Hemodynamic and Metabolic Responses to Moderate and Vigorous Cycle Ergometry in Men Who Have Had Transtibial Amputation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Assessment of VO2max and VT
2.4. Assessment of Thoracic Impedance
2.5. MICE and HIIE Sessions
2.6. Assessment of RPE and FS
2.7. Statistical Analysis
3. Results
3.1. Maximal Data in TTAs and CONs
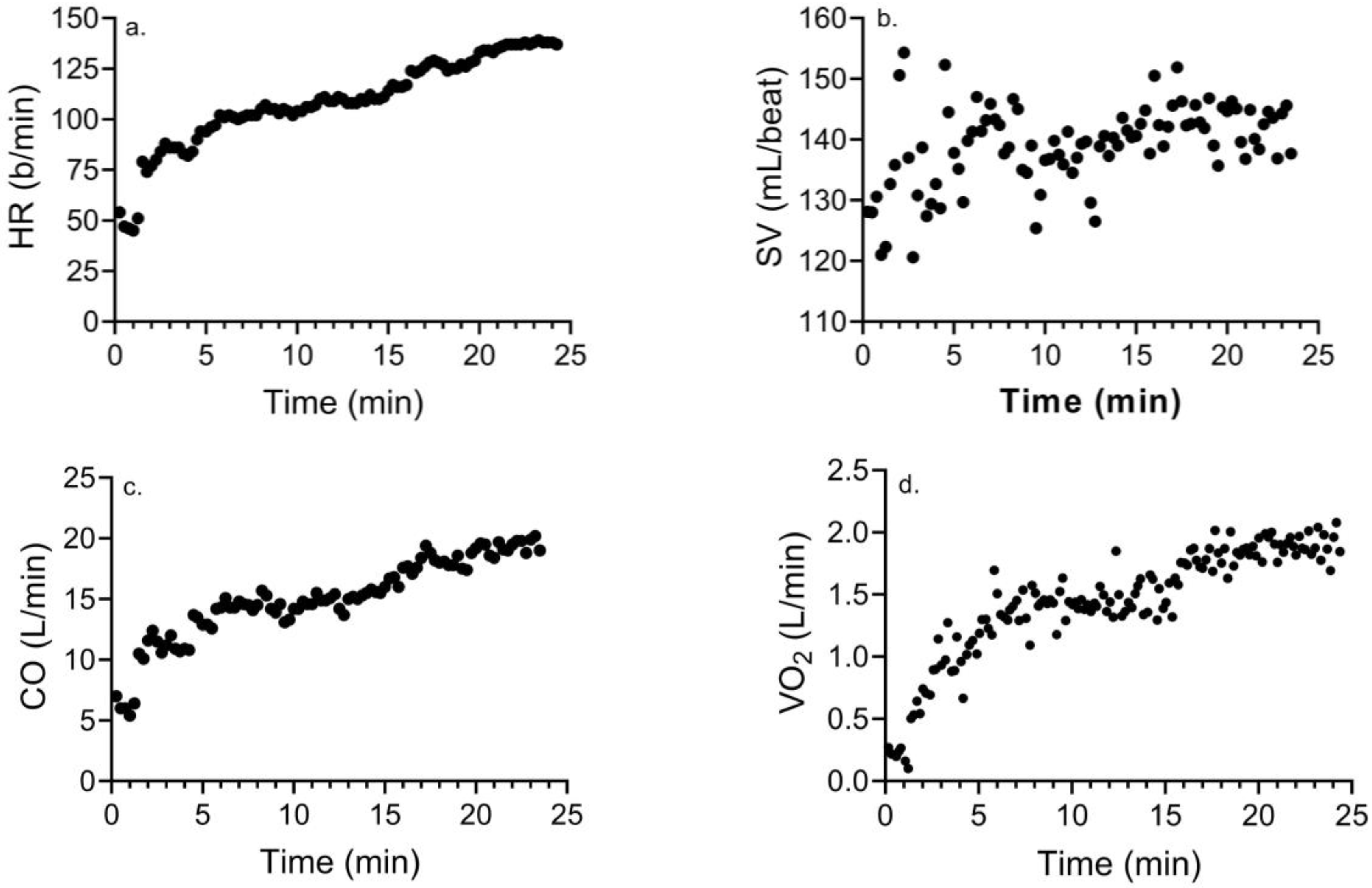
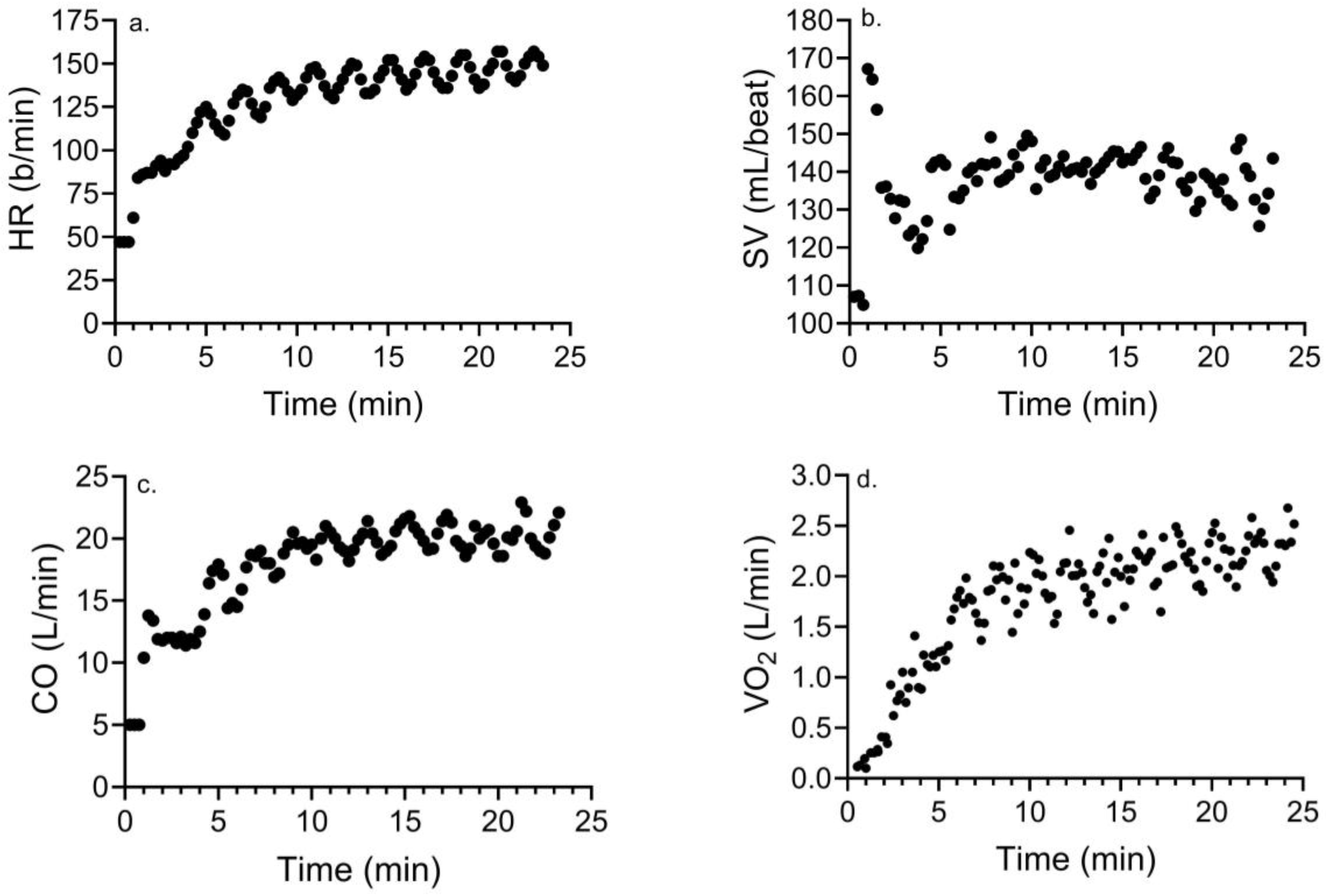
3.2. Changes in Hemodynamic and Cardiometabolic Responses with MICE and HIIE
3.3. Changes in Perceptual Responses to MICE and HIIE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Limb Loss Research Center, Washington, DC, USA. Available online: https://www.amputee-coalition.org/limb-loss-resource-center/ (accessed on 10 July 2023).
- Wong, C.K.; Rissland, M.S.; Madagan, D.M.; Jones, K.N. A scoping review of physical activity in people with lower-limb loss: 10,000 steps per day? Phys. Ther. 2021, 101, 115. [Google Scholar] [CrossRef] [PubMed]
- Deans, S.; Burns, D.; McGarry, A.; Murray, K.; Mutrie, N. Motivations and barriers to prosthesis users participation in physical activity, exercise and sport: A review of the literature. Pros. Orthotic Int. 2012, 36, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Ladlow, P.; Nightingale, T.E.; McGuigan, P.M.; Bennett, A.N.; Phillip, R.D.; Bilzon, J.L.J. Predicting ambulatory energy expenditure in lower limb amputees using multi-sensor methods. PLoS ONE 2019, 14, e209249. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Sawamura, S.; Fujita, H.; Nakajima, S.; Oyabu, H.; Nagakura, Y.; Ojima, I.; Otsuka, H.; Nakagawa, A. Physical fitness of lower limb amputees. Am. J. Phys. Med. Rehabil. 2002, 81, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Gjovaag, T.; Starholm, I.M.; Mirtaheri, P.; Hegge, F.W.; Skjetne, K. Assessment of aerobic capacity and walking economy of unilateral transfemoral amputees. Prosthet. Orthot. Int. 2014, 38, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Sawamura, S.; Fujita, H.; Nakajima, S.; Ojima, I.; Oyabu, H.; Nagakura, Y.; Otsuka, H.; Nakagawa, A. Effect of endurance training program based on anaerobic threshold (AT) for lower limb amputees. J. Rehabil. Res. Dev. 2001, 38, 7–11. [Google Scholar] [PubMed]
- Nevin, J.; Smith, P. The effectiveness of a 30-week concurrent strength and endurance training program in preparation for an ultra-endurance handcycling challenge: A case study. Int. J. Sports Physiol. Perf. 2021, 16, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Starholm, I.M.; Gjovaag, T.; Mengshoel, A.M. Energy expenditure of transfemoral amputees walking on a horizontal and tilted treadmill simulating different outdoor walking conditions. Prosthet. Orthot. Int. 2017, 34, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Kurdibaylo, S. Cardiorespiratory status and movement capabilities in adults with limb amputation. J. Rehabil. Res. Dev. 1994, 31, 222–235. [Google Scholar]
- Ewing-Garber, C.; Blissmer, B.; Deschenes, M.R.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1360. [Google Scholar] [CrossRef]
- Astorino, T.A.; Causer, E.; Hazell, T.J.; Arhen, B.B.; Gurd, B.J. Change in central cardiovascular function in response to intense interval training: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2022, 54, 1991–2004. [Google Scholar] [CrossRef]
- Jelleyman, C.; Yates, T.; O’Donovan, G.; Gray, L.J.; King, J.A.; Khunti, K.; Davies, M.J. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 2015, 16, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Batacan, R.B., Jr.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017, 51, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, Z.; Sporis, G.; Weston, M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L. Generalized equations for predicting body density of men. Br. J. Nutr. 1978, 40, 497–504. [Google Scholar] [CrossRef]
- Astorino, T.A.; White, A.C.; Dalleck, L.C. Supramaximal testing to confirm attainment of VO2max in sedentary men and women. Int. J. Sports Med. 2009, 30, 279–284. [Google Scholar] [CrossRef]
- Caiozzo, V.J.; Davis, J.A.; Ellis, J.F.; Azus, J.L.; Vandagriff, R.; Prietto, C.A.; McMaster, W.C.; Wilhelm, E.N.; González-Alonso, J.; Parris, C.; et al. A comparison of gas exchange indices used to detect the anaerobic threshold. J. Appl. Physiol. 1982, 53, 1184–1189. [Google Scholar] [CrossRef]
- Charloux, A.; Lonsdorfer-Wolf, E.; Richard, R.; Lampert, E.; Oswald-Mammosser, M.; Mettauer, B.; Geny, B.; Lonsdorfer, J. A new impedance cardiographic device for the non-invasive evaluation of cardiac output at rest and during exercise: A comparison with the “direct” Fick method. Eur. J. Appl. Physiol. 2000, 82, 313–320. [Google Scholar] [CrossRef]
- Richard, R.; Lonsdorfer-Wolf, E.; Charloux, A.; Doutreleau, S.; Buchheit, M.; Oswald-Mammosser, M.; Lampert, E.; Mettauer, B.; Geny, B.; Lonsdorfer, J. Non-invasive cardiac output during maximal progressive exercise test by a new impedance cardiograph device. Eur. J. Appl. Physiol. 2001, 85, 202–207. [Google Scholar] [CrossRef] [PubMed]
- De Revere, J.; Clausen, R.D.; Astorino, T.A. Changes in VO2max and cardiac output in response to short-term high-intensity interval training in Caucasian and Hispanic young women: A pilot study. PLoS ONE 2021, 16, e0244850. [Google Scholar] [CrossRef] [PubMed]
- Zafeiridis, A.; Kounoupis, A.; Dipla, K.; Kyparos, A.; Nikolaidis, M.G.; Smilios, I.; Vrabas, I.S. Oxygen delivery and muscle deoxygenation during continuous, long- and short-interval exercise. Int. J. Sports Med. 2015, 36, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.J.; Rejeski, W.J. Not what, but how one feels: The measurement of affect during exercise. J. Sport. Exerc. Psychol. 1989, 11, 304–317. [Google Scholar] [CrossRef]
- Kendzierski, D.; DeCarlo, K.J. Physical activity enjoyment scale: Two validation studies. J. Sport. Exerc. Psychol. 1991, 13, 50–64. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Imboden, M.T.; Arena, R.; Myers, J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: Data from the fitness registry and the importance of exercise national database (FRIEND) registry. Mayo Clin. Proc. 2017, 92, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Iannetta, D.; Inglis, E.C.; Mattu, A.T.; Fontana, F.Y.; Pogliaghi, S.; Keir, D.A.; Murias, J.M. A critical evaluation of current methods for exercise prescription in women and men. Med. Sci. Sports Exerc. 2020, 52, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.W.; McNaughton, L.R. Time at or near VO2max during continuous and intermittent running. A review with special reference to considerations for the optimisation of training protocols to elicit the longest time at or near VO2max. J. Sports Med. Phys. Fit. 2006, 46, 1–14. [Google Scholar]
- Thum, J.S.; Parsons, G.; Whittle, T.; Astorino, T.A. High-intensity interval training elicits higher enjoyment than moderate intensity continuous exercise. PLoS ONE 2017, 12, e0166299. [Google Scholar] [CrossRef]
- Astorino, T.A.; Thum, J.S. Higher enjoyment in response to high intensity interval training in spinal cord injury. J. Spinal Cord. Med. 2018, 41, 77–84. [Google Scholar] [CrossRef]
- Jarvis, H.L.; Bennett, A.N.; Twiste, M.; Phillip, R.D.; Etherington, J.; Baker, R. Temporal spatial and metabolic measures of walking in highly functional individuals with lower limb amputations. Arch. Phys. Med. Rehabil. 2017, 98, 1389–1399. [Google Scholar] [CrossRef]
- van Schaik, L.; Geertzen, J.H.B.; Dijkstra, P.U.; Dekker, R. Metabolic costs of activities of daily living in persons with a lower limb amputation: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0213256. [Google Scholar] [CrossRef] [PubMed]
- Wezenberg, D.S.; van der Woude, L.H.; Faber, W.X.; de Haan, A.; Houdijk, H. Relation between aerobic capacity and walking ability in older adults with a lower-limb amputation. Arch. Phys. Med. Rehabil. 2013, 94, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Wezenberg, D.; de Hann, A.; Faber, W.X.; Slootman, H.J.; van der Woude, L.H.; Houdijk, H. Peak oxygen consumption in older adults with a lower limb amputation. Arch. Phys. Med. Rehabil. 2012, 93, 1924–1929. [Google Scholar] [CrossRef]
- Vella, C.A.; Robergs, R.A. A review of the stroke volume response to upright exercise in healthy subjects. Br. J. Sports Med. 2005, 39, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Wade, K.H.; Chiesa, S.T.; Hughes, A.D.; Chaturvedi, N.; Charakida, M.; Rapala, A.; Muthurangu, V.; Khan, T.; Finer, N.; Sattar, N.; et al. Assessing the causal role of body mass index on cardiovascular health in young adults. Circulation 2018, 138, 2187–2201. [Google Scholar] [CrossRef]
- Brooks, G.A.; Mercier, J. Balance of carbohydrate and lipid utilization during exercise: The “crossover” concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef]
- Simim, M.A.M.; da Mota, G.R.; Marocolo, M.; da Silva, B.V.; de Mello, M.T.; Bradley, P.S. The demands of amputee soccer impair muscular endurance and power indices but not match physical performance. Adapt. Phys. Activ Q. 2018, 35, 76–92. [Google Scholar] [CrossRef]
- Mohr, M.; Krustrup, P.; Bangsbo, J. Fatigue in soccer: A brief review. J. Sports Sci. 2000, 23, 593–599. [Google Scholar] [CrossRef]
- Spriet, L.L.; Howlett, R.A.; Heigenhauser, G.J. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med. Sci. Sports Exerc. 2000, 32, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Dunsiger, S.; Ciccoli, J.T.; Lewis, B.A.; Albrecht, A.E.; Marcus, B.H. Acute affective responses to a moderate-intensity exercise stimulus predicts physical activity participation 6 and 12 months later. Psychol. Sport. Exerc. 2008, 9, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Reichert, F.F.; Barros, A.J.; Domingues, M.R.; Hallal, P.C. The role of perceived personal barriers to engagement in leisure-time physical activity. Am. J. Public. Health 2007, 97, 168–173. [Google Scholar] [CrossRef] [PubMed]


| Outcome | TTAs | Range | CONs | Range | p Value |
|---|---|---|---|---|---|
| Age (yr) | 39 ± 15 | 21–61 | 32 ± 11 | 21–50 | 0.37 |
| TSA (yr) | 8 ± 5 | 0.5–12.0 | NA | ||
| Body mass (kg) | 75 ± 10 | 60–86 | 86 ± 11 | 69–99 | 0.11 |
| BMI (kg/m2) | 23.8 ± 3.5 | 20.3–29.1 | 27.0 ± 3.4 | 23.0–33.3 | 0.08 |
| Body fat (%) | 14.4 ± 6.9 | 7.1–26.1 | 18.3 ± 6.1 | 15.3–25.2 | 0.37 |
| Physical activity (h/wk) | 7.8 ± 4.5 | 1–12 | 5.3 ± 1.4 | 3–7 | 0.22 |
| Outcome | TTAs | Range | CONs | Range | p Value |
|---|---|---|---|---|---|
| Wmax (W) | 256 ± 44 | 191–302 | 298 ± 48 | 251–359 | 0.16 |
| VO2max (mL/kg/min) | 39.2 ± 7.5 | 28–46 | 40.2 ± 3.6 | 35–46 | 0.76 |
| VO2max (L/min) | 2.89 ± 0.42 | 2.38–3.49 | 3.43 ± 0.50 | 2.80–4.05 | 0.09 |
| VEmax (L/min) | 151.1 ± 9.5 | 137–160 | 147.7 ± 20.5 | 115–218 | 0.85 |
| RER | 1.30 ± 0.05 | 1.24–1.37 | 1.24 ± 0.10 | 1.12–1.36 | 0.27 |
| HRmax (b/min) | 181 ± 11 | 169–193 | 182 ± 9 | 166–191 | 0.89 |
| SVmax (mL/beat) | 118 ± 25 | 82–142 | 134 ± 24 | 117–180 | 0.30 |
| COmax (L/min) | 22.0 ± 4.1 | 15–26 | 23.8 ± 2.7 | 20–28 | 0.23 |
| BLa (mM) | 11.1 ± 1.5 | 8.9–12.6 | 10.7 ± 1.4 | 8.2–12.1 | 0.70 |
| VT (%VO2 max) | 65 ± 4 | 61–72 | 66 ± 5 | 55–70 | 0.86 |
| Outcome | TTAs | CONs | Bouts | BoutsXgroup | ||
|---|---|---|---|---|---|---|
| MICE | HIIE | MICE | HIIE | |||
| EE (kcal) | 159 ± 16 | 183 ± 42 * | 196 ± 31 | 216 ± 29 * | 0.02 | 0.72 |
| Mean HR (b/min) | 130 ± 14 | 144 ± 12 * | 126 ± 10 | 141 ± 12 * | <0.001 | 0.76 |
| Mean SV (mL) | 114 ± 17 | 113 ± 16 | 114 ± 18 | 127 ± 20 a | 0.054 | 0.02 |
| Mean CO (L/min) | 14.7 ± 1.3 | 16.1 ± 2.1 * | 14.4 ± 1.8 | 17.4 ± 2.8 * | 0.001 | 0.08 |
| Mean VO2 (L/min) | 1.47 ± 0.15 | 1.83 ± 0.40 * | 1.84 ± 0.25 | 2.10 ± 0.30 * | <0.001 | 0.42 |
| Peak HR (b/min) | 149 ± 17 | 161 ± 14 * | 144 ± 12 | 160 ± 10 * | 0.002 | 0.55 |
| Peak SV (mL) | 118 ± 16 | 120 ± 13 | 119 ± 12 | 134 ± 17 *,a | 0.006 | 0.02 |
| Peak CO (L/min) | 17.4 ± 1.4 | 19.0 ± 1.9 * | 17.0 ± 1.9 | 21.5 ± 2.5 *,a | <0.001 | 0.007 |
| Peak VO2 (L/min) | 1.82 ± 0.21 | 2.17 ± 0.49 * | 2.27 ± 0.31 | 2.58 ± 0.40 * | <0.001 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storey, K.K.; Geschwindt, A.; Astorino, T.A. Hemodynamic and Metabolic Responses to Moderate and Vigorous Cycle Ergometry in Men Who Have Had Transtibial Amputation. Int. J. Environ. Res. Public Health 2024, 21, 450. https://doi.org/10.3390/ijerph21040450
Storey KK, Geschwindt A, Astorino TA. Hemodynamic and Metabolic Responses to Moderate and Vigorous Cycle Ergometry in Men Who Have Had Transtibial Amputation. International Journal of Environmental Research and Public Health. 2024; 21(4):450. https://doi.org/10.3390/ijerph21040450
Chicago/Turabian StyleStorey, Kionte K., Adam Geschwindt, and Todd A. Astorino. 2024. "Hemodynamic and Metabolic Responses to Moderate and Vigorous Cycle Ergometry in Men Who Have Had Transtibial Amputation" International Journal of Environmental Research and Public Health 21, no. 4: 450. https://doi.org/10.3390/ijerph21040450
APA StyleStorey, K. K., Geschwindt, A., & Astorino, T. A. (2024). Hemodynamic and Metabolic Responses to Moderate and Vigorous Cycle Ergometry in Men Who Have Had Transtibial Amputation. International Journal of Environmental Research and Public Health, 21(4), 450. https://doi.org/10.3390/ijerph21040450







