Chi-Squared Automatic Interaction Detection Decision Tree Analysis of Social Determinants for Low Birth Weight in Virginia
Abstract
:1. Introduction
2. Methods
2.1. Data and Variables
2.2. Data Analysis
3. Results
3.1. Demographic Profile
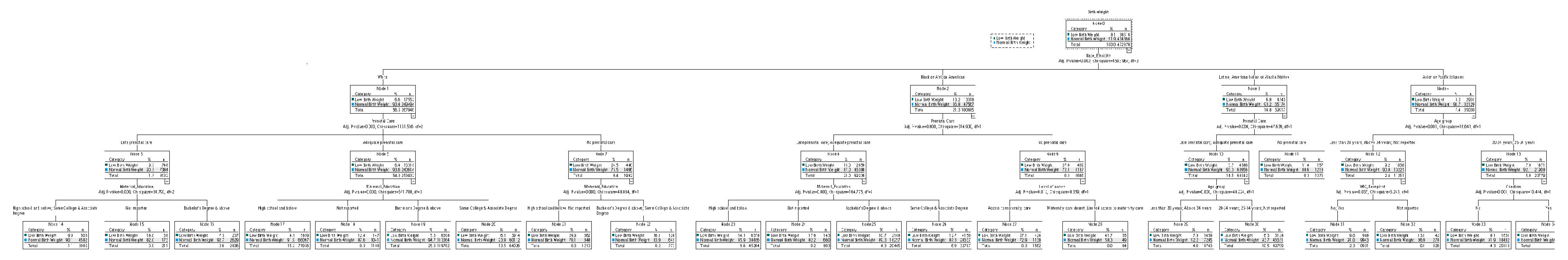
3.2. Chi-Squared Automatic Interaction Detection Decision Tree Analysis of Low Birth Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. 2023. Available online: https://www.who.int/data/nutrition/nlis/info/low-birth-weight (accessed on 12 December 2023).
- Kramer, M.S. Determinants of low birth weight: Methodological assessment and meta-analysis. Bull. World Health Organ. 1987, 65, 663–737. [Google Scholar] [PubMed]
- Ely, D.M.; Driscoll, A.K. Infant mortality in the United States, 2017: Data from the period linked birth/infant death file. Natl. Vital Stat. Rep. 2019, 68, 1–20. [Google Scholar] [PubMed]
- Jacob, J.A. US infant mortality rate declines but still exceeds other developed countries. JAMA 2016, 315, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.F.; Indurkhya, A.; McCormick, M.C. Prevalence, stability and predictors of clinically significant behavior problems in low birthweight children at 3, 5 and 8 years of age. Pediatrics 2004, 114, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Gosdin, L.K.; Deputy, N.P.; Kim, S.Y.; Dang, E.P.; Denny, C.H. Alcohol Consumption and Binge Drinking During Pregnancy Among Adults Aged 18–49 Years—United States, 2018–2020. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Pintican, D.; Poienar, A.A.; Strilciuc, S.; Mihu, D. Effects of maternal smoking on human placental vascularization: A systematic review. Taiwan J. Obs. Gynecol. 2019, 58, 454–459. [Google Scholar] [CrossRef]
- Simhan, H.N.; Caritis, S.N. Prevention of Preterm Delivery. N. Engl. J. Med. 2007, 357, 477–487. [Google Scholar] [CrossRef]
- Krukowski, R.A.; Jacobson, L.T.; John, J.; Kinser, P.; Campbell, K.; Ledoux, T.; Gavin, K.L.; Chiu, C.Y.; Wang, J.; Kruper, A. Correlates of early prenatal care access among us women: Data from the pregnancy risk assessment monitoring system (PRAMS). Matern. Child Health J. 2022, 26, 328–341. [Google Scholar] [CrossRef]
- Thompson, E.L.; Barnett, T.E.; Litt, D.M.; Spears, E.C.; Lewis, M.A. Discordance between perinatal alcohol use among women and provider counseling for alcohol use: An assessment of the Pregnancy Risk Assessment Monitoring System. Public Health Rep. 2021, 136, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.M.; Dinsmoor, M.J.; Lin, P.Y. Preventable risk factors for the delivery of very low birth weight infants in Richmond, Virginia. Prim. Care Update OB/GYNS 2001, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- March of Dimes. March of Dimes Report Card. 2022. Available online: https://www.marchofdimes.org/sites/default/files/2022-11/2022-MarchofDimes-ReportCard-UnitedStates.pdf (accessed on 11 January 2024).
- Shmueli, A.; Cullen, M.R. Birth weight, maternal age, and education: New observations from Connecticut and Virginia. Yale J. Biol. Med. 1999, 72, 245. [Google Scholar]
- Virginia Department of Health. 2010. Available online: https://apps.vdh.virginia.gov/HealthStats/stats.htm (accessed on 15 April 2024).
- Virginia Division of Population Health Data. Virginia Pregnancy Risk Assessment Monitoring System; Virginia Department of Health: Richmond, VA, USA, 2020. [Google Scholar]
- Shah, M.K.; Austin, K.R. Do home visiting services received during pregnancy improve birth outcomes? Findings from Virginia PRAMS 2007–2008. Public Health Nurs. 2014, 31, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Veith, S.T.; Chisholm, C.; Novicoff, W.; Rheuban, K.; Cohn, W. Perinatologists and Advanced Practice Nurses Collaborate to Provide High-Risk Prenatal Care in Rural Virginia Communities. J. Obstet. Gynecol. Neonatal Nurs. 2014, 43, S26–S27. [Google Scholar] [CrossRef]
- Ye, F.; Chen, Z.H.; Chen, J.; Liu, F.; Zhang, Y.; Fan, Q.Y.; Wang, L. Chi-squared Automatic Interaction Detection Decision Tree Analysis of Risk Factors for Infant Anemia in Beijing, China. Chin. Med. J. 2016, 129, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Milovic, B.; Milovic, M. Prediction and Decision Making in Health Care using Data Mining. Int. J. Public Health Sci. (IJPHS) 2012, 1, 69–78. [Google Scholar] [CrossRef]
- Shanthi, D.; Sahoo, G.; Saravanan, N. Feature Selection using Hybrid Neuro Genetic Approach in the diagnosis of Stroke. Int. J. Comput. Sci. Netw. Secur. 2008, 8, 99–107. [Google Scholar]
- Vlahou, A.; Schorge, J.O.; Gregory, B.W.; Coleman, R.L. Diagnosis of Ovarian Cancer Using Decision Tree Classification of Mass Spectral data. J. Biotechnol. 2003, 5, 308–314. [Google Scholar]
- Tao, J.; Yuan, Z.; Sun, L.; Yu, K.; Zhang, Z. Fetal birthweight prediction with measured data by a temporal machine learning method. BMC Med. Inform. Decis. Mak. 2021, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bracken, M.B. Tree-based risk factor analysis of preterm delivery and small-for-gestational-age birth. Am. J. Epidemiol. 1995, 141, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Collin, D.F.; Guan, A.; Hamad, R. Predictors of WIC uptake among low-income pregnant individuals: A longitudinal nationwide analysis. Am. J. Clin. Nutr. 2023, 117, 1331–1341. [Google Scholar] [CrossRef]
- Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). USDA. 2023. Available online: https://www.fns.usda.gov/wic (accessed on 16 January 2024).
- Qin, C.; Deng, Y.; Chen, W.T.; Mi, C.; Wang, W.; Sun, M.; Chen, J.; Liu, W.; Tang, S. Does previous cesarean section influence neonatal birth weight? A path analysis in China. Women Birth J. Aust. Coll. Midwives 2019, 32, e71–e76. [Google Scholar] [CrossRef]
- Silver, R.M. Implications of the first cesarean: Perinatal and future reproductive health and subsequent cesareans, placentation issues, uterine rupture risk, morbidity, and mortality. In Seminars in Perinatology; WB Saunders: Philadelphia, PA, USA, 2012; Volume 36, pp. 315–323. [Google Scholar]
- Kotelchuck, M. The adequacy of prenatal care utilization index: Its US distribution and association with low birthweight. Am. J. Public Health 1994, 84, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.A.; Cameron, N.A.; Culler, K.; Grobman, W.A.; Khan, S.S. Rural–Urban Disparities in Adverse Maternal Outcomes in the United States, 2016–2019. Am. J. Public Health 2023, 113, 224–227. [Google Scholar] [CrossRef]
- National Center for Health Workforce Analysis Report. 2022. Available online: https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/maternal-health-workforce-brief-2022.pdf (accessed on 17 January 2024).
- Jean-Louis, F. Lost in the Maternity Care Desert: The Worsening Crisis in Rural Obstetrics Care, RTI Health Advance. 2022. Available online: https://healthcare.rti.org/insights/maternity-care-deserts-rural-obstetrics (accessed on 12 December 2023).
- Kozhimannil, K.B.; Henning-Smith, C.; Hung, P.; Casey, M.M.; Prasad, S. Ensuring access to high-quality maternity care in rural America. Women’s Health Issues 2016, 26, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Maternity Care Target Area (MCTA). Available online: https://bhw.hrsa.gov/workforce-shortage-areas/shortage-designation#mcta (accessed on 17 January 2024).
- Kass, G.V. An exploratory technique for investigating large quantities of categorical data. Appl. Stat. 1980, 29, 119–127. [Google Scholar] [CrossRef]
- March of Dimes, Nowhere to Go: Maternity Care Deserts across the U.S.; 2020 Report. 2020. Available online: https://www.marchofdimes.org/materials/2020_Annual_Report_Financials.pdf (accessed on 5 May 2023).
- Virginia Vital Records and Health Statistics. Electronic Birth Certificates; Virginia Department of Health: Richmond, VA, USA, 2020. [Google Scholar]
- Green, T.L.; Bodas, M.V.; Jones, H.A.; Masho, S.W.; Hagiwara, N. Disparities in self-reported prenatal counseling: Does immigrant status matter? J. Community Health 2018, 43, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Osterman, M.J.K.; Martin, J.A. Timing and adequacy of prenatal care in the United States, 2016. Natl. Vital Stat. Rep. 2018, 67, 1–13. [Google Scholar] [PubMed]
- Svikis, D.S.; Kelpin, S.S.; Keyser-Marcus, L.; Bishop, D.L.; Parlier-Ahmad, A.B.; Jones, H.; Villalobos, G.; Varner, S.B.; Lanni, S.M.; Karjane, N.W.; et al. Increasing Prenatal Care Compliance in At-Risk Black Women: Findings from a RCT of Patient Navigation and Behavioral Incentives. J. Racial Ethn. Health Disparities 2022, 9, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Bater, J.; Lauer, J.M.; Ghosh, S.; Webb, P.; Agaba, E.; Bashaasha, B.; Turyashemererwa, F.M.; Shrestha, R.; Duggan, C.P. Predictors of low birth weight and preterm birth in rural Uganda: Findings from a birth cohort study. PLoS ONE 2020, 15, e0235626. [Google Scholar] [CrossRef] [PubMed]
- Fall, C.H.; Sachdev, H.S.; Osmond, C.; Restrepo-Mendez, M.C.; Victora, C.; Martorell, R.; Stein, A.D.; Sinha, S.; Tandon, N.; Adair, L.; et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: A prospective study in five low-income and middle-income countries (COHORTS collaboration). Lancet Glob. Health 2015, 3, e366–e377. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Hacker, M.R. Birth outcome racial disparities: A result of intersecting social and environmental factors. Semin. Perinatol. 2017, 41, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Mazul, M.C.; Ward, T.C.S.; Ngui, E.M. Anatomy of good prenatal care: Perspectives of low-income African American women on barriers and facilitators to prenatal care. J. Racial Ethn. Health Disparities 2017, 4, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.H.; Hummer, R.A.; Sierra, G.; Hargrove, T.; Powers, D.A.; Rogers, R.G. Race/ethnicity, maternal educational attainment, and infant mortality in the United States. Biodemography Soc. Biol. 2020, 66, 1–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hueston, W.J.; Gilbert, G.E.; Davis, L.; Sturgill, V. Delayed prenatal care and the risk of low birth weight delivery. J. Community Health 2003, 28, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kozhimannil, K.B.; Interrante, J.D.; Henning-Smith, C.; Admon, L.K. Rural-urban differences in severe maternal morbidity and mortality in the US, 2007–2015. Health Aff. 2019, 38, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.M.; Jones, K.A.; Yee, L.M.; Feinglass, J. Modeling the Likelihood of Low Birth Weight: Findings from a Chicago-Area Health System. J. Racial Ethn. Health Disparities 2023, 10, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, K.; Wiseman, M. Birth weights and the geography of poverty. Focus 1989, 12, 7. [Google Scholar]
- Nobile, C.G.; Raffaele, G.; Altomare, C.; Pavia, M. Influence of maternal and social factors as predictors of low birth weight in Italy. BMC Public Health 2007, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Virginia Rural Health Plan 2022–2026. 2022. Available online: https://www.vdh.virginia.gov/content/uploads/sites/76/2022/01/Virginia-Rural-Health-Plan_Book_POST_1-24-22_LR.pdf (accessed on 15 April 2024).
- Choi, H.Y.; Kim, E.Y.; Kim, J. Prognostic factors in diabetes: Comparison of Chi-square automatic interaction detector (CHAID) decision tree technology and logistic regression. Medicine 2022, 101, e31343. [Google Scholar] [CrossRef]
- Welder, E.; Powers, J.; Walter, A.C.; Bedell, D.; Shen, W.W. Providing Prenatal Care for Patients with Limited Medical Insurance Coverage. J. Community Health 2022, 47, 974–980. [Google Scholar] [CrossRef]
| Characteristic | N | Proportion (%) |
|---|---|---|
| All | 472,878 | 100 |
| Maternal age * | ||
| <20 | 14,359 | 3.0 |
| 20–24 | 79,490 | 16.8 |
| 25–34 | 275,228 | 58.2 |
| >34 | 103,246 | 21.8 |
| Race/Ethnicity | ||
| Non-Hispanic White | 267,046 | 56.5 |
| Non-Hispanic Black | 100,885 | 21.3 |
| Non-Hispanic, American Indian or Alaskan native | 809 | 0.2 |
| Non-Hispanic Asian or Pacific Islander | 35,030 | 7.4 |
| Hispanic | 69,108 | 14.6 |
| WIC Recipients | ||
| Yes | 109,598 | 23.2 |
| No | 343,003 | 72.5 |
| Unknown | 20,277 | 4.3 |
| Maternal education | ||
| High school and below | 171,092 | 36.2 |
| Some College and Associate Degree | 118,226 | 25.0 |
| Bachelor’s Degree and above | 183,560 | 38.8 |
| Prenatal care | ||
| Adequate prenatal care | 449,402 | 95.0 |
| Late prenatal care | 18,329 | 3.9 |
| No prenatal care | 5147 | 1.1 |
| Level of access | ||
| Access to maternity care | 437,725 | 92.6 |
| Limited access to maternity care | 8993 | 1.9 |
| Maternity care desert | 26,160 | 5.5 |
| Birth weight | ||
| Normal birth weight | 434,364 | 91.9 |
| Low birth weight | 38,514 | 8.1 |
| Previous Cesarean section | ||
| Yes | 81,937 | 17.3 |
| No | 390,941 | 82.7 |
| Characteristic | Low Birth Weight N (%) | Normal Birth Weight N (%) | p |
|---|---|---|---|
| Maternal age | 0.000 * | ||
| <20 | 1441 (10.0) | 12,918 (90.0) | |
| 20–24 | 7139 (9.0) | 72,351 (91.0) | |
| 25–34 | 20,898 (7.6) | 254,330 (92.4) | |
| >34 | 8981 (8.7) | 94,265 (91.3) | |
| Race/Ethnicity | 0.000 * | ||
| Non-Hispanic White | 17,552 (6.6) | 249,494 (93.4) | |
| Non-Hispanic Black | 13,318 (13.2) | 87,567 (86.8) | |
| Non-Hispanic, American Indian, or Alaskan native | 57 (7.0) | 752 (93.0) | |
| Non-Hispanic Asian or Pacific Islander | 2901 (8.3) | 32,129 (91.7) | |
| Hispanic | 4686 (6.8) | 64,422 (93.2) | |
| WIC Recipients | 0.000 * | ||
| Yes | 10,481 (9.6) | 99,117 (90.4) | |
| No | 25,905 (7.6) | 317,098 (92.4) | |
| Unknown | 2128 (10.5) | 18,149 (89.5) | |
| Maternal education | 0.000 * | ||
| High school and below | 16,627 (9.7) | 154,465 (90.3) | |
| Some College and Associate Degree | 9692 (8.2) | 108,534 (91.8) | |
| Bachelor’s Degree and above | 12,195 (6.6) | 171,365 (93.4) | |
| Prenatal care | 0.000 * | ||
| Adequate prenatal care | 35,636 (7.9) | 413,766 (92.1) | |
| Late prenatal care | 1756 (9.6) | 16,573 (90.4) | |
| No prenatal care | 1122 (21.8) | 4025 (78.2) | |
| Level of access | 0.000 * | ||
| Access to maternity care | 35,507 (8.1) | 402218 (91.9) | |
| Limited access to maternity care | 604 (6.7) | 8389 (93.3) | |
| Maternity care desert | 2403 (9.2) | 23,757 (90.8) | |
| Previous Cesarean section | 0.044 ** | ||
| Yes | 6530 (8.0) | 75,407 (92.0) | |
| No | 31,984 (8.2) | 358,957 (91.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattath, P.; Maynor, M.R.; Anson-Dwamena, R. Chi-Squared Automatic Interaction Detection Decision Tree Analysis of Social Determinants for Low Birth Weight in Virginia. Int. J. Environ. Res. Public Health 2024, 21, 1060. https://doi.org/10.3390/ijerph21081060
Pattath P, Maynor MR, Anson-Dwamena R. Chi-Squared Automatic Interaction Detection Decision Tree Analysis of Social Determinants for Low Birth Weight in Virginia. International Journal of Environmental Research and Public Health. 2024; 21(8):1060. https://doi.org/10.3390/ijerph21081060
Chicago/Turabian StylePattath, Priyadarshini, Meagan Robinson Maynor, and Rexford Anson-Dwamena. 2024. "Chi-Squared Automatic Interaction Detection Decision Tree Analysis of Social Determinants for Low Birth Weight in Virginia" International Journal of Environmental Research and Public Health 21, no. 8: 1060. https://doi.org/10.3390/ijerph21081060




