were below the Estimated Average Requirement for iodine preconception (95% CI 0–11%), compared with 15% prior to fortification (95% CI 3–27%). |
| Mallard et al. (2012) [57] | Māori, Not stated/unclear | Mandatory folic acid bread fortification (modelled) | Cross-sectional survey of postpartum women to understand bread intake prior to and during pregnancy and model effects of proposed mandatory folic acid fortification | 723 women who had given birth in birthing centres across New Zealand in March-April 2011, including 93 Māori women | Assuming mandatory bread fortification, mean preconception folic acid intake from bread for Māori women would be 173.1 mcg/day (95% CI 144.8–201.5). However, 47.1% of Māori women would not be consuming the target of an additional 140 mcg of folic acid from bread (95% CI 35.6–58.7%). |
| United States | |||||
| Charron-Prochownik et al. (2023) [61] | AI/AN, Urban and rural | ‘Stopping GDM’—gestational diabetes preconception risk reduction and counselling intervention for AI/AN teenagers at high risk of GDM | Analysis of baseline data from a GDM risk reduction intervention for at-risk AI/AN teens, ‘Stopping GDM’, to explore associations between GDM risk reduction awareness, knowledge, health beliefs, and behaviours | Included 149 female AI/AN young women, 12–24 years old, and their mothers (or adult female caregivers) who participated in Stopping GDM | At baseline, mothers had greater knowledge (measured as a percentage of questioned answered correctly) of GDM prevention than their daughters (mean score on a 0 to 100 scale: mothers 49.2, SD 20.9, daughters: 20.9, SD 20.4, p < 0.001) and perceived greater susceptibility than their daughters (mean score on a 0 to 20 scale: mothers 8.9, SD 4.0, daughters 7.0, SD 3.8, p < 0.001). Daughters reported moderate levels of self-confidence (self-efficacy) in their ability to engage in healthy living (mean score on an 8 to 80 scale: 48.2, SD 13.9) but low mean scores on healthy eating (mean score, 9.0, SD 5.6) and physical activity (mean score: 3.4, SD 2.2). Mothers were more likely to initiate GDM communication with daughters than vice versa (mean score: mothers 2.9, SD 0.9, daughters 2.3, SD 1.3, p < 0.001) |
| Deutsch et al. (2021) [63] | AI/AN, Not stated/unclear | Brief alcohol-exposed pregnancy reduction program (OST CHOICES) | Qualitative analysis of alcohol exposure prevention strategies by AI/AN women | Included 160 AI/AN women of 18–42 years who participated in OST CHOICES | Common prevention strategies included positive social supports and avoiding negative or alcohol-involved social environments. Other strategies used circular logic (i.e., drinking less to reduce drinking). Traditional and Western cultural strengths were identified as important resources, although many participants did not have a cultural resource strategy. |
| Gorman et al. (2013) [65] | AI/AN, Not stated/unclear | Brief online program for screening and prevention of prenatal alcohol use | Qualitative focus groups and interviews with AI/AN women and key informants to modify a brief online program for screening and prevention of prenatal alcohol use | Included 15 AI/AN women of childbearing age and 6 key informants | Participants reported that the program and format were acceptable and raised issues of confidentiality, the importance of family and culture, and tailored content and recommended including information on the negative impacts of alcohol on child health. |
| Hanson et al. (2011) [68] | American Indian, Not stated/unclear | Alcohol-exposed pregnancy prevention and awareness media campaign | Mixed methods study including focus groups with American Indian women, elders, and community members and a survey of American Indian women to develop and evaluate an alcohol-exposed pregnancy prevention and awareness media campaign in the Northern Plains | Included 5 American Indian women, 18–44, 10 elder women, and 25 community members, who participated in focus groups and 119 American Indian women of 18–44 years who completed a survey | Focus group participants identified the importance of including traditional language and themes as well as positive messaging in the campaign. Overall, 91.6% of survey participants agreed that the campaign increased their knowledge of Foetal Alcohol Syndrome, and 71.8% agreed that the campaign decreased their drinking (no further statistical analysis conducted). |
| Hanson et al. (2013) [69] | American Indian, Rural | Telephone-based alcohol-exposed pregnancy reduction program | Longitudinal survey (baseline, 3, 6, 9, and 12 months) | Included 231 non-pregnant American Indian women of 18–44 years who participated in a phone-based intervention modified from Project CHOICES program | The proportion of participants at risk of alcohol-exposed pregnancy reduced from 54% at baseline to 20% at 12 months (difference between baseline and all other visits p < 0.001). Alcohol consumption decreased across all behavioural measures over the intervention duration (average change −26% to −17%, 99% CIs −41% to −7%), and the proportion of participants reporting no birth control decreased from baseline to 3 months (29–10%, p < 0.001). |
| Hanson et al. (2015) [70] | American Indian, Rural | Group-based brief alcohol-exposed pregnancy reduction program (CHOICES Group) | Mixed-methods study, including survey of non-pregnant American Indian women of 18–44 years and observations to evaluate group mode of delivery for CHOICES Group | Included 33 non-pregnant American Indian women of 18–44 years who participated in the pilot of CHOICES Group | Participants reported that sessions positively engaged members (median engagement score session 1: 4.8/6, session 2: 4.4/6), had low levels of conflict (median conflict score session 1: 0.6/6, session 2: 0.4/6), and had average levels of avoidance of personal responsibility (median avoidance score session 1: 3.7/6, session 2: 3.3/6). Observations found that group leaders possessed some motivational interview skills, with improvement needed in other motivational interviewing and leadership skills. |
| Hanson et al. (2017) [71] | American Indian, Urban and rural | Brief alcohol-exposed pregnancy reduction program (OST CHOICES) | Longitudinal survey (baseline and 3 and 6 months post-intervention) of non-pregnant American Indian women to evaluate OST CHOICES | Included 99 non-pregnant American Indian women of 18–44 years who participated in OST CHOICES | All women were at risk at baseline. This dropped to 25.4–47.2% at 3 months and 18.1–66.3% at 6 months depending on modelling assumptions. For those who reduced AEP risk, this was most commonly due to using effective birth control (3 months: 67.7, 6 months: 61.5%) rather than lowering binge drinking (3 months: 9.8%, 6 months: 20.0%) or both behaviours (3 months: 22.6%, 6 months: 18.5%). |
| Jensen et al. (2016) [74] | American Indian, Urban and rural | Brief alcohol-exposed pregnancy reduction program (OST CHOICES) | Qualitative interviews with health and social service professionals and focus groups with American Indian men and women to inform the expansion of OST CHOICES | Included 25 health and social service professionals and 58 American Indian men and women | Participants suggested a focus on younger people through schooling and other channels and raised the role of family and culture in preventing alcohol-exposed pregnancies. |
| Lowrey et al. (2019) [77] | American Indian, Urban and rural | Brief alcohol-exposed pregnancy reduction program (OST CHOICES) | Qualitative written responses of American Indian women to understand participant experiences using a transtheoretical model | Included 203 American Indian women who participated in OST CHOICES | Participants’ responses indicated progression through stages of change from participating in the program. Participants reported that their children, education, and work were motivators to decrease unhealthy behaviours. Common barriers to behaviour change were reported and included stress and temptation to drink socially. |
| Montag et al. (2015) [78] | AI/AN, Not stated/unclear | Online Screening, brief intervention, and referral to treatment through alcohol-exposed pregnancy prevention intervention | Randomised controlled trial with survey data at baseline and 1, 3, and 6 months post-intervention) of AI/AN women | Included 247 AI/AN women of 18–45 years who received an online alcohol-exposed pregnancy prevention intervention or usual treatment | Both groups decreased self-reported risky drinking behaviour (risk of alcohol-exposed pregnancy p < 0.001 at 6 months post-intervention), with no significant difference between groups. |
| Montag et al. (2017) [79] | AI/AN, Not stated/unclear | Peer-to-peer screening, brief intervention and referral to alcohol-exposed pregnancy prevention intervention | Qualitative focus groups and interviews with American Indian women, community leaders, Tribal Elders, and health staff to inform adaption of online screening, brief intervention, and referral to treatment alcohol-exposed pregnancy prevention intervention to a peer-to-peer, motivational interviewing format | Included 10 focus groups (n = 54) and 3 interviews with American Indian women of 18–45 years, community leaders, Tribal Elders, and health staff | Participants identified adaptions to make the intervention appropriate for the peer-to-peer format, culturally appropriate, and relevant for the target audience. Participants suggested making changes to content about assessing alcohol consumption, assessing why women drink, and assessing why women do not drink. |
| Moore et al. (2019) [80] | AI/AN, Urban | ‘Stopping GDM’—gestational diabetes preconception risk reduction and counselling intervention for AI/AN teenagers at high risk of GDM | Qualitative focus groups with AI/AN adolescent females at risk of gestational diabetes (and their mothers) about their awareness and understanding of GDM and reproductive health to inform the adaption of a preconception counselling and diabetes education intervention | Included 13 AI/AN adolescent females at risk of gestational diabetes and their mothers | Participants identified a lack of awareness and knowledge of GDM, a need for pregnancy planning and culturally responsive GDM and reproductive health resources, and importance of empowerment to promote positive reproductive health behaviours. |
| Moore et al. (2021) [81] | AI/AN, Not stated/unclear | ‘Stopping GDM’—gestational diabetes preconception risk reduction and counselling intervention for AI/AN teenagers at high risk of GDM | Qualitative focus groups with AI/AN tribal leaders and health care administrators to inform the development of Stopping GDM | Included 12 AI/AN tribal leaders and health care administrators who attended the 2015 National Indian Health Board Conference in Washington, DC | Participants made recommendations regarding effective communication with AI/AN youth (avoiding directive language, including health information, using relevant imagery and language), the importance of family and community engagement, and incorporating diverse traditional AI/AN cultural values and practices. |
| Nadeau et al. (2020) [83] | AI/AN, Urban | ‘Stopping GDM’—gestational diabetes preconception risk reduction and counselling intervention for AI/AN teenagers at high risk of GDM | Pre–post study of AI/AN daughters and their mothers to evaluate Stopping GDM prior to a randomised controlled trial | Included 11 AI/AN daughters at risk of GDM and their mothers recruited through an urban Indian health program | Mean knowledge relating to diabetes prevention increased pre-intervention to post-intervention (mothers: 70% to 80%, daughters: 45% to 58%), as did reproductive health and GDM knowledge (mothers: 65% to 92%, daughters: 34% to 71%). Daughters’ self-efficacy increased for healthy living (47.2 ± 11.1 to 56.6 ± 14.3, possible score 8–80) and pregnancy planning (57.0 ± 9.2 to 66.4 ± 17.7, possible score 12–120). Satisfaction for the materials was moderately high to very high. |
| Richards and Mousseau (2012) [84] | American Indian, Not stated/unclear | Preconception health educational intervention aimed at American Indian high school students | Randomised controlled evaluation | Included 58 American Indian high school students who attended a summer high school residential academic program (28 exposed and 30 in control group) | The intervention group scored higher than the non-intervention group in overall preconception health knowledge (96% vs. 90%, p = 0.03) and obesity knowledge post-intervention (44% vs. 33%, p = 0.01). Other health areas (smoking, alcohol, diabetes, contraception) were not significant.There were significant changes in obesity and diabetes knowledge for the intervention group from pre- to post-intervention (obesity: 28% vs. 48%, p = 0.01, diabetes: 36% vs. 72%, p = 0.02). Other health areas (general preconception health, smoking, alcohol, contraception) were not significant. |
| Shreshtha et al. (2018) [87] | American Indian, Urban and rural | Brief alcohol-exposed pregnancy reduction program (OST CHOICES) | Mixed-methods study, including behavioural survey and qualitative analysis of drinking behaviours and attitudes of non-pregnant American Indian women participating in OST CHOICES | Included 264 non-pregnant American Indian women of 18–44 years who participated in OST CHOICES | Mean alcohol self-efficacy was −0.04 (range: −23 to 24). Participants reported positive aspects of drinking including escaping from problems, socializing, and relaxation. Negative aspects included impact on families and domestic violence. |
| Stotz et al. (2019) [88] | AI/AN, Urban | ‘Stopping GDM’—gestational diabetes preconception risk reduction and counselling intervention for AI/AN teenagers at high risk of GDM | Qualitative interviews and focus groups with AI/AN women with type 2 diabetes or a history of GDM to inform the development of Stopping GDM | Included 5 AI/AN women with type 2 diabetes or a history of GDM | Participants raised a lack of knowledge on GDM and risk factors, importance of culture and family, suggestions for communication with AI/AN girls to reduce risk of GDM (open non-judgemental conversation, using stories, and connecting to culture), and the emotional impact of GDM diagnosis. |
| Stotz et al. (2021) [89] | AI/AN, Not stated/unclear | ‘Stopping GDM’—gestational diabetes preconception risk reduction and counselling intervention for AI/AN teenagers at high risk of GDM | Qualitative interviews and focus groups with AI/AN women, AI/AN girls and their mothers, health care providers and administrators, AI/AN tribal leaders, and health system administrators to understand how stakeholders understand perceptions on food and nutrition in terms of gestational diabetes risk reduction for AI/AN adolescent girls | Included 5 AI/AN women with a history of GDM, 14 AI/AN girls at risk of GDM and their mothers (n = 11), 16 health care providers and administrators, 12 AI/AN tribal leaders, and health system administrators | Participants raised that AI/AN women were not aware of how healthy nutrition and healthy weight gain during pregnancy were linked to reducing risk of GDM, the need for education on the role of nutrition and weight management in GDM risk reduction, and challenges of healthful eating before and during pregnancy for AI/AN women. |
| Stotz et al. (2023) [90] | AI/AN, Not stated/unclear | ‘Stopping GDM’—gestational diabetes preconception risk reduction and counselling intervention for AI/AN teenagers at high risk of GDM | Secondary analysis of randomised controlled trial of a GDM risk reduction intervention for at-risk AI/AN teens, ‘Stopping GDM’, to explore food insecurity as a potential moderator of the effect of Stopping GDM on healthy eating behaviours and self-efficacy | Included 149 female AI/AN young women, 12–24 years old, and their mothers (or adult female caregivers) who participated in Stopping GDM | At 3 months post-intervention, the intervention group had increases in times eating vegetables per week (mean change on a scale of 0 to 24: intervention = 1.0, 95% CI −0.2 to 2.2, control = −0.9 95% CI −2.0 to 0.2, p = 0.022) and fruit (mean change on a scale of 0 to 6: intervention 0.5, 95% CI 0.6 to 0.9, control −0.3, 95% CI −0.8 to 0.2, p = 0.015), whereas the control group had declines. Food insecurity did not moderate the group by time interaction for healthy eating self-efficacy (p ≥ 0.05) but did moderate the group by time interaction for times drinking soda (p = 0.004) and days eating breakfast (p = 0.013). |
| Terry et al. (2020) [92] | AI/AN, Urban and rural | ‘Stopping GDM’—gestational diabetes preconception risk reduction and counselling intervention for AI/AN teenagers at high risk of GDM | Qualitative interviews and focus groups with health professionals to inform the adaption of a preconception counselling and diabetes education intervention for AI/AN teens | Included 16 health professionals with expertise in AI/AN youth, adolescent health, and gestational diabetes | Participants felt that the content would be appropriate for AI/AN teens and their mothers or another adult. Participants made recommendations regarding effective communication with AI/AN teens, including culturally appropriate images, using non-directive language, and education with a community focus. Concerns included: socioeconomic issues that affect AI/AN people such as food and housing insecurity, abuse, and historical trauma. |
| Author and Date | Setting (Indigenous Population, Geographical Remoteness) | Study Description | Participants/Sample | Key Results |
|---|---|---|---|---|
| Australia | ||||
| Gilbert et al. (2021) [43] | Aboriginal and Torres Strait Islander, Rural | Mixed methods study including Aboriginal and Torres Strait Islander and non-Indigenous youth to understand Aboriginal and Torres Strait Islander youth preconception awareness and knowledge and to co-design a preconception health resource | Included 4 Aboriginal and Torres Strait Islander and 3 non-Indigenous youth of 18–25 years living in Darwin and employed as trainees or undergraduate research assistant cadets, who participated in a youth working group and 24 residents of the NT of 16–22 years who completed a survey | Participants of consultation meetings identified an interest in fertility and a need for more information on lifestyle factors associated with infertility. Participants were interested in learning more about preconception health, including regarding the opposite sex, and using information that incorporates current local knowledge and world views. Of survey respondents, 45% had a pre-existing understanding of preconception health. Optimising lifestyle behaviours prior to pregnancy was perceived as important for women (88%), but less so for men (67%). |
| Griffiths et al. (2020) [44] | Aboriginal, Rural | Retrospective audit of medical records of Aboriginal women to understand preconception health care delivery | Included 127 Aboriginal women who attended a remote Australian Aboriginal Community Controlled Health Service and who had at least one pregnancy from 2011 to 2018. | In total, 121/177 (68%) of confirmed pregnancies had received preconception care prior to the pregnancy. Sexually transmissible infection screening (71%) was the most common care delivered, followed by folic acid prescription (57%) and smoking cessation support (43%). Nutrition and weight (36%), alcohol and illicit substances (26%), chronic disease management (17%), and vaccinations (12%) were less common preconception care interventions. Preconception care was usually patient-initiated (63%), conducted by a nurse or Aboriginal Health Worker (59%) and increased significantly over the audit period (number of consultations p = 0.003).There were no differences in the likelihood of receiving preconception care by age group, pregnancy outcome, gestation at first antenatal visit, parity, diabetes or albuminuria status, BMI, or smoking behaviour. Younger women were less likely to be screened for chronic diseases (linear trend across age groups p < 0.01). |
| New Zealand | ||||
| Tromop-van Dalen et al. (2021) [59] | Māori, Not stated/unclear | Mixed methods study including 1. review of clinical letters for women with heart disease and 2. survey of Wellington Hospital cardiologists to understand frequency of preconception counselling and barriers | Included 199 women of 15–52 years with heart disease who attended Wellington Hospital cardiology clinics in 2015–2020 (clinical letter review) and 16 Wellington Hospital cardiologists, who completed a survey | In total, 32% of Māori women had documented contraception and preconception counselling discussions.Barriers to discussing contraception were time limitations, cultural barriers, presence of family members, not considered, considered outside of expertise. |
| United States | ||||
| Lammers et al. (2017) [76] | Native American, Rural | Cross-sectional survey of women, including Native American women, to understand awareness and access to preconception health care | Included 868 women of 18–45 years, including 258 Native American women, who were receiving community health services from the South Dakota Department of Health | In total, 79.1% of Native American women reported receiving 1+ preconception health care interventions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, C.; Begum, T.; Boyle, J.A.; Ward, J.; Barzi, F. Preconception Health of Indigenous Peoples in Australia, Canada, New Zealand, and the United States: A Scoping Review. Int. J. Environ. Res. Public Health 2024, 21, 345. https://doi.org/10.3390/ijerph21030345
Walker C, Begum T, Boyle JA, Ward J, Barzi F. Preconception Health of Indigenous Peoples in Australia, Canada, New Zealand, and the United States: A Scoping Review. International Journal of Environmental Research and Public Health. 2024; 21(3):345. https://doi.org/10.3390/ijerph21030345
Chicago/Turabian StyleWalker, Clara, Tahmina Begum, Jacqueline A Boyle, James Ward, and Federica Barzi. 2024. "Preconception Health of Indigenous Peoples in Australia, Canada, New Zealand, and the United States: A Scoping Review" International Journal of Environmental Research and Public Health 21, no. 3: 345. https://doi.org/10.3390/ijerph21030345
APA StyleWalker, C., Begum, T., Boyle, J. A., Ward, J., & Barzi, F. (2024). Preconception Health of Indigenous Peoples in Australia, Canada, New Zealand, and the United States: A Scoping Review. International Journal of Environmental Research and Public Health, 21(3), 345. https://doi.org/10.3390/ijerph21030345






